Abstract
Melatonin, as an endocrine neurotransmitter, can promote the development of the ovary. Meanwhile, it also has protective effect on the ovary as an antioxidant. Thyroid hormone (TH) is essential for normal human reproductive function. Many studies have shown that 3,5,3′-triiodothyronine (T3) regulates the development of ovarian granulosa cells. However, little is known about the specific mechanisms by which melatonin combines with T3 to regulate granulosa cell development. The aim of present study was to investigate the effects and the possible mechanisms of melatonin and T3 on ovarian granulosa cell development. In the present study, cell development and apoptosis were detected by CCK8, EdU and TUNEL, respectively. The levels of related proteins were analyzed by Western blotting. The results showed that oxidative stress (OS) and reactive oxygen species (ROS) were induced by H2O2 in granulosa cells, and cell apoptosis was also increased accompanied with the decreased cellular proliferation and viability. Melatonin protects granulosa cells from H2O2-induced apoptosis and OS by downregulating ROS levels, especially in the presence of T3. Co-treatment of cell with melatonin and T3 also promotes the expression of GRP78 and AMH, while inhibiting CHOP, Caspase-3, and P16. It was demonstrated that melatonin alone or in combination with T3 had positive effect on the development of granulosa cells. In addition, the AMPK/SIRT1 signaling pathway is involved in the process of melatonin/T3 promoting granulosa cell development.
1. Introduction
Mammalian follicular development from the primordial follicle to ovulation is a highly complex and tightly regulated process [1,2], which is also accompanied by the follicle atresia [3]. Granulosa cells are crucial for the follicular development, and their apoptosis is closely related to follicle atresia [4,5,6,7].
It is well known that incomplete reduction of oxygen leads to the generation of reactive oxygen species (ROS), causing oxidative stress (OS) and affecting normal cell function [8]. Studies have shown that H2O2 can induce granulosa cell apoptosis through the ROS-JNK-p53 pathway [9]. OS has significant effects on mitochondrial activity, proliferation, differentiation and cell cycle in granulosa cells [10]. Endoplasmic reticulum stress (ERS) also affects ovarian granulosa cell development. The excessive ERS may trigger cell apoptosis by regulating the expression of CHOP and caspase-3 [11]. In goat follicles, ERS-related apoptotic proteins are increased with the upregulation of GRP78 (Glucose-regulated protein 78), which plays many important roles in cell survival and apoptosis [12]. Increased evidence has shown that OS and ERS interact with each other to regulate cellular development. Ovarian dysfunction in patients with endometriosis is associated with ERS combined with OS by regulating granulosa cell apoptosis [13]. In neuronal cells, excessive OS induces ERS and then increases cell apoptosis [14]. However, the regulation of ovarian granulosa cells by OS and ERS still remains to be explored.
It has been reported that melatonin can act against OS and regulate ovarian development [15]. A previous study has shown that melatonin has a positive effect on granulosa cells and stimulates the synthesis of estradiol [16]. Moreover, melatonin can interact with thyroid hormone (TH) to regulate ovarian functions. It has been reported that dysregulation of TH impairs ovarian function, and TH indirectly or directly regulates follicular development [17,18]. Our previous studies have shown that 3,5,3′-triiodothyronine (T3) combined with follicle-stimulating hormone (FSH) inhibits cell apoptosis and promotes cell proliferation by increasing steroid hormone synthesis and glucose uptake in granulosa cells [11,19,20,21]. Although many studies have reported that melatonin and TH are important in granulosa cell development, the exact mechanisms by which melatonin and T3 regulate ovarian granulosa cell development are still unclear.
Anti-Mullerian hormone (AMH) is a reproductive hormone representing ovarian reserve capacity, which inhibits follicle recruitment and affects cells normal growth and differentiation. Lower concentration of AMH and impaired oocyte quality during female aging are due to the increased cellular OS and DNA damage [22]. Multiple tumor suppressor 1 (P16/MTS) inhibits cells division and is considered a marker of tissue and cellular senescence. P16 expression is related to OS and is increased by the high OS levels in the ovary, thereby inducing ovarian aging [23,24,25]. Accumulating studies suggest that OS contributes to cellular senescence; however, the mechanisms underlying the potential alleviating effects of melatonin and T3 on this phenomenon remain unknown.
The Adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK)/Sirtuin1 (SIRT1) pathway is a well-established important biological metabolic pathway that regulates many processes in numerous cells. Moreover, AMPK and SIRT1 also interact with each other and participate in cell aging, life span regulation, and other functions. The SIRT1/AMPK axis is involved in autophagy activation and promotes ovarian development [26]. The AMPK/SIRT1 signaling pathway also plays a key role in delaying premature ovarian failure (POF) [27]. The regulation of AMPK/SIRT1 is diverse and complex. However, whether and how AMPK and SIRT1 modulate follicular development is not known.
In this study, we found that melatonin and T3 play positive roles in the development of granulosa cells by reducing OS and ERS. Moreover, the AMPK/SIRT1 signaling pathway is involved in these regulations.
2. Materials and Methods
2.1. Reagents and Antibodies
Unless otherwise specified, most of the reagents and chemicals used in the present study were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal anti-AMH (ab229212), rabbit monoclonal anti-SIRT1 (ab189494), and rabbit monoclonal anti-AMPK (ab32047) were purchased from Abcam (Cambridge, MA, USA). Rabbit polyclonal anti-GRP78 (sc-13968), mouse monoclonal anti-CHOP (sc-7351), rabbit polyclonal anti-Caspase-3 (sc-7148), and mouse monoclonal anti-P16 (sc-1661) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit monoclonal anti-p-AMPK (#2537) was obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal anti-β-actin (BE39995), horse radish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse IgG were from Bio-easy (Beijing, China). The inhibitors of AMPK (Compound C) and SIRT1 (EX527) were purchased from Selleck (Houston, TX, USA). Medium 199 (M199) was purchased from Biological Industries (Beit Haemek, Israel). Fetal bovine serum was from PAN-Biotech (Bavaria, Aidenbach, Germany). The penicillin and streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). The enhanced chemiluminescence (ECL) detection kit was obtained from LABLEAD (Beijing, China).
