Practical Guidelines by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) on Nutritional Management of Patients with Chronic Obstructive Pulmonary Disease: A Review
Abstract
1. Introduction
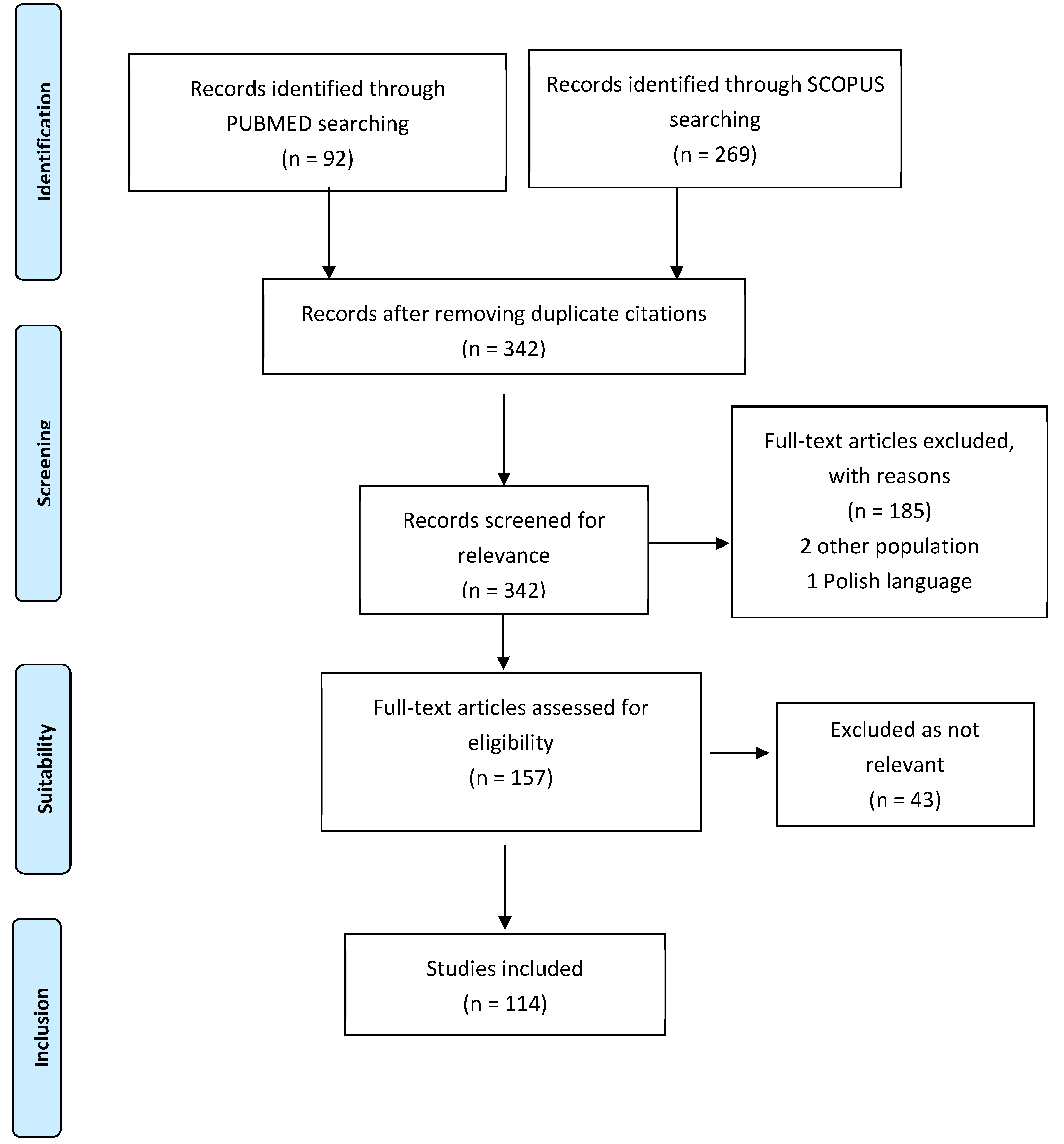
2. Methodology
3. Results and Discussion
3.1. General
3.1.1. Should Food Intake Be Assessed?
- Comment:
3.1.2. When Should COPD Patients’ Weight Loss Be Assessed?
- Comment:
3.1.3. Is It Possible to Establish a BMI Range for COPD Patients?
- Comment:
3.1.4. Should Body Composition Be Assessed in the COPD Patient and When Should It Be Done?
- Comment:
3.2. Nutritional Screening and Morphofuntional Assessment
3.2.1. In Which Patients under Follow-Up for COPD Should Nutritional Screening Be Performed?
- Comment:
3.2.2. What Nutritional Screening Tool Should Be Used?
- Comment:
3.2.3. How to Establish the Degree of Malnutrition after Screening?
- Comment:
3.2.4. Is It Necessary to Screen for Sarcopenia in These Patients?
- Comment:
3.2.5. Which Tool Should We Use for the Diagnosis of Sarcopenia?
- Comment:
3.2.6. How Should Muscle Function Be Assessed?
- Comment:
3.2.7. How to Assess Muscle Mass?
- Comment:
3.3. Nutritional Requirements
3.3.1. How to Measure Energy Requirements in Adult COPD Patients?
- Comment:
3.3.2. What Are the Protein Requirements of the COPD Patient?
- Comment:
3.3.3. What Is the Ideal Macronutrient Ratio in This Patient Group?
- There is insufficient evidence to make recommendations.
- Comment:
3.4. Nutritional Management
3.4.1. What Type of Diet Would Be Indicated?
- Comment
3.4.2. What Is the Role of Dietary Advice?
- Comment:
3.4.3. In Patients with COPD and Malnutrition, Is Nutritional Supplementation Associated with an Improvement in Morphofunctional Nutritional Parameters and Disease Progression?
- Comment—Weight and lean mass increase:
- Comment—Potency of effect in physical exercise:
- Comment—Improved muscle and lung function:
- Comment—Improved quality of life:
- Comment—Improvement in laboratory parameters:
- Comment—Decreased mortality:
3.4.4. Is There a Specific ONS for This Group of Patients?
- There is insufficient evidence to make any recommendations about specific supplements in this group of patients.
- Comment:
3.4.5. What Is the Role of Enteral and Parenteral Nutrition in Exacerbation Episodes in COPD Patients?
- Comment:
3.4.6. Is There Sufficient Evidence to Recommend Any Form of Micronutrient or Trace Element Supplementation?
- Comment—HMB and essential amino acids:
- Comment—Omega-3, Vit D and leucine:
- Comment—Antioxidant vitamins:
3.5. Physical Activity
3.5.1. What Is the Best Strategy in the Rehabilitation of the COPD Patient, Associated with Nutritional Therapy?
- Comment:
3.5.2. Is It Possible to Make a Specific Recommendation on Physical Exercise?
- Comment:
3.6. Quality of Life
What Are the Most Commonly Used Quality of Life Questionnaires?
4. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A. Glim Criteria and SGA

| Phenotypic Criteria a | |||
|---|---|---|---|
| Weight Loss (%) | Low Body Mass Index (kg/m2) b | Reduced Muscle Mass c | |
| Stage 1 (Moderate Malnutrition) | 5–10% within the past 6 mo, | <20 if < 70 yr, <22 if ≥70 yr | Mild to moderate deficit |
| (Requires 1 phenotypic criterion that meets this grade) | or 10–20% beyond 6 mo | (per validated assessment methods—see below) | |
| Stage 2 (Severe Malnutrition) | >10% within the past 6 mo, | <18.5 if < 70 yr, <20 if ≥70 yr | Severe deficit |
| (Requires 1 phenotypic criterion that meets this grade) | or >20% beyond 6 mo | (per validated assessment methods—see below) | |
| (a) Severity grading is based upon the noted phenotypic criteria while the etiologic criteria described in the text and Figure 1 are used to provide the context to guide intervention and anticipated outcomes. (b) Further research is needed to secure consensus reference BMI data for Asian populations in clinical settings. (c) For example appendicular lean mass index (ALMI, kg/m2) by dual-energy absorptiometry or corresponding standards using other body composition methods like bioelectrical impedance analysis (BIA), CT or MRI. When not available or by regional preference, physical examination or standard anthropometric measures like mid-arm muscle or calf circumferences may be used. Functional assessments like hand-grip strength may be used as a supportive measure. | |||

Appendix B. EWGSOP2 and SARC-F

| Component | Question | Scoring |
|---|---|---|
| Strength | How much difficulty do you have in lifting and carrying 10 pounds? | None = 0, Some = 1, A lot or unable = 2 |
| Assistance in walking | How much difficulty do you have walking across a room? | None = 0, Some = 1, A lot, use aids, or unable = 2 |
| Rise from a chair | How much difficulty do you have transferring from a chair or bed? | None = 0, Some = 1, A lot or unable without help = 2 |
| Climb stairs | How much difficulty do you have climbing a flight of 10 stairs? | None = 0, Some = 1, A lot or unable = 2 |
| Falls | How many times have you fallen in the past year? | None = 0, 1–3 falls = 1, ≥4 falls = 2 |
References
- WHO. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 23 March 2023).
- GBD. GBD DALYs Hale Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden. Lancet 2018, 392, 1852–1922. [Google Scholar]
- Yang, K.; Wu, Y.; Chen, D.; Liu, S.; Chen, R. The Impact of Lung Function on Extra-Pulmonary Diseases and All-Cause Mortality in US Adult Population with and without COPD. Clin. Epidemiol. 2020, 12, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Wouters, E.F.M. An update on pulmonary rehabilitation techniques for patients with chronic obstructive pulmonary disease. Expert Rev. Respir. Med. 2020, 14, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Thorsdottir, I.; Gunnarsdottir, I.; Eriksen, B. Screening method evaluated by nutritional status measurements can be used to detect malnourishment in chronic obstructive pulmonary disease. J. Am. Diet. Assoc. 2001, 101, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Vermeeren, M.; Creutzberg, E.; Schols, A.; Postma, D.; Pieters, W.; Roldaan, A.; Wouters, E. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir. Med. 2006, 100, 1349–1355. [Google Scholar] [CrossRef]
- Marco, E.; Sánchez-Rodríguez, D.; Dávalos-Yerovi, V.N.; Duran, X.; Pascual, E.M.; Muniesa, J.M.; Rodríguez, D.A.; Aguilera-Zubizarreta, A.; Escalada, F.; Duarte, E. Malnutrition according to ESPEN consensus predicts hospitalizations and long-term mortality in rehabilitation patients with stable chronic obstructive pulmonary disease. Clin. Nutr. 2019, 38, 2180–2186. [Google Scholar] [CrossRef]
- Jo, Y.S.; Kim, Y.H.; Lee, J.Y.; Kim, K.; Jung, K.S.; Yoo, K.H.; Rhee, C.K. Impact of BMI on exacerbation and medical care expenses in subjects with mild to moderate airflow obstruction. Int. J. COPD 2018, 13, 2261–2269. [Google Scholar] [CrossRef]
- Hoong, J.M.; Ferguson, M.; Hukins, C.; Collins, P.F. Economic and operational burden associated with malnutrition in chronic obstructive pulmonary disease. Clin. Nutr. 2017, 36, 1105–1109. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.S.; Kim, K.; Oh, Y.M.; Yoo, K.H.; Rhee, C.K.; Lee, J.H. Socioeconomic impact of asthma, chronic obstructive pulmonary disease and asthma-COPD overlap syndrome. J. Thorac. Dis. 2017, 9, 1547–1556. [Google Scholar] [CrossRef]
- de Blasio, F.; Di Gregorio, A.; de Blasio, F.; Bianco, A.; Bellofiore, B.; Scalfi, L. Malnutrition and sarcopenia assessment in patients with chronic obstructive pulmonary disease according to international diagnostic criteria, and evaluation of raw BIA variables. Respir. Med. 2018, 134, 1–5. [Google Scholar] [CrossRef]
- Ingadottir, A.R.; Beck, A.M.; Baldwin, C.; Weekes, C.E.; Geirsdottir, O.G.; Ramel, A.; Gislason, T.; Gunnarsdottir, I. Association of energy and protein intakes with length of stay, readmission and mortality in hospitalised patients with chronic obstructive pulmonary disease. Br. J. Nutr. 2018, 119, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Benedik, B.; Farkas, J.; Kosnik, M.; Kadivec, S.; Lainscak, M. Mini nutritional assessment, body composition, and hospitalisations in patients with chronic obstructive pulmonary disease. Respir. Med. 2011, 105, S38–S43. [Google Scholar] [CrossRef]
- Mete, B.; Pehlivan, E.; Gülbaş, G.; Günen, H. Prevalence of malnutrition in COPD and its relationship with the parameters related to disease severity. Int. J. COPD 2018, 13, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Schols, A.M.; Ferreira, I.M.; Franssen, F.M.; Gosker, H.R.; Janssens, W.; Muscaritoli, M.; Pison, C.; Rutten-Van Mölken, M.; Slinde, F.; Steiner, M.C.; et al. Nutritional assessment and therapy in COPD: A European respiratory society statement. Eur. Respir. J. 2014, 44, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Collins, P.F.; Pavey, T.G.; Nguyen, N.V.; Pham, T.D.; Gallegos, D.L. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int. J. COPD 2019, 14, 215–226. [Google Scholar] [CrossRef]
- Gupta, S.S.; Gothi, D.; Narula, G.; Sircar, J. Correlation of BMI and oxygen saturation in stable COPD in Northern India. Lung India 2014, 31, 29–34. [Google Scholar] [CrossRef]
- Lee, H.M.; Liu, M.; Lee, K.; Luo, Y.; Wong, N.D. Does low vitamin D amplify the association of COPD with total and cardiovascular disease mortality? Clin. Cardiol. 2014, 37, 473–478. [Google Scholar] [CrossRef]
- Agustí, A.; Sisó-Almirall, A.; Roman, M.; Vogelmeier, C.F.; Anzueto, A.; Barnes, P.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Halpin, D.; et al. Gold 2023: Highlights for primary care. npj Prim. Care Respir. Med. 2023, 33, 28. [Google Scholar] [CrossRef]
- Cheng, S.L.; Lin, C.H.; Chu, K.A.; Chiu, K.L.; Lin, S.H.; Lin, H.C.; Ko, H.K.; Chen, Y.C.; Chen, C.H.; Sheu, C.C.; et al. Update on guidelines for the treatment of COPD in Taiwan using evidence and GRADE system-based recommendations. J. Formos. Med. Assoc. 2021, 120, 1821–1844. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; Zu Wallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.C.; et al. An official American thoracic society/European respiratory society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, 889–1042. [Google Scholar] [CrossRef]
- Celli, B.R.; Decramer, M.; Wedzicha, J.A.; Wilson, K.C.; Agustí, A.A.; Criner, G.J.; MacNee, W.; Make, B.J.; Rennard, S.I.; Stockley, R.A.; et al. An official American thoracic society/ European respiratory society statement: Research questions in COPD. Eur. Respir. Rev. 2015, 24, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, A.; Keith, R. Clinical guideline highlights for the hospitalist: The GOLD and NICE Guidelines for the Management of COPD. J. Hosp. Med. 2020, 15, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Soler-Cataluña, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Trigueros, J.A.; Cosío, B.G.; Casanova, C.; Riesco, J.A.; et al. Spanish COPD Guidelines (GesEPOC) 2017. Pharmacological Treatment of Stable Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2017, 53, 324–335. [Google Scholar] [CrossRef]
- Soler-Cataluña, J.J.; Piñera, P.; Trigueros, J.A.; Calle, M.; Casanova, C.; Cosío, B.G.; López-Campos, J.L.; Molina, J.; Almagro, P.; Gómez, J.-T.; et al. Actualización 2021 de la guía española de la EPOC (GesEPOC). Diagnóstico y tratamiento del síndrome de agudización de la EPOC. Arch. Bronconeumol. 2022, 58, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Scottish Intercollegiate Guidelines Network. A Guideline Developer’s Handbook. (SIGN Publication no. 50) [Internet]. 2019. Available online: http://www.sign.ac.uk (accessed on 14 February 2023).