2.2. Animal Treatments
The 21-day-old Sprague Dawley (SD) female rats were purchased from the Beijing Vital Laboratory Animal Technology (Beijing, China) and kept at constant temperature (24–26 °C) and humidity (60% ± 2%) with a light/dark cycle of 12/12 h. Ovaries were collected after administration of diethylstilbestrol (DES) (10 mg/mL) to immature SD rats by intraperitoneal injection for three consecutive days [28]. All animals were euthanized using carbon dioxide inhalation. All procedures were approved by the Institutional Animal Care and Use Committee of Capital Normal University and conducted in accordance with the Principles of the Care and Use of Laboratory Animals and China Council on Animal Care.
2.3. Rat Granulosa Cell Isolation and Culture
The processes of granulosa cell isolation and culture were performed as described before [29]. The culture medium was prepared using M199 culture medium and supplemented with 10% fetal bovine serum and 1% antibiotics. The cells were maintained under a humidified atmosphere of 95% air and 5% CO2 at 37 °C. Briefly, the ovaries were punctured with a 1 mL syringe needle under a stereoscope, and then the cell suspensions were filtered through a nylon cell strainer. Approximately 9 × 105 viable granulosa cells were isolated from 2–3 ovaries in each group (per well in a 6-well plate). The cells were centrifuged and subsequently plated in pre-equilibrated culture medium, followed by 24 h of adhesion culture. Then, cells were pretreated with H2O2 for 1 h and subsequently cultured with melatonin (0.1 μM) (Lot # WXBC9956V, purity 99%) and/or T3 (1.0 nM) (Lot # BCCC9589, purity 99%) for 48 h. Some groups were treated with melatonin (0.1 μM) and T3 (1.0 nM) for 0 min, 30 min, 1 h, 6 h, 12 h or 24 h. For some experiments, cells were pretreated with AMPK or SIRT1 inhibitors (10 μM) for 24 h prior to being cultured with melatonin (0.1 μM) and T3 (1.0 nM) for 48 h.
2.4. Protein Extraction and Western Blotting
Western blotting analysis was performed as described previously [30]. The cell pellets were lysed by incubation with lysis buffer at 0 °C for 30 min, and the supernatant was collected for experiments after centrifugation (15,000× g, 4 °C, 30 min). Protein concentration was determined by the BCA Protein Assay kit (Beyotime Biotechnology, Shanghai, China). Total proteins (20 µg) were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Roche, Basel, Switzerland). Subsequently, the membranes were incubated with 5% bovine serum protein for 1 h at room temperature, and then incubated with diluted primary antibody [polyclonal anti-AMH (1:500), monoclonal anti-AMPK (1:500), monoclonal anti-p-AMPK (1:500), monoclonal anti-CHOP (1:500), polyclonal anti-Caspase-3 (1:500), polyclonal anti-GRP78 (1:1000), monoclonal anti-P16 (1:200), monoclonal anti-SIRT1 (1:500), or polyclonal anti-β-actin (1:500)] overnight at 4 °C, followed by incubation with HRP-conjugated secondary antibody (1:2000–1:5000) for 2 h at room temperature. Peroxidase activity was observed and visualized with the ECL kit and ImageQuant LAS 4000 Mini imaging system (Cytiva, Marlborough, MA, USA) according to the manufacturer’s instructions. The immune response signals were analyzed using AlphaEaseFC 4.0 (Alpha Innotech, San Leandro, CA, USA).
2.5. Detection of ROS
In accordance with instructions, DCFH-DA (1:1000) was diluted to a final concentration of 10 μM. After the treatment drug was removed, the cells were washed 3 times with 1× phosphate-buffered saline (1×PBS), and then diluted DCFH-DA solution was added to fully cover all the cells. Subsequently, the cells were incubated at 37 °C for 30 min. The adherent growth cells were examined under a fluorescence microscope and photographed. The experimental results were analyzed using ImageJ 1.53e.
2.6. Analysis of Cell Viability
CCK-8 (Dojindo, Kumamoto, Japan) was used to detect the cell viability, as described previously [20]. The cells were incubated with 10 µL CCK-8 solution for 2–3 h at 37 °C, and optical density values were measured by a microplate reader at 450 nm. The mean optical density value of each treatment was used as an index of cell viability.
2.7. EdU Incorporation Assay
EdU cell proliferation assays were performed according to the manufacturer’s instructions (Beyotime Biotechnology, Shanghai, China). Briefly, cells were cultured and incubated with 50 µM EdU for 2 h, followed by three washes in 1×PBS. After fixation with 4% paraformaldehyde for 30 min at room temperature and permeabilization with 0.5% Triton X-100 for 10 min, cells were stained for EdU. Cell nuclei were counterstained with Hoechst 33342 for 30 min. EdU-positive nuclei were detected using a laser scanning microscope, LSM 780 (Zeiss, Jena, Germany). The proportion of nucleated cells containing EdU in five high-power fields/well was used to calculate the rate of cell proliferation.
2.8. TUNEL Analysis
Apoptotic cells were identified using a TUNEL Apoptosis Assay Kit (KeyGEN, Beijing, China). In brief, cells were fixed with 4% paraformaldehyde for 30 min at room temperature and then permeated with 1% Triton X-100 for 5 min. After washing with 1×PBS, cells were incubated in TdT buffer for 1 h at 37 °C in a humidified chamber. Then, 50 µL of the Streptavidin-TRITC labeling solution was added to each well and incubated in the dark at 37 °C for 30 min. To stain the nuclei, cells were incubated with diluted DAPI solution (1:200) for 10 min at room temperature in dark. Subsequently, the cells were photographed with a laser scanning microscope, LSM 780 (Zeiss, Jena, Germany), and the images were recorded using Zeiss ZEN lite software (ZEN 2, blue edition).
2.9. Statistical Analysis
The experiments were repeated at least three times. All experimental data are presented as mean ± SEM. The data were analyzed by GraphPad Prism 8.3.0 software (GraphPad Software, La Jolla, CA, USA) and the statistical differences between groups were calculated with a t-test and one-way (repeated-measures) ANOVA. Means were compared by the Bonferroni post-test when significant differences were found. Additionally, statistically significant differences were considered at p < 0.05.
3. Results
3.1. Effect of H2O2 on Granulosa Cells
To explore the effect of OS on the development of granulosa cells, cells were treated by H2O2 with different concentrations (0, 100, 200, 400 and 800 μM) for 1 h, and cell viability was determined by CCK8 assay. As shown in Figure 1, cell viability was significantly decreased by H2O2 in a dose–response manner (p < 0.0001), which suggests that cell growth was affected by higher OS.
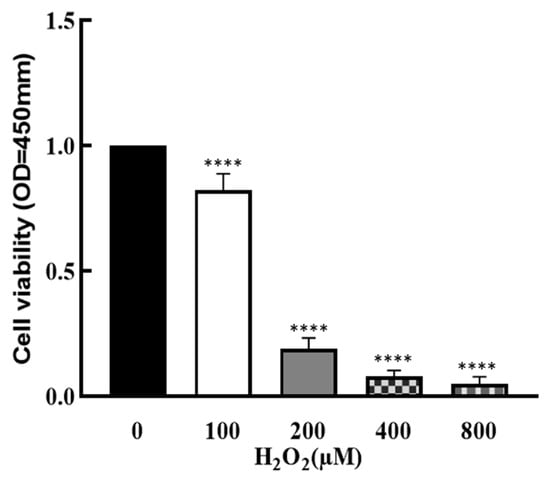
Figure 1.
Effect of H2O2 on granulosa cell viability. The ovarian granulosa cells were pretreated with different concentrations of H2O2 (0, 100, 200, 400 and 800 μM) for 1 h, and a CCK-8 assay was used to analyze the cell viability. **** p < 0.0001, compared with 0 μM group.
3.2. Effect of Melatonin/T3 on H2O2-Induced ROS in Granulosa Cells
To explore the effect of melatonin/T3 on OS in granulosa cells, cells were pre-treated with H2O2 for 1 h, and then treated with melatonin (0.1 μM) and/or T3 (1.0 nM) for 48 h. The concentrations of melatonin and T3 were selected based on the preliminary experiments (Figures S1 and S2). The fluorescence intensity of DCF was detected, and the content of ROS was measured. As shown in Figure 2, ROS levels were significantly increased by H2O2 (p < 0.0001). However, this induction was dramatically reduced by melatonin and T3 alone (p < 0.001). Moreover, the combination of melatonin and T3 was more effective in reducing ROS than hormone alone (p < 0.01).
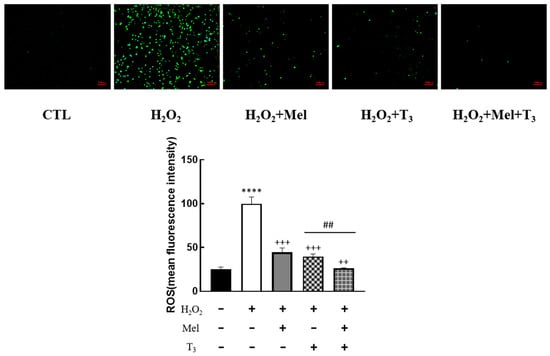
Figure 2.
Effects of melatonin/T3 on ROS in rat granulosa cells. After granulosa cells were induced by H2O2 (100 μM) for 1 h, granulosa cells were cultured with melatonin (0.1 μM) and/or T3 (1.0 nM) for 48 h, and the ROS levels were measured. **** p < 0.0001, compared with CTL; ++ p < 0.01, +++ p < 0.001, compared with H2O2; ## p < 0.01, compared with H2O2 + T3. Scale bars = 100 μm.
3.3. Effects of Melatonin/T3 on Cell Growth of H2O2-Treated Granulosa Cells
To demonstrate the effect of melatonin/T3 on cellular development in rat granulosa cells under OS, cell viability and proliferation were investigated after hormone treatment. As shown in Figure 3, granulosa cell viability was significantly reduced by H2O2 (p < 0.01, Figure 3A), and the decreasing effect was reversed by melatonin, T3 (p < 0.01, p < 0.001, Figure 3A), and especially by the combination of these hormones (p < 0.05, Figure 3A).
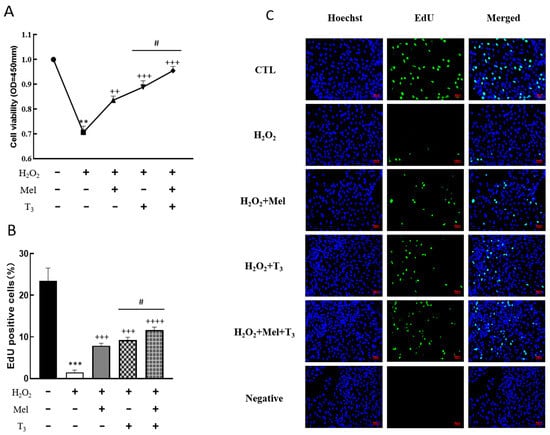
Figure 3.