- Bischoff, S.C.; Singer, P.; Koller, M.; Barazzoni, R.; Cederholm, T.; van Gossum, A. Standard operating procedures for ESPEN guidelines and consensus papers. Clin. Nutr. 2015, 34, 1043–1051. [Google Scholar] [CrossRef]
- Hartley, J. Some thoughts on Likert-type scales. Int. J. Clin. Health Psychol. 2014, 14, 83–86. [Google Scholar] [CrossRef]
- Jung, J.W.; Yoon, S.W.; Lee, G.E.; Shin, H.G.; Kim, H.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Poor nutritional intake is a dominant factor for weight loss in chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2019, 23, 631–637. [Google Scholar] [CrossRef]
- Laudisio, A.; Costanzo, L.; Di Gioia, C.; Delussu, A.S.; Traballesi, M.; Gemma, A.; Antonelli Incalzi, R. Dietary intake of elderly outpatients with chronic obstructive pulmonary disease. Arch. Gerontol. Geriatr. 2016, 64, 75–81. [Google Scholar] [CrossRef]
- Collins, P.F.; Yang, I.A.; Chang, Y.C.; Vaughan, A. Nutritional support in chronic obstructive pulmonary disease (COPD): An evidence update. J. Thorac. Dis. 2019, 11, S2230–S2237. [Google Scholar] [CrossRef]
- Nordén, J.; Grönberg, A.M.; Bosaeus, I.; Forslund, H.B.; Hulthén, L.; Rothenberg, E.; Karlsson, J.; Wallengren, O.; Slinde, F. Nutrition impact symptoms and body composition in patients with COPD. Eur. J. Clin. Nutr. 2015, 69, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Leem, A.Y.; Lee, S.H.; Song, J.H.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; Chung, K.S. Comorbidities in obstructive lung disease in Korea: Data from the fourth and fifth Korean national health and nutrition examination survey. Int. J. COPD 2015, 10, 1571–1582. [Google Scholar] [CrossRef]
- Ingadottir, A.R.; Beck, A.M.; Baldwin, C.; Weekes, C.E.; Geirsdottir, O.G.; Ramel, A.; Gislason, T.; Gunnarsdottir, I. Two components of the new ESPEN diagnostic criteria for malnutrition are independent predictors of lung function in hospitalized patients with chronic obstructive pulmonary disease (COPD). Clin. Nutr. 2018, 37, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Park, J.; Do Lee, S.; Oh, Y.M. Comorbidities of chronic obstructive pulmonary disease in Koreans: A population-based study. J. Korean Med. Sci. 2012, 27, 901–906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barreiro, E.; Bustamante, V.; Cejudo, P.; Gáldiz, J.B.; Gea, J.; de Lucas, P.; Martínez-Llorens, J.; Ortega, F.; Puente-Maestu, L.; Roca, J.; et al. Guidelines for the Evaluation and Treatment of Muscle Dysfunction in Patients with Chronic Obstructive Pulmonary Disease. Arch. Bronconeumol. 2015, 51, 384–395. [Google Scholar] [CrossRef]
- Wu, T.D.; Ejike, C.O.; Wise, R.A.; McCormack, M.C.; Brigham, E.P. Investigation of the Obesity Paradox in Chronic Obstructive Pulmonary Disease, According to Smoking Status, in the United States. Am. J. Epidemiol. 2019, 188, 1977–1983. [Google Scholar] [CrossRef]
- Hogan, D.; Lan, L.T.T.; Diep, D.T.N.; Gallegos, D.; Collins, P.F. Nutritional status of Vietnamese outpatients with chronic obstructive pulmonary disease. J. Hum. Nutr. Diet. 2017, 30, 83–89. [Google Scholar] [CrossRef]
- Gea, J.; Casadevall, C.; Pascual, S.; Orozco-Levi, M.; Barreiro, E. Respiratory diseases and muscle dysfunction. Expert Rev. Respir. Med. 2012, 6, 75–90. [Google Scholar] [CrossRef]
- Pezzuto, A.; Ricci, A.; D’ascanio, M.; Moretta, A.; Tonini, G.; Calabrò, N.; Minoia, V.; Pacini, A.; De Paolis, G.; Chichi, E.; et al. Short-Term Benefits of Smoking Cessation Improve Respiratory Function and Metabolism in Smokers. Int. J. COPD 2023, 18, 2861–2865. [Google Scholar] [CrossRef]
- Doo, J.H.; Kim, S.M.; Park, Y.J.; Kim, K.H.; Oh, Y.H.; Kim, J.S.; Park, S.M. Smoking cessation after diagnosis of COPD is associated with lower all-cause and cause-specific mortality: A nationwide population-based cohort study of South Korean men. BMC Pulm. Med. 2023, 23, 237. [Google Scholar] [CrossRef]
- Okazaki, R.; Watanabe, R.; Inoue, D. Osteoporosis Associated with Chronic Obstructive Pulmonary Disease. J. Bone Metab. 2016, 23, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Majid, H.; Kanbar-Agha, F.; Sharafkhaneh, A. COPD: Osteoporosis and sarcopenia. COPD Res. Pract. 2016, 2, 3. [Google Scholar] [CrossRef]
- Lee, I.S.; Leem, A.Y.; Lee, S.H.; Rhee, Y.; Ha, Y.; Kim, Y.S. Relationship between pulmonary function and bone mineral density in the Korean National Health and Nutrition Examination Survey. Korean J. Intern. Med. 2016, 31, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Horadagoda, C.; Dinihan, T.; Roberts, M.; Kairaitis, K. Body composition and micronutrient deficiencies in patients with an acute exacerbation of chronic obstructive pulmonary disease. Intern. Med. J. 2017, 47, 1057–1063. [Google Scholar] [CrossRef]
- McDonald, V.M.; Gibson, P.G.; Scott, H.A.; Baines, P.J.; Hensley, M.J.; Pretto, J.J.; Wood, L.G. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology 2016, 21, 875–882. [Google Scholar] [CrossRef]
- Liu, Y.; Pleasants, R.A.; Croft, J.B.; Lugogo, N.; Ohar, J.; Heidari, K.; Strange, C.; Wheaton, A.G.; Mannino, D.M.; Kraft, M. Body mass index, respiratory conditions, asthma, and chronic obstructive pulmonary disease. Respir. Med. 2015, 109, 851–859. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-K.; Heo, E.Y.; Kim, D.K.; Chung, H.S. The effect of obesity on patients with mild chronic obstructive pulmonary disease: Results from KNHANES 2010 to 2012. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 757–763. [Google Scholar] [CrossRef][Green Version]
- Joppa, P.; Tkacova, R.; Franssen, F.M.E.; Hanson, C.; Rennard, S.I.; Silverman, E.K.; McDonald, M.L.N.; Calverley, P.M.A.; Tal-Singer, R.; Spruit, M.A.; et al. Sarcopenic Obesity, Functional Outcomes, and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. J. Am. Med. Dir. Assoc. 2016, 17, 712–718. [Google Scholar] [CrossRef]
- Venkatesan, P. GOLD COPD report: 2024 update. Lancet. Respir. Med. 2024, 12, 15–16. [Google Scholar] [CrossRef]