Effects of melatonin/T3 on the viability and proliferation of rat granulosa cells. Granulosa cells were induced by H2O2 (100 μM for 1 h) and cultured with melatonin (0.1 μM) and/or T3 (1.0 nM) for 48 h. Cell viability (A) and proliferation (B,C) were detected by CCK-8 assay and EdU, respectively. The nucleus was stained with Hoechst (blue fluorescence). Data are presented as mean ± standard error of the mean of three independent experiments. ** p < 0.01, *** p < 0.001, compared with CTL; ++ p < 0.05, +++ p < 0.001, ++++ p < 0.0001, compared with H2O2; # p < 0.05, compared with H2O2 + T3. Scale bars = 50 μm.
3.4. Effect of Hormones on Granulosa Cells’ Apoptosis
To explore the effect of melatonin/T3 on cellular apoptosis, granulosa cells were induced by H2O2 before hormone treatment. As shown in Figure 4, H2O2 significantly increased granulosa cell apoptosis (p < 0.001). However, H2O2-induced apoptosis was significantly inhibited by melatonin (p < 0.01) and T3 (p < 0.01) alone. The combined hormone treatment showed the most pronounced effect.
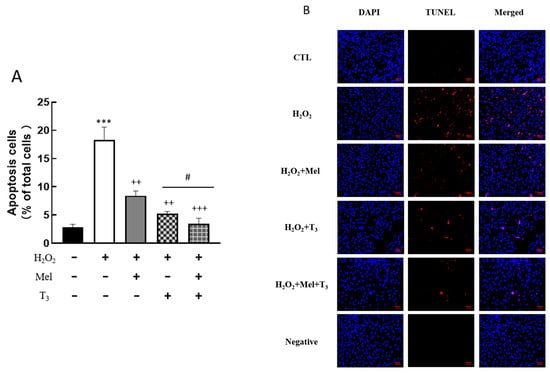
Figure 4.
Effects of melatonin/T3 on apoptosis of rat granulosa cells. Granulosa cells were induced by H2O2 (100 μM for 1 h) and then cultured with melatonin (0.1 μM) and/or T3 (1.0 nM) for 48 h. A TUNEL assay was used to measure the apoptosis (A,B) of granulosa cells in different groups. The nucleus was stained with DAPI (blue fluorescence). *** p < 0.001, compared with CTL; ++ p < 0.01, +++ p < 0.001, compared with H2O2; # p < 0.05, compared with H2O2 + T3. Scale bars = 50 μm.
3.5. Effects of Melatonin/T3 on the Expression of AMH and P16 in Granulosa Cells
It is well known that AMH is produced by the granulosa cells of small, growing follicles, which are regarded as the marker of ovarian reserve. In order to investigate the effects of hormones on AMH expression, cells were pretreated with H2O2 before hormone treatment. The results showed that H2O2 treatment significantly suppressed AMH expression (p < 0.05, Figure 5A). This suppression was effectively countered by subsequent treatment with either melatonin or T3 (p < 0.05, p < 0.01, Figure 5A), and especially by combined treatment with both hormones (p < 0.05, Figure 5A).
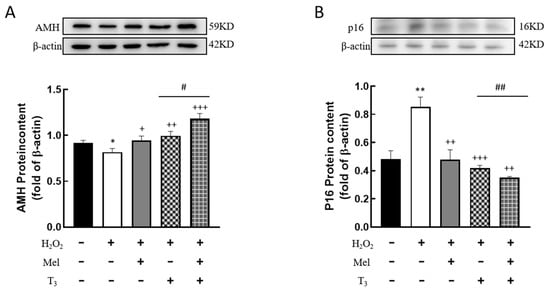
Figure 5.
Effects of melatonin/T3 on AMH and P16 content in granulosa cells. The proteins expression of AMH (A) and P16 (B) were determined by Western blotting. * p < 0.05, ** p < 0.01, compared with CTL; + p < 0.05, ++ p < 0.01, +++ p < 0.001, compared with H2O2; # p < 0.05, ## p < 0.01, compared with H2O2 + T3.
3.6. Effects of Melatonin/T3 on ERS in Granulosa Cells
To further detect the effect of H2O2 on ERS in granulosa cells, the expression of ERS-related proteins, GRP78, CHOP, and Caspase-3, were investigated. The results showed that H2O2 significantly downregulated the expression of the ERS-associated protein GRP78 (p < 0.01, Figure 6A), and the inhibitory effect was mitigated by individual treatments with melatonin or T3 alone (p < 0.05, p < 0.01, Figure 6A). Notably, co-treatment with both hormones further increased GRP78 expression (p < 0.05, Figure 6A).
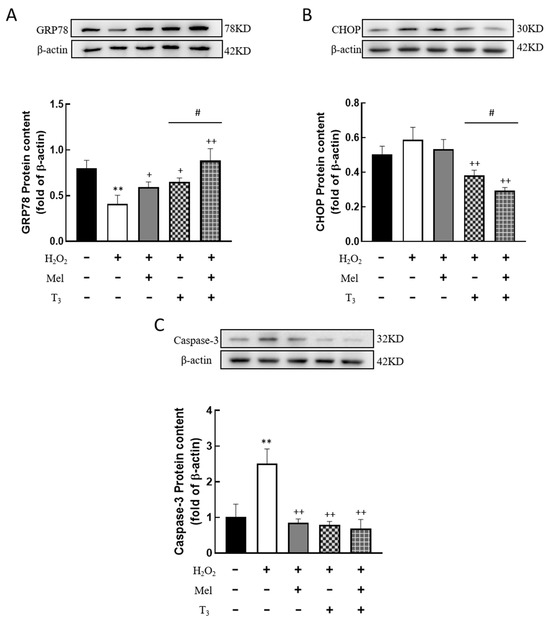
Figure 6.