- Lei, Y.; Liang, Y.; Chen, Y.; Liu, X.; Liao, X.; Luo, F. Increased circulating obestatin in patients with chronic obstructive pulmonary disease. Multidiscip. Respir. Med. 2014, 9, 5. [Google Scholar] [CrossRef]
- Yee, N.; Locke, E.R.; Pike, K.C.; Chen, Z.; Lee, J.; Huang, J.C.; Nguyen, H.Q.; Fan, V.S. Frailty in chronic obstructive pulmonary disease and risk of exacerbations and hospitalizations. Int. J. COPD 2020, 15, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.R.; Albay, A.B.; Tee, M.L. Body composition of Filipino chronic obstructive pulmonary disease (COPD) patients in relation to their lung function, exercise capacity and quality of life. Int. J. COPD 2019, 14, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Verhage, T.L.; Heijdra, Y.; Molema, J.; Vercoulen, J.; Dekhuijzen, R. Associations of muscle depletion with health status. Another gender difference in COPD? Clin. Nutr. 2011, 30, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hallin, R.; Janson, C.; Arnardottir, R.H.; Olsson, R.; Emtner, M.; Branth, S.; Boman, G.; Slinde, F. Relation between physical capacity, nutritional status and systemic inflammation in COPD. Clin. Respir. J. 2011, 5, 136–142. [Google Scholar] [CrossRef]
- Matkovic, Z.; Cvetko, D.; Rahelic, D.; Esquinas, C.; Zarak, M.; Miravitlles, M.; Tudoric, N. Nutritional Status of Patients with Chronic Obstructive Pulmonary Disease in Relation to their Physical Performance. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 626–634. [Google Scholar] [CrossRef]
- Bordejé Laguna, M.L. Nuestros grandes olvidados, los enfermos respiratorios crónicos. Nutr. Hosp. 2017, 34, 38–45. [Google Scholar] [CrossRef]
- Farooqi, N.; Sandström, T.; Slinde, F.; Håglin, L.; Carlsson, M. Predicting energy requirement with pedometer-determined physical-activity level in women with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1129–1137. [Google Scholar] [CrossRef][Green Version]
- Shan, X.; Liu, J.; Luo, Y.; Xu, X.; Han, Z.; Li, H. Relationship between nutritional risk and exercise capacity in severe chronic obstructive pulmonary disease in male patients. Int. J. COPD 2015, 10, 1207–1212. [Google Scholar] [CrossRef]
- López-López, L.; Torres-Sánchez, I.; González-Jiménez, E.; Díaz-Pelegrina, A.; Merlos-Navarro, S.; Valenza, M.C. Severe chonic obstructive pulmonary disease and malnutrition: Effect on symptoms and function. Nutr. Hosp. 2016, 33, 319–323. [Google Scholar] [CrossRef]
- Battaglia, S.; Spatafora, M.; Paglino, G.; Pedone, C.; Corsonello, A.; Scichilone, N.; Antonelli-Incalzi, R.; Bellia, V. Ageing and COPD affect different domains of nutritional status: The ECCE study. Eur. Respir. J. 2011, 37, 1340–1345. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Fujita, Y.; Yamamoto, Y.; Yamauchi, M.; Tomoda, K.; Koyama, N.; Kimura, H. Mini Nutritional Assessment Short-Form predicts exacerbation frequency in patients with chronic obstructive pulmonary disease. Respirology 2014, 19, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Mitani, Y.; Oki, Y.; Fujimoto, Y.; Ohira, M.; Kaneko, H.; Kawashima, T.; Nishio, M.; Ishikawa, A. Comparison of Geriatric Nutritional Risk Index scores on physical performance among elderly patients with chronic obstructive pulmonary disease. Heart Lung J. Acute Crit. Care 2015, 44, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xing, L.; You, C.; Ou, X. Prediction of prognosis in chronic obstructive pulmonary disease patients with respiratory failure: A comparison of three nutritional assessment methods. Eur. J. Intern. Med. 2018, 57, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Hasenboehler, F.; Cantone, J.; Hersberger, L.; Bargetzi, A.; Bargetzi, L.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; Hoess, C.; et al. Effect of nutritional support in patients with lower respiratory tract infection: Secondary analysis of a randomized clinical trial. Clin. Nutr. 2021, 40, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Eftekhari, M.H.; Mazloom, Z.; Masoompour, M.; Fararooei, M.; Eskandari, M.H.; Mehrabi, S.; Bedeltavana, A.; Famouri, M.; Zare, M.; et al. Fortified whey beverage for improving muscle mass in chronic obstructive pulmonary disease: A single-blind, randomized clinical trial. Respir. Res. 2020, 21, 216. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef]
- Matheson, E.M.; Nelson, J.L.; Baggs, G.E.; Luo, M.; Deutz, N.E. Specialized oral nutritional supplement (ONS) improves handgrip strength in hospitalized, malnourished older patients with cardiovascular and pulmonary disease: A randomized clinical trial. Clin. Nutr. 2021, 40, 844–849. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Pavey, T.G.; Collins, P.F.; Nguyen, N.V.; Pham, T.D.; Gallegos, D. Effectiveness of Tailored Dietary Counseling in Treating Malnourished Outpatients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. J. Acad. Nutr. Diet. 2020, 120, 778–791.e1. [Google Scholar] [CrossRef]
- Nogay, N.H. The relationship og subjetive global assessmente with respiratory function and other nutrition parameters in COPD. Healthmed 2012, 6, 2013–2017. [Google Scholar]
- Al-dorzi, H.M.; Aldawood, A.S.; Tamim, H.; Haddad, S.H.; Jones, G.; Mcintyre, L.; Solaiman, O.; Sakhija, M.; Sadat, M.; Afesh, L.; et al. Clinical Nutrition ESPEN Caloric intake and the fat-to-carbohydrate ratio in hypercapnic acute respiratory failure: Post-hoc analysis of the PermiT trial. Clin. Nutr. ESPEN 2018, 29, 175–182. [Google Scholar] [CrossRef]