Effects of melatonin/T3 on ERS related proteins in granulosa cells. The protein expression of GRP78 (A), CHOP (B) and Caspase-3 (C) was determined by Western blotting. ** p < 0.01, compared with CTL; + p < 0.05, ++ p < 0.01, compared with H2O2; # p < 0.05, compared with H2O2 + T3.
3.7. Effect of Melatonin/T3 on the Expression of AMPK and SIRT1 in Granulosa Cells
To investigate whether H2O2 affects the expression of AMPK and SIRT1, the protein contents were detected. H2O2 treatment significantly decreased the p-AMPK protein level (p < 0.05, Figure 7A), and the effect was substantially reversed by hormone treatment (p < 0.01, Figure 7A). Similarly, H2O2 suppressed SIRT1 expression (p < 0.05, Figure 7B), and this suppression was also counteracted by T3 (p < 0.05, Figure 7B), especially in the presence of melatonin (p < 0.05, Figure 7B).
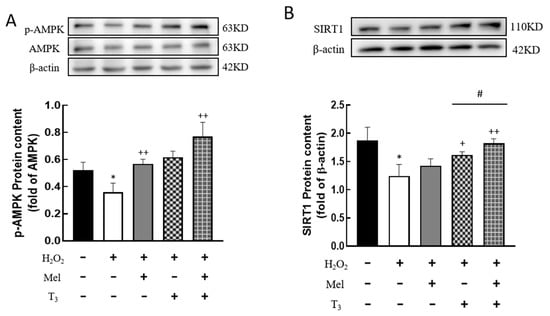
Figure 7.
Effects of melatonin/T3 on AMPK and SIRT1 in granulosa cells. The protein expression of AMPK (A) and SIRT1 (B) was determined by Western blotting. * p < 0.05, compared with CTL; + p < 0.05, ++ p < 0.01, compared with H2O2; # p < 0.05, compared with H2O2 + T3.
3.8. The AMPK/SIRT1 Signaling Pathway Is Involved in the Regulation of Cellular Development by Melatonin/T3
To examine whether melatonin/T3 regulate AMPK and SIRT1, granulosa cells were treated with melatonin and T3, and then the expression levels of p-AMPK and SIRT1 were detected. The results showed that the expression level of p-AMPK peaked at 1 h after melatonin and T3 co-treatment (p < 0.05, Figure 8A), and the expression level of SIRT1 peaked at 6 h after hormone treatment (p < 0.01, Figure 8B).
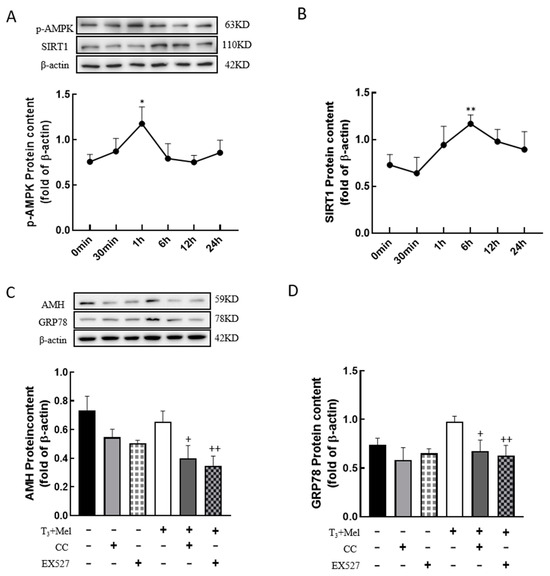
Figure 8.
The AMPK/SIRT1 signaling pathway is involved in the regulation of granulosa cells via melatonin/T3. A/B protein was extracted at 0 min, 30 min, 1 h, 6 h, 12 h and 24 h after melatonin/T3 co-treatment. The protein expression of AMPK (A), SIRT1 (B), AMH (C) and GRP78 (D) was determined by Western blotting. * p < 0.05, ** p < 0.01, compared with 0 min; + p < 0.05, ++ p < 0.01, compared with melatonin/T3.
In order to determine whether the AMPK/SIRT1 signaling pathway is involved in the regulation of cellular development, granulosa cells were pretreated with Compound C (AMPK inhibitor, 10 μM) or EX527 (SIRT1 inhibitor, 10 μM) for 24 h and then treated with melatonin and T3 for 48 h. As shown in Figure 8C,D, the expression of AMH and GRP78 in granulosa cells was significantly reduced by the inhibitors after melatonin and T3 treatment (p < 0.05, p < 0.01, Figure 8C,D).
4. Discussion
In the present study, we investigated the effects of melatonin and/or T3 on ovarian granulosa cells. Our results demonstrated that melatonin and T3 have protective effects against hydrogen peroxide (H2O2)-induced oxidative damage to granulosa cells, especially in the presence of both hormones. Additionally, the AMPK/SIRT1 signaling pathway is involved in this regulation.
Studies have found that OS is an imbalance between oxidative and anti-oxidative processes in vivo, which is involved in the pathogenesis of different diseases. OS also regulates the survival and development of granulosa cells [12]. In the present experiment, H2O2 induced higher levels of ROS. Additionally, cell proliferation and viability were significantly reduced with increased apoptosis, which caused negative effects on cells.
It has been reported that the occurrence of POF may be closely related to OS in the ovary [31,32]. Our results showed that H2O2 decreased the expression of AMH as a marker of ovarian aging [25]. Moreover, the expression of P16 was significantly increased in granulosa cells after H2O2 treatment. As a cell cycle suppressor gene, P16 is highly expressed in the process of inhibiting cell differentiation [28], and P16 is currently considered a tissue and cellular marker of senescence [33,34,35]. Some studies have found that the P16 protein is expressed at low levels in the tissues of young animals, and its expression increases with age [36]. These results indicate that H2O2 could induce POF by increasing cellular senescence and downregulating AMH content.
OS is also implicated in the cellular apoptosis. Autophagy is promoted by activating ROS and ERS in mouse granulosa cells [37]. Some forms of ROS can interfere with protein folding in endoplasmic reticulum and induce ERS [38]. GRP78 plays an important role in protein synthesis and is the central regulator of ERS [11]. It participates in protein assembly and folding and binds to misfolded proteins to prevent protein agglutination. GRP78 can regulate the balance between cell survival and apoptosis in ERS cells [39]. The GRP78 receptor can be detected on the surface of rat ovarian granulosa cells and regulates cell proliferation and survival [40,41]. It was found that ERS enhanced the expression of GRP78 in mouse granulosa cells and triggered cell apoptosis [42]. Some studies have also shown that ERS-related apoptotic proteins are increased with the expression of GRP78, which regulates granulosa cell apoptosis and participates in the process of follicular atresia in goats [12]. In the present study, the expression of GRP78 in granulosa cells after H2O2 treatment was significantly decreased, and the expression of CHOP and Caspase-3 were dramatically increased. However, these effects induced by H2O2 were significantly reversed by melatonin alone or in combination with T3. These results indicate that GRP78 may play protective roles in cellular development after H2O2 treatment.
Melatonin has extensive physiological functions in different tissues and cells. Melatonin also has protective effects on OS-induced apoptosis in different cells. The antioxidant effect of melatonin is closely related to the removal of OS [35,43]. It has been reported that melatonin is essential in reducing the production of cellular OS to promote oocyte maturation and granulosa cell development [11]. Meanwhile, melatonin is considered -an anti-aging agent based on its cytoprotective effect. In the current study, melatonin improved the development of granulosa cells. The attenuation of apoptosis by melatonin in granulosa cells might be due to decreased ROS production. Moreover, the same positive effects were also shown after T3 treatment in H2O2-induced granulosa cells. Interestingly, the protective effects of the combination of melatonin and T3 on granulosa cells were significantly higher than that of melatonin or T3 alone. The effects of these hormones may be due to the antioxidant and anti-aging properties [22]. In addition, melatonin is a seasonal hormone, which is regulated by photoperiod [44]. The effects of melatonin regulated by photoperiod on reproduction still need to be further investigated in the future. Whether hormones alleviate ovarian dysfunction by reducing OS in vivo remains to be explored.
It has been reported that OS is closely related to energy transfer, and AMPK is the main energy sensor for energy homeostasis in eukaryotes [45,46]. AMPK is significantly reduced when cells undergo OS [34,47], which regulates downstream pathways. SIRT1, as a downstream regulator of AMPK, plays roles in regulating gene transcription and DNA repair [19,48]. Many studies have shown that AMPK/SIRT1 plays an important role in promoting cell signal transduction, and this pathway is involved in the regulation of follicular maturation and development [49,50]. In the present study, H2O2 decreased AMPK and SIRT1 expression in granulosa cells. Moreover, the expressions of AMH and GRP78 were significantly decreased by AMPK or SIRT1 inhibitors, respectively. These results indicate that the AMPK/SIRT1 pathway is involved in the upregulation of AMH and GRP78 expression by melatonin/T3.
5. Conclusions
Our findings demonstrate that OS and ERS significantly impair the viability and developmental potential of rat granulosa cells, as evidenced by H2O2-induced apoptosis and reduced cell proliferation. Melatonin and T3, and particularly the combination of both hormones, have protective effects on granulosa cell development. In addition, the AMPK/SIRT1 signaling pathway is involved in the regulation of AMH and GRP78 expression in granulosa cells by melatonin and T3. Our results enhance our understanding of the roles of melatonin and thyroid hormone in suppressing ERS and OS to promote the development of granulosa cells. Our findings provide valuable insights into the protective mechanisms of granulosa cells and lay the groundwork for improving ovarian health and fertility.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16183085/s1, Figure S1: Effects of melatonin on ROS in rat granulosa cells; Figure S2: Effects of T3 on ROS in rat granulosa cells.
Author Contributions
Conceptualization, M.W., Y.Y. (Yilin Yao) and C.Z.; methodology, M.W., Y.Y. (Yilin Yao), R.C., B.F. and C.Z.; validation, Y.S., Y.Y. (Yakun Yu) and Y.L.; formal analysis, R.C., H.F. and S.G.; investigation, M.W. and B.F.; resources, C.Z.; data curation, R.C. and Y.Y. (Yilin Yao); writing—original draft preparation, M.W.; writing—review and editing, C.Z.; visualization, M.W.; supervision, C.Z. and Y.Y. (Yanzhou Yang); project administration, Y.Y. (Yanzhou Yang), B.F. and C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32171109.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Capital Normal University, approval code: IACUC NO. 2021025 (date of 14 June 2021) and conducted in accordance with the Principles of the Care and Use of Laboratory Animals and China Council on Animal Care.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and materials that support the findings of this study are available in the methods of this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
AMH, anti-Mullerian hormone; AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; AP, ammonium persulfate; DES, diethylstilbestrol; ECL, enhanced chemiluminescence; ERS, endoplasmic reticulum stress; FSH, follicle-stimulating hormone; GRP78, glucose-regulated protein 78; HRP, horse radish peroxidase; MEL, melatonin; M199, medium 199; OS, oxidative stress; PBS, phosphate-buffered saline; POF, premature ovarian failure; P16/MTS, multiple tumor suppressor 1; ROS, reactive oxygen species; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; SIRT1, sirtuin1; TH, thyroid hormone; TUNEL, terminal deoxynucleotidyl transferase 2′-deoxyuridine, 5′-triphosphate nick-end labeling; T3, 3,5,3′-triiodothyronine.