- Jeon, Y.K.; Shin, M.J.; Kim, M.H.; Mok, J.H.; Kim, S.S.; Kim, B.H.; Kim, S.-J.; Kim, Y.K.; Chang, J.H.; Shin, Y.B.; et al. Low pulmonary function is related with a high risk of sarcopenia in community-dwelling older adults: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2011. Osteoporos. Int. 2015, 26, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Borda, M.G.; Celis-Preciado, C.A.; Pérez-Zepeda, M.U.; Ríos-Zuluaga, J.D.; Cano-Gutiérrez, C.A. Sarcopenia en ancianos con antecedente de EPOC/asma: Resultados del estudio SABE—Bogotá. Rev. Esp. Geriatr. Gerontol. 2017, 52, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Jin, H.J.; Shin, K.C.; Chung, J.H.; Lee, H.W.; Lee, K.H. Presence of sarcopenia in asthma–COPD overlap syndrome may be a risk factor for decreased bone-mineral density, unlike asthma: Korean national health and nutrition examination survey (KNHANES) IV and V (2008–2011). Int. J. COPD 2017, 12, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Kim, M.C.; Ahn, J.H. Sarcopenia is an independent risk factor for NAFLD in COPD: A nationwide survey (KNHANES 2008–2011). Int. J. COPD 2020, 15, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Hwang, H.J.; Han, C.H.; Son, B.S.; Kim, D.H.; Park, M.S. Association between sarcopenia and metabolic syndrome in chronic obstructive pulmonary disease: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2008 to 2011. COPD J. Chronic Obstr. Pulm. Dis. 2015, 12, 82–89. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Nijholt, W.; ter Beek, L.; Hobbelen, J.S.M.; van der Vaart, H.; Wempe, J.B.; van der Schans, C.P.; Jager-Wittenaar, H. The added value of ultrasound muscle measurements in patients with COPD: An exploratory study. Clin. Nutr. ESPEN 2019, 30, 152–158. [Google Scholar] [CrossRef]
- Neo, H.Y.; Xu, H.Y.; Wu, H.Y.; Hum, A. Prediction of Poor Short-Term Prognosis and Unmet Needs in Advanced Chronic Obstructive Pulmonary Disease: Use of the Two-Minute Walking Distance Extracted from a Six-Minute Walk Test. J. Palliat. Med. 2017, 20, 821–828. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Han, Y.X.; Niu, M.E.; Chen, Y.; Zhang, X.Q.; Qian, H.Y. Handgrip strength is associated with dyspnoea and functional exercise capacity in male patients with stable COPD. Int. J. Tuberc. Lung Dis. 2019, 23, 428–432. [Google Scholar] [CrossRef]
- McDonald, M.L.N.; Diaz, A.A.; Ross, J.C.; Estepar, R.S.J.; Zhou, L.; Regan, E.A.; Eckbo, E.; Muralidhar, N.; Come, C.E.; Cho, M.H.; et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease: A cross-sectional study. Ann. Am. Thorac. Soc. 2014, 11, 326–334. [Google Scholar] [CrossRef]
- Diaz, A.A.; Martinez, C.H.; Harmouche, R.; Young, T.P.; McDonald, M.L.; Ross, J.C.; Han, M.L.; Bowler, R.; Make, B.; Regan, E.A.; et al. Pectoralis muscle area and mortality in smokers without airflow obstruction. Respir. Res. 2018, 19, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.H.; Kwon, S.O.; Han, S.S.; Kim, W.J. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: A case control study. Respir. Res. 2019, 20, 226. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.C.; Hsu, J.W.-C.; Bandi, V.; Hanania, N.A.; Kheradmand, F.; Jahoor, F. Glucose and pyruvate metabolism in severe chronic obstructive pulmonary disease. J. Appl. Physiol. 2012, 112, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.Y.; Wu, X.T.; Wang, M.Y.; Hu, W. Comparison between measured and predicted resting energy expenditure in mechanically ventilated patients with COPD. Asia Pac. J. Clin. Nutr. 2012, 21, 338–346. [Google Scholar] [PubMed]
- Kovarik, M.; Najpaverova, S.; Koblizek, V.; Zadak, Z.; Hronek, M. Association of resting energy expenditure and nutritional substrate oxidation with COPD stage and prediction indexes. Respir. Med. 2020, 174, 106174. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Tabira, K.; Takemura, H.; Inaba, S.; Kusuki, H.; Hashitsume, Y.; Suzuki, Y.; Tenpaku, Y.; Yasuma, T.; D’alessandro-Gabazza, C.N.; et al. A prediction equation to assess resting energy expenditure in Japanese patients with COPD. J. Clin. Med. 2020, 9, 3455. [Google Scholar] [CrossRef]
- Bendavid, I.; Lobo, D.N.; Barazzoni, R.; Cederholm, T.; Coëffier, M.; de van der Schueren, M.; Fontaine, E.; Hiesmayr, M.; Laviano, A.; Pichard, C.; et al. The centenary of the Harris–Benedict equations: How to assess energy requirements best? Recommendations from the ESPEN expert group. Clin. Nutr. 2021, 40, 690–701. [Google Scholar] [CrossRef]
- Jonker, R.; Deutz, N.E.P.; Ligthart-Melis, G.C.; Zachria, A.J.; Veley, E.A.; Harrykissoon, R.; Engelen, M.P.K.J. Preserved anabolic threshold and capacity as estimated by a novel stable tracer approach suggests no anabolic resistance or increased requirements in weight stable COPD patients. Clin. Nutr. 2019, 38, 1833–1843. [Google Scholar] [CrossRef]
- Engelen, M.P.K.J.; Wouters, E.F.M.; Deutz, N.E.P.; Menheere, P.P.C.A.; Schols, A.M.W.J. Factors contributing to alterations in skeletal muscle and plasma amino acid profiles in patients with chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2000, 72, 1480–1487. [Google Scholar] [CrossRef]
- Calder, P.C.; Laviano, A.; Lonnqvist, F.; Muscaritoli, M.; Öhlander, M.; Schols, A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: A randomized, controlled trial. J. Cachexia. Sarcopenia Muscle 2018, 9, 28–40. [Google Scholar] [CrossRef]
- Deutz, N.E.; Ziegler, T.R.; Matheson, E.M.; Matarese, L.E.; Tappenden, K.A.; Baggs, G.E.; Nelson, J.L.; Luo, M.; Hegazi, R.; Jonnalagadda, S.S. Reduced mortality risk in malnourished hospitalized older adult patients with COPD treated with a specialized oral nutritional supplement: Sub-group analysis of the NOURISH study. Clin. Nutr. 2021, 40, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Jonker, R.; Deutz, N.E.P.; Schols, A.M.W.J.; Veley, E.A.; Harrykissoon, R.; Zachria, A.J.; Engelen, M.P.K.J. Whole body protein anabolism in COPD patients and healthy older adults is not enhanced by adding either carbohydrates or leucine to a serving of protein. Clin. Nutr. 2019, 38, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.S.; Choi, S.M.; Lee, J.; Park, Y.S.; Lee, S.M.; Yim, J.J.; Yoo, C.G.; Kim, Y.W.; Han, S.K.; Lee, C.H. The relationship between chronic obstructive pulmonary disease and comorbidities: A cross-sectional study using data from KNHANES 2010–2012. Respir. Med. 2015, 109, 96–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williams, M.T.; Effing, T.W.; Paquet, C.; Gibbs, C.A.; Lewthwaite, H.; Katrina Li, L.S.; Phillips, A.C.; Johnston, K.N. Counseling for health behavior change in people with COPD: Systematic review. Int. J. COPD 2017, 12, 2165–2178. [Google Scholar] [CrossRef]