References
- Adashi, E.Y. Endocrinology of the ovary. Hum. Reprod. 1994, 9, 815–827. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [PubMed]
- Hsueh, A.J.; Billig, H.; Tsafriri, A. Ovarian follicle atresia: A hormonally controlled apoptotic process. Endocr. Rev. 1994, 15, 707–724. [Google Scholar] [PubMed]
- Takagi, K.; Yamada, T.; Miki, Y.; Umegaki, T.; Nishimura, M.; Sasaki, J. Histological observation of the development of follicles and follicular atresia in immature rat ovaries. Acta Medica Okayama 2007, 61, 283–298. [Google Scholar] [PubMed]
- Orisaka, M.; Tajima, K.; Tsang, B.K.; Kotsuji, F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J. Ovarian Res. 2009, 2, 9. [Google Scholar] [CrossRef]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Amsterdam, A.; Keren-Tal, I.; Aharoni, D.; Dantes, A.; Land-Bracha, A.; Rimon, E.; Sasson, R.; Hirsh, L. Steroidogenesis and apoptosis in the mammalian ovary. Steroids 2003, 68, 861–867. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Li, H.; Mu, H.; Zeng, L.; Cai, S.; Su, P.; Li, H.; Zhang, L.; Xiang, W. miR-484 mediates oxidative stress-induced ovarian dysfunction and promotes granulosa cell apoptosis via SESN2 downregulation. Redox Biol. 2023, 62, 102684. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Yang, D.; Ren, D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 2017, 8, 25310–25322. [Google Scholar] [CrossRef]
- Saeed-Zidane, M.; Linden, L.; Salilew-Wondim, D.; Held, E.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS ONE 2017, 12, e0187569. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Zhang, C. Effects of thyroid hormone on ovarian cell apoptosis in the rat. Reprod. Fertil. Dev. 2020, 32, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yang, Y.; Li, X.; Chen, F.; Cui, C.; Hu, L.; Li, Q.; Liu, W.; Jin, Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol. Reprod. Dev. 2012, 79, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Kunitomi, C.; Harada, M.; Takahashi, N.; Azhary, J.M.K.; Kusamoto, A.; Nose, E.; Oi, N.; Takeuchi, A.; Wada-Hiraike, O.; Hirata, T.; et al. Activation of endoplasmic reticulum stress mediates oxidative stress-induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol. Hum. Reprod. 2020, 26, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Qin, Y.; Li, D.; Cai, N.; Wu, J.; Jiang, L.; Jie, L.; Zhou, Z.; Xu, J.; Wang, H. Inhibition of PDE4 protects neurons against oxygen-glucose deprivation-induced endoplasmic reticulum stress through activation of the Nrf-2/HO-1 pathway. Redox Biol. 2020, 28, 101342. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Cao, Y.; Jiang, Y.; Wei, Y.; Liu, H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation-independent mechanism. Redox Biol. 2018, 18, 138–157. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Li, W.; Ao, H.; Zhang, Y.; Zhou, R.; Li, K. Effects of melatonin on the synthesis of estradiol and gene expression in pig granulosa cells. J. Pineal Res. 2019, 66, e12546. [Google Scholar] [CrossRef]
- Wakim, A.N.; Polizotto, S.L.; Buffo, M.J.; Marrero, M.A.; Burholt, D.R. Thyroid hormones in human follicular fluid and thyroid hormone receptors in human granulosa cells. Fertil. Steril. 1993, 59, 1187–1190. [Google Scholar] [CrossRef]
- Allshouse, A.A.; Semple, A.L.; Santoro, N.F. Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause 2015, 22, 166–174. [Google Scholar] [CrossRef]
- Xu, K.; Tian, Y.; Weng, X.; Hu, X.; Heng, D.; Xia, G.; Zhang, C. Effect of thyroid dysfunction on NOS expression in the female rat. Cell Tissue Res. 2020, 379, 291–300. [Google Scholar] [CrossRef]
- Tian, Y.; Ding, Y.; Liu, J.; Heng, D.; Xu, K.; Liu, W.; Zhang, C. Nitric Oxide-Mediated Regulation of GLUT by T3 and Follicle-Stimulating Hormone in Rat Granulosa Cells. Endocrinology 2017, 158, 1898–1915. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Y.; Ding, Y.; Heng, D.; Xu, K.; Liu, W.; Zhang, C. Role of CYP51 in the Regulation of T3 and FSH-Induced Steroidogenesis in Female Mice. Endocrinology 2017, 158, 3974–3987. [Google Scholar] [CrossRef] [PubMed]
- Buratini, J.; Dellaqua, T.T.; Dal Canto, M.; La Marca, A.; Carone, D.; Mignini Renzini, M.; Webb, R. The putative roles of FSH and AMH in the regulation of oocyte developmental competence: From fertility prognosis to mechanisms underlying age-related subfertility. Hum. Reprod. Update 2022, 28, 232–254. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, Y.; Fu, H.; Zheng, Y.; Bao, D.; Yin, Y.; Chen, Q.; Nie, X.; Hao, Q.; Hou, D.; et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J. Mol. Endocrinol. 2018, 60, 261–271. [Google Scholar] [CrossRef]
- Qin, X.; Du, D.; Chen, Q.; Wu, M.; Wu, T.; Wen, J.; Jin, Y.; Zhang, J.; Wang, S. Metformin prevents murine ovarian aging. Aging 2019, 11, 3785–3794. [Google Scholar] [CrossRef]