- Varraso, R.; Barr, R.G.; Willett, W.C.; Speizer, F.E.; Camargo, C.A. Fish intake and risk of chronic obstructive pulmonary disease in 2 large US cohorts. Am. J. Clin. Nutr. 2015, 101, 354–361. [Google Scholar] [CrossRef]
- Kim, T.; Choi, H.; Kim, J. Association between Dietary Nutrient Intake and Chronic Obstructive Pulmonary Disease Severity: A Nationwide Population-Based Representative Sample. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 49–58. [Google Scholar] [CrossRef]
- Whyand, T.; Hurst, J.R.; Beckles, M.; Caplin, M.E. Pollution and respiratory disease: Can diet or supplements help? A review. Respir. Res. 2018, 19, 79. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, C.Y.; Lee, M.G.; Kim, Y.S. Effects of dietary antioxidant vitamins on lung functions according to gender and smoking status in Korea: A population-based cross-sectional study. BMJ Open 2018, 8, e020656. [Google Scholar] [CrossRef]
- Park, H.J.; Byun, M.K.; Kim, H.J.; Kim, J.Y.; Kim, Y.I.; Yoo, K.H.; Chun, E.M.; Jung, J.Y.; Lee, S.H.; Ahn, C.M. Dietary vitamin C intake protects against COPD: The Korea National Health and Nutrition Examination Survey in 2012. Int. J. COPD 2016, 11, 2721–2728. [Google Scholar] [CrossRef]
- Salari-Moghaddam, A.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Processed red meat intake and risk of COPD: A systematic review and dose-response meta-analysis of prospective cohort studies. Clin. Nutr. 2019, 38, 1109–1116. [Google Scholar] [CrossRef]
- Min, J.E.; Huh, D.A.; Moon, K.W. The joint effects of some beverages intake and smoking on chronic obstructive pulmonary disease in Korean adults: Data analysis of the Korea national health and nutrition examination survey (KNHANES), 2008–2015. Int. J. Environ. Res. Public Health 2020, 17, 2611. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, N.; Nordström, L.; Lundgren, R.; Sandström, T.; Håglin, L. Changes in body weight and physical performance after receiving dietary advice in patients with chronic obstructive pulmonary disease (COPD): 1-year follow-up. Arch. Gerontol. Geriatr. 2011, 53, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Buys, D.R.; Campbell, A.D.; Godfryd, A.; Flood, K.; Kitchin, E.; Kilgore, M.L.; Allocca, S.; Locher, J.L. Meals Enhancing Nutrition After Discharge: Findings from a Pilot Randomized Controlled Trial. J. Acad. Nutr. Diet. 2017, 117, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Torre, G.L.A.; Cocchiara, R.A.; Sordo, E.L.O.; Chiarini, M.; Siliquini, R.; Firenze, A.; Maurici, M.; Agati, L.; Saulle, R.; Mannocci, A. Counseling intervention to improve quality of life in patients with pre-existing acute myocardial infarction (AMI) or chronic obstructive pulmonary disease (COPD): A pilot study. J. Prev. Med. Hyg. 2018, 59, E153–E158. [Google Scholar] [PubMed]
- Ferreira, I.M.; Brooks, D.; White, J.; Goldstein, R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2012, 12, CD000998. [Google Scholar] [CrossRef]
- van Beers, M.; Rutten-van Mölken, M.P.M.H.; van de Bool, C.; Boland, M.; Kremers, S.P.J.; Franssen, F.M.E.; van Helvoort, A.; Gosker, H.R.; Wouters, E.F.; Schols, A.M.W.J. Clinical outcome and cost-effectiveness of a 1-year nutritional intervention programme in COPD patients with low muscle mass: The randomized controlled NUTRAIN trial. Clin. Nutr. 2020, 39, 405–413. [Google Scholar] [CrossRef]
- Benito Martínez, M.d.P.; Guarro Riba, M.; La Serna Infantes, J.E.; Camere Colarossi, D.M.; Camere Torrealva, M.A.; Tort, G. Estado Nutricional y funcional en pacientes con enfermedad pulmonar obstructiva crónica: Efectos de la suplementación nutricional oral (Estudio OFOS). Nutr. Hosp. 2017, 34, 776–783. [Google Scholar]
- Khan, N.A.; Kumar, N.; Daga, M.K. Effect of dietary supplementation on body composition, pulmonary function and health-related quality of life in patients with stable COPD. Tanaffos 2016, 15, 225–235. [Google Scholar]
- van de Bool, C.; Rutten, E.P.A.; van Helvoort, A.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A.M.W.J. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J. Cachexia. Sarcopenia Muscle 2017, 8, 748–758. [Google Scholar] [CrossRef]
- Sugawara, K.; Takahashi, H.; Kashiwagura, T.; Yamada, K.; Yanagida, S.; Homma, M.; Dairiki, K.; Sasaki, H.; Kawagoshi, A.; Satake, M.; et al. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir. Med. 2012, 106, 1526–1534. [Google Scholar] [CrossRef]
- Van De Bool, C.; Mattijssen-Verdonschot, C.; Van Melick, P.P.M.J.; Spruit, M.A.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A.M.W.J.; Rutten, E.P.A. Quality of dietary intake in relation to body composition in patients with chronic obstructive pulmonary disease eligible for pulmonary rehabilitation. Eur. J. Clin. Nutr. 2014, 68, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Payne, C.; Wiffen, P.; Martin, S. Interventions for fatigue and weight loss in adults with advanced progressive illness: A cochrane overview of systematic reviews. Cochrane Database Syst. Rev. 2017, 2017, CD008427. [Google Scholar]
- Korkmaz, C.; Demirbas, S.; Vatansev, H.; Yildirim, E.; Teke, T.; Zamani, A. Effects of comprehensive and intensive pulmonary rehabilitation and nutritional support on quality of life and functional status in patients with chronic obstructive pulmonary disease. J. Int. Med. Res. 2020, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jonker, R.; Deutz, N.E.P.; Erbland, M.L.; Anderson, P.J.; Engelen, M.P.K.J. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin. Nutr. 2014, 33, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.; White, H.; Sosnowski, K.; Tran, K.; Jones, M.; Palmer, M. Energy and protein intakes of hospitalised patients with acute respiratory failure receiving non-invasive ventilation. Clin. Nutr. 2014, 33, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, T.; Marui, S.; Miura, E.; Sugiura, M.; Matsuyama, W.; Aoshima, Y.; Kasamatsu, N.; Ogiku, M.; Ikematsu, Y. Effect of eicosapentaenoic acid on prevention of lean body mass depletion in patients with exacerbation of chronic obstructive pulmonary disease: A prospective randomized controlled trial. Clin. Nutr. ESPEN 2018, 28, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wandrag, L.; Brett, S.J.; Frost, G.; Hickson, M. Impact of supplementation with amino acids or their metabolites on muscle wasting in patients with critical illness or other muscle wasting illness: A systematic review. J. Hum. Nutr. Diet. 2015, 28, 313–330. [Google Scholar] [CrossRef]