- Nie, X.; Dai, Y.; Zheng, Y.; Bao, D.; Chen, Q.; Yin, Y.; Fu, H.; Hou, D. Establishment of a Mouse Model of Premature Ovarian Failure Using Consecutive Superovulation. Cell. Physiol. Biochem. 2018, 51, 2341–2358. [Google Scholar] [CrossRef] [PubMed]
- Emidio, G.D.; Placidi, M.; Rea, F.; Rossi, G.; Falone, S.; Cristiano, L.; Nottola, S.; D’Alessandro, A.M.; Amicarelli, F.; Palmerini, M.G.; et al. Methylglyoxal-Dependent Glycative Stress and Deregulation of SIRT1 Functional Network in the Ovary of PCOS Mice. Cells 2020, 9, 209. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 2019, 66, e12550. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Weng, X.; Hu, X.; Wang, Q.; Tian, Y.; Ding, Y.; Zhang, C. Roles of different n-3/n-6 PUFA ratios in ovarian cell development and steroidogenesis in PCOS rats. Food Funct. 2019, 10, 7397–7406. [Google Scholar] [CrossRef]
- Liu, J.; Han, Y.; Tian, Y.; Weng, X.; Hu, X.; Liu, W.; Heng, D.; Xu, K.; Yang, Y.; Zhang, C. Regulation by 3,5,3′-tri-iodothyronine and FSH of cytochrome P450 family 19 (CYP19) expression in mouse granulosa cells. Reprod. Fertil. Dev. 2018, 30, 1225–1233. [Google Scholar] [CrossRef]
- Heng, D.; Wang, Q.; Ma, X.; Tian, Y.; Xu, K.; Weng, X.; Hu, X.; Liu, W.; Zhang, C. Role of OCT4 in the Regulation of FSH-Induced Granulosa Cells Growth in Female Mice. Front. Endocrinol. 2019, 10, 915. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Cao, L.Q.; Chen, H.Y. Protective effects ROS up-regulation on premature ovarian failure by suppressing ROS-TERT signal pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6198–6204. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf expression is a biomarker of aging. J. Clin. Investig. 2004, 114, 1299–1307. [Google Scholar] [CrossRef]
- Hudgins, A.D.; Tazearslan, C.; Tare, A.; Zhu, Y.; Huffman, D.; Suh, Y. Age- and Tissue-Specific Expression of Senescence Biomarkers in Mice. Front. Genet. 2018, 9, 59. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; You, S.; Farris, J.; Kong, B.-W.; Christman, S.A.; Foster, L.K.; Foster, D.N. Expression profiles of p53-, p16(INK4a)-, and telomere-regulating genes in replicative senescent primary human, mouse, and chicken fibroblast cells. Exp. Cell Res. 2002, 272, 199–208. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhang, S.; Huang, H.; Wu, J.; Wang, Y.; Yuan, L.; Liu, C.; Zeng, X.; Cheng, X.; et al. Oxidative Stress Mediates Microcystin-LR-Induced Endoplasmic Reticulum Stress and Autophagy in KK-1 Cells and C57BL/6 Mice Ovaries. Front. Physiol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019, 25, 101047. [Google Scholar] [CrossRef]
- Simmons, D.G.; Kennedy, T.G. Induction of glucose-regulated protein 78 in rat uterine glandular epithelium during uterine sensitization for the decidual cell reaction. Biol. Reprod. 2000, 62, 1168–1176. [Google Scholar] [CrossRef]
- Sato, M.; Yao, V.J.; Arap, W.; Pasqualini, R. GRP78 signaling hub a receptor for targeted tumor therapy. Adv. Genet. 2010, 69, 97–114. [Google Scholar] [CrossRef]
- Gonzalez-Gronow, M.; Selim, M.A.; Papalas, J.; Pizzo, S.V. GRP78: A multifunctional receptor on the cell surface. Antioxid. Redox Signal. 2009, 11, 2299–2306. [Google Scholar] [CrossRef]
- Chen, Z.; Lei, L.; Wen, D.; Yang, L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J. Ovarian Res. 2019, 12, 43. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocr. J. 2013, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hazlerigg, D.G.; Simonneaux, V.; Dardente, H. Melatonin and Seasonal Synchrony in Mammals. J. Pineal Res. 2024, 76, e12996. [Google Scholar] [CrossRef]
- Carling, D.; Thornton, C.; Woods, A.; Sanders, M.J. AMP-activated protein kinase: New regulation, new roles? Biochem. J. 2012, 445, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; de Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, Y.; Teng, F.; Li, J.; Guan, Y.; Xu, J.; Lv, X.; Guan, F.; Zhang, M.; Chen, L. Berberine Improves Cognitive Deficiency and Muscular Dysfunction via Activation of the AMPK/SIRT1/PGC-1a Pathway in Skeletal Muscle from Naturally Aging Rats. J. Nutr. Health Aging 2018, 22, 710–717. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, J.; Lv, Q.; Shi, D.; Yang, S.; Xing, Q.; Zhang, R.; Cheng, J.; Deng, Y. Follicle-stimulating hormone regulates glycolysis of water buffalo follicular granulosa cells through AMPK/SIRT1 signalling pathway. Reprod. Domest. Anim. 2022, 57, 185–195. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Liu, K.; Zhu, H.; Qiao, M.; Sun, X.; Li, G. Metformin Protects Against Inflammation, Oxidative Stress to Delay Poly I:C-Induced Aging-Like Phenomena in the Gut of an Annual Fish. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 77, 276–282. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).