- Jonker, R.; Ep Deutz, N.; Erbland, M.L.; Anderson, P.J.; Pkj Engelen, M. Effectiveness of essential amino acid supplementation in stimulating whole body net protein anabolism is comparable between COPD patients and healthy older adults HHS Public Access continuous IV infusion of L-[ring-2 H 5 ]-PHE and L-[ring-2 H2]-tyrosine. Metabolism 2017, 69, 120–129. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Testa, A.; Aquilani, R.; Tognella, S.; Pasini, E.; Barbieri, A.; Boschi, F. Essential amino acid supplementation in patients with severe COPD: A step towards home rehabilitation. Monaldi Arch. Chest Dis. Pulm. Ser. 2012, 77, 67–75. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, Q.Y. Vitamin D should be supplemented more actively in elderly patients with coronary heart disease combined with COPD. Int. J. COPD 2016, 11, 1359–1365. [Google Scholar] [CrossRef]
- Hendryx, M.; Luo, J. A test of vitamin D benefits on respiratory health mediated through inflammatory markers. Chron. Respir. Dis. 2015, 12, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Gouzi, F.; Maury, J.; Héraud, N.; Molinari, N.; Bertet, H.; Ayoub, B.; Blaquière, M.; Bughin, F.; De Rigal, P.; Poulain, M.; et al. Additional effects of nutritional antioxidant supplementation on peripheral muscle during pulmonary rehabilitation in COPD patients: A randomized controlled trial. Oxid. Med. Cell. Longev. 2019, 2019, 5496346. [Google Scholar] [CrossRef] [PubMed]
- Guertin, K.A.; Grant, R.K.; Arnold, K.B.; Burwell, L.; Hartline, J.; Goodman, P.J.; Minasian, L.M.; Lippman, S.M.; Klein, E.; Cassano, P.A. Effect of long-term vitamin E and selenium supplementation on urine F2-isoprostanes, a biomarker of oxidative stress. Free Radic. Biol. Med. 2016, 95, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, I.; Zakynthinos, S. The physiological basis of rehabilitation in chronic heart and lung disease. J. Appl. Physiol. 2013, 115, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Constantin, D.; Menon, M.K.; Houchen-Wolloff, L.; Morgan, M.D.; Singh, S.J.; Greenhaff, P.; Steiner, M.C. Skeletal muscle molecular responses to resistance training and dietary supplementation in COPD. Thorax 2013, 68, 625–633. [Google Scholar] [CrossRef]
- Meys, R.; Stoffels, A.A.F.; de Brandt, J.; van Hees, H.W.H.; Franssen, F.M.E.; Sillen, M.J.H.; Wouters, E.F.M.; Burtin, C.; Klijn, P.; Bij de Vaate, E.; et al. Beta-alanine supplementation in patients with COPD receiving non-linear periodised exercise training or neuromuscular electrical stimulation: Protocol of two randomised, double-blind, placebo-controlled trials. BMJ Open 2020, 10, e038836. [Google Scholar] [CrossRef]
- Coppi, F.; Nasi, M.; Farinetti, A.; Manenti, A.; Gallina, S.; Mattioli, A.V. Physical activity, sedentary behaviour, and diet in menopausal women: Comparison between COVID19 “first wave” and “second wave” of pandemic in Italy. Prog. Nutr. 2021, 23, e2021194. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Walker, J.F. Increased daily movement associates with reduced mortality among COPD patients having systemic inflammation. Int. J. Clin. Pract. 2016, 70, 286–291. [Google Scholar] [CrossRef]
- Fekete, M.; Kerti, M.; Fazekas-Pongor, V.; Balazs, P.; Csizmadia, Z.; Nemeth, A.N.; Tarantini, S.; Varga, J.T. Effect of interval training with non-invasive ventilation in severe chronic obstructive pulmonary disease—A prospective cohort study with matched control group. Ann. Palliat. Med. 2021, 10, 5289–5298. [Google Scholar] [CrossRef]
- Emtner, M.; Hallin, R.; Arnardottir, R.H.; Janson, C. Effect of physical training on fat-free mass in patients with chronic obstructive pulmonary disease (COPD). Ups. J. Med. Sci. 2015, 120, 52–58. [Google Scholar] [CrossRef]
- Paulin, F.V.; Zagatto, A.M.; Chiappa, G.R.; Müller, P. de T. Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: A randomized and controlled study. Respir. Med. 2017, 122, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.R.; Liu, J.; Roberts, M.B.; Eaton, C.B. Is inflammatory chronic obstructive pulmonary disease a coronary heart disease risk equivalent? A longitudinal analysis of the third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. BMC Pulm. Med. 2014, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Larson, J.L. The relationship between physical activity and metabolic syndrome in people with chronic obstructive pulmonary disease. J. Cardiovasc. Nurs. 2014, 29, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Yentes, J.M.; Sayles, H.; Meza, J.; Mannino, D.M.; Rennard, S.I.; Stergiou, N. Walking abnormalities are associated with COPD: An investigation of the NHANES III dataset. Respir. Med. 2011, 105, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Lewthwaite, H.; Olds, T.; Williams, M.T.; Effing, T.W.; Dumuid, D. Use of time in chronic obstructive pulmonary disease: Longitudinal associations with symptoms and quality of life using a compositional analysis approach. PLoS ONE 2019, 14, e214058. [Google Scholar] [CrossRef]
- Shah, A.M.; Shah, R.B.; Kachoria, S. Health-related quality of life and associated factors in patients with chronic obstructive pulmonary disease. Drugs Ther. Perspect. 2019, 35, 241–249. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Walker, J.F.; Lee, H. Association between physical activity and inflammatory markers among U.S. Adults with chronic obstructive pulmonary disease. Am. J. Health Promot. 2014, 29, 81–88. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK566045/ (accessed on 16 January 2023).
- Susanthy, D.; Erna, K.; Rahayu, W.K.S. Effect of Ophiocephalus striatus Extract on the Levels of TNF-α, CRP, Leptin, Adiponectin, and COPD Assessment Test (CAT) Score in Stable COPD Patients with Muscle Wasting. Turk. J. Immunol. 2018, 6, 23–29. [Google Scholar] [CrossRef]
- Ingadottir, A.R.; Beck, A.M.; Baldwin, C.; Weekes, C.E.; Geirsdottir, O.G.; Ramel, A.; Gislason, T.; Gunnarsdottir, I. Oral nutrition supplements and between-meal snacks for nutrition therapy in patients with COPD identified as at nutritional risk: A randomised feasibility trial. BMJ Open Respir. Res. 2019, 6, e000349. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, S.J.; Han, Y.; Ryu, Y.J.; Lee, J.H.; Chang, J.H. Hand grip strength and chronic obstructive pulmonary disease in Korea: An analysis in KNHANES VI. Int. J. COPD 2017, 12, 2313–2321. [Google Scholar] [CrossRef]
- Jeong, M.; Kang, H.K.; Song, P.; Park, H.K.; Jung, H.; Lee, S.-S.; Koo, H.-K. Hand grip strength in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Poot, C.C.; Meijer, E.; Kruis, A.L.; Smidt, N.; Chavannes, N.H.; Honkoop, P.J. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2021, 2021, CD009437. [Google Scholar] [CrossRef]
- Valentijn, P.I.M.P.; Tymchenko, L.; Arends, R.Y. Effectiveness of Integrated Care for Diabetes Mellitus Type 2, Cardiovascular and Chronic Respiratory Diseases: A Systematic Review and Meta-Analysis. Int. J. Integr. Care 2024, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Beijers, R.J.H.C.G.; Steiner, M.C.; Schols, A.M.W.J. The role of diet and nutrition in the management of COPD. Eur. Respir. Rev. 2023, 32, 230003. [Google Scholar] [CrossRef]
- Allard, J.P.; Keller, H.; Gramlich, L.; Jeejeebhoy, K.N.; Laporte, M.; Duerksen, D.R. GLIM criteria has fair sensitivity and specificity for diagnosing malnutrition when using SGA as comparator. Clin. Nutr. 2020, 39, 2771–2777. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| 1++ | High-quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias |
| 1+ | Meta-analyses, systematic reviews of well-conducted RCTs, or RCTs with low risk of bias |
| 1− | Meta-analyses, systematic reviews of well-conducted RCTs, or RCTs at high risk of bias |
| 2++ | High-quality systematic reviews of case-control studies; cohort or case-control studies with a low risk of confounding, bias, or chance and a high probability that the relationship is causal |
| 2+ | Well-conducted case-control or cohort studies with a low risk of confounding, bias, or chance and a moderate probability that the relationship is causal |
| 2− | Case-control or cohort studies with a high risk of confounding, bias, or chance and a significant risk that the relationship is causal |
| 3 | Non-analytical studies, such as case reports and case series |
| 4 | Expert opinion |
| A | At least one meta-analysis, systematic review, or RCT rated 1++ and directly applicable to the target population; or A body of evidence consisting primarily of studies rated 1+, directly applicable to the target population, and demonstrating the overall consistency of the results |
| B | A body of evidence that includes studies rated as 2++, directly applicable to the target population; or a body of evidence that includes studies rated as 2+, directly applicable to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated 1++ or 1+ |
| O | Level of evidence 3 or 4; or extrapolated evidence from studies rated 2++ or 2+ |
| GPP | Good Practice Point/Expert Consensus: Recommended best practice based on the clinical experience of the expert panel |
| 1 | Strongly disagree |
| 2 | Disagree |
| 3 | Neither agree nor disagree |
| 4 | Agree |
| 5 | Strongly agree |
| Level of Study | |
|---|---|
| Dual-energy X-ray absorptiometry (DEXA) | *** |
| Magnetic resonance imaging (MRI) | ** |
| Computed tomography (CT) | ** |
| Muscle ultrasound | ** |
| Bioelectric impedance analysis | ** |
| Anthropometry | * |
|
| Objectives to Be Taken into Account | Grade of Recommendation |
|---|---|
| Weight increase | *** |
| Lean mass increase | *** |
| Potency of effect in physical exercise | *** |
| Improvement in muscle functionality | ** |
| Improvement in lung function | ** |
| Improvement in quality of life | ** |
| Improvement of laboratory parameters | * |
| Reduction of post-hospitalisation mortality | * |
| ONS (kcal/prot) | (N) and Time | Grade COPD | Comparator | Effects | References |
|---|---|---|---|---|---|
| Normo/Normo Omega-3/6 DHA FOS (Prebiotic fiber) | (99) 3 months | Stable outpatient Mild/moderate/severe | PCB | ↑ weight ↑ FEV1 ↑ Grip strength ↑ 6MWT | Benito Martinez et al. [109] |
| High/High Leucine Omega-3 [111] PUFAs [108] Vitamin D | (81) 4 months [108,111] (45) 3 months [92] | Stable outpatient Moderate/severe +High-intensity exercise | PCB [108,111] Isocaloric formula [92] | ↑ Vit D, EPA, DHA, Omega-3 ↑ Weight, ↑ FM ↑ FEV1 ↓ Depression Score without difference with isocaloric formula * [92] | Van de Bool et al. [111] Van Beers et al. [108] Calder et al. [92] |
| High/High HMB | (652) 3 months [68,69] (214) 3 months [93] | Hospital COPD > 65 years + other pathologies. Up to 90 days post-H | PCB P (comp form 48 kcal standard + Vit C) [68] | ↓ 30–60–90 day mortality (NNT 20) ↑ SGA scale ↑ Weight ↑ Vit D ↑ Grip strength ↑ nutritional markers | Deutz et al. [68] Deutz et al. [93] (sub-analysis of [68]) Matheson et al. [69] |
| Normo/High Whey protein Omega-3 Vitamins A,C,E | (36) 3 months (12) 2 weeks | Stable outpatient Moderate/severe +Low-intensity exercise | PCB [112] Hydrolysed casein [113] | ↓ Inflammatory markers ↑ 6MWT ↑ Weight, ↑ FM ↑ Quadriceps strength ↑ Inspiratory pressure max ↑ Emotional Function and Quality of Life score No difference between the two types of protein [113] | Sugawara et al. [112] Jonker et al. [116] |
| Low (0.45)/High Whey protein Magnesium + Vitamin C | (44) 2 months | Stable outpatient Moderate/severe | PCB | ↑ FFM ↑ Vit C ↑ St. George’s Respiratory Questionnaire ↓ Inflammatory markers | Ahmadi et al. [67] |
| Grade of Recommendation | |
|---|---|
| Monitor levels of ions, especially magnesium, calcium, phosphorus | *** |
| Vit D if deficient (<20 ng/mL) | ** |
| HMB and essential amino acids | * |
| Omega-3, Vit D and leucine | N |
| Antioxidant vitamins A, C and E, and selenium | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Justel Enríquez, A.; Rabat-Restrepo, J.M.; Vilchez-López, F.J.; Tenorio-Jiménez, C.; García-Almeida, J.M.; Irles Rocamora, J.-A.; Pereira-Cunill, J.L.; Martínez Ramírez, M.J.; Molina-Puerta, M.J.; Molina Soria, J.B.; et al. Practical Guidelines by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) on Nutritional Management of Patients with Chronic Obstructive Pulmonary Disease: A Review. Nutrients 2024, 16, 3105. https://doi.org/10.3390/nu16183105
Justel Enríquez A, Rabat-Restrepo JM, Vilchez-López FJ, Tenorio-Jiménez C, García-Almeida JM, Irles Rocamora J-A, Pereira-Cunill JL, Martínez Ramírez MJ, Molina-Puerta MJ, Molina Soria JB, et al. Practical Guidelines by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) on Nutritional Management of Patients with Chronic Obstructive Pulmonary Disease: A Review. Nutrients. 2024; 16(18):3105. https://doi.org/10.3390/nu16183105
Chicago/Turabian StyleJustel Enríquez, Alicia, Juana M. Rabat-Restrepo, Francisco J. Vilchez-López, Carmen Tenorio-Jiménez, José M. García-Almeida, José-Antonio Irles Rocamora, José L. Pereira-Cunill, María J. Martínez Ramírez, María J. Molina-Puerta, Juan B. Molina Soria, and et al. 2024. "Practical Guidelines by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) on Nutritional Management of Patients with Chronic Obstructive Pulmonary Disease: A Review" Nutrients 16, no. 18: 3105. https://doi.org/10.3390/nu16183105
APA StyleJustel Enríquez, A., Rabat-Restrepo, J. M., Vilchez-López, F. J., Tenorio-Jiménez, C., García-Almeida, J. M., Irles Rocamora, J.-A., Pereira-Cunill, J. L., Martínez Ramírez, M. J., Molina-Puerta, M. J., Molina Soria, J. B., Rebollo-Pérez, M. I., Olveira, G., & García-Luna, P. P. (2024). Practical Guidelines by the Andalusian Group for Nutrition Reflection and Investigation (GARIN) on Nutritional Management of Patients with Chronic Obstructive Pulmonary Disease: A Review. Nutrients, 16(18), 3105. https://doi.org/10.3390/nu16183105






