Combined Aerobic Exercise Training and Chlorella Intake Reduces Arterial Stiffness through Enhanced Arterial Nitric Oxide Production in Obese Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Protocol
2.2. Carotid–Femoral Pulse Wave Velocity and Blood Pressures
2.3. Aerobic Exercise Training Protocol
2.4. Citrate Synthase Activity
2.5. Western Blot Analysis
2.6. Griess Assay
2.7. Statistical Analysis
3. Results
Animal Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar]
- Jia, G.; Aroor, A.R.; DeMarco, V.G.; Martinez-Lemus, L.A.; Meininger, G.A.; Sowers, J.R. Vascular stiffness in insulin resistance and obesity. Front. Physiol. 2015, 6, 231. [Google Scholar] [CrossRef] [PubMed]
- Cecelja, M.; Chowienczyk, P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc. Dis. 2012, 1, 012016. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Inoue, K.; Fujie, S.; Hasegawa, N.; Horii, N.; Uchida, M.; Iemitsu, K.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Aerobic exercise training-induced irisin secretion is associated with the reduction of arterial stiffness via nitric oxide production in adults with obesity. Appl. Physiol. Nutr. Metab. 2020, 45, 715–722. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, M.K. Effect of Chlorella vulgaris on glucose metabolism in Wistar rats fed high fat diet. J. Med. Food 2009, 12, 1029–1037. [Google Scholar] [CrossRef]
- Merchant, R.E.; Andre, C.A. Dietary Supplementation with Chlorella pyrenoidosa Produces Positive Results in Patients with Cancer or Suffering from Certain Common Chronic Illnesses. Townsend Lett. Dr. Patients 2001, 213, 74. [Google Scholar]
- Sherafati, N.; Bideshki, M.V.; Behzadi, M.; Mobarak, S.; Asadi, M.; Sadeghi, O. Effect of supplementation with Chlorella vulgaris on lipid profile in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther. Med. 2022, 66, 102822. [Google Scholar] [CrossRef]
- Vecina, J.F.; Oliveira, A.G.; Araujo, T.G.; Baggio, S.R.; Torello, C.O.; Saad, M.J.; Queiroz, M.L. Chlorella modulates insulin signaling pathway and prevents high-fat diet-induced insulin resistance in mice. Life Sci. 2014, 95, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Shimizu, K.; Maeda, S. Changes in arterial stiffness and nitric oxide production with Chlorella-derived multicomponent supplementation in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Fujie, S.; Hasegawa, N.; Horii, N.; Inoue, K.; Uchida, M.; Iemitsu, M. Effects of combined exercise training and Chlorella intake on vasorelaxation mediated by nitric oxide in aged mice. Appl. Physiol. Nutr. Metab. 2021, 46, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tousoulis, D.; Antoniades, C.; Stefanadi, E.; Stefanadis, C. L-Arginine, the substrate for NO synthesis: An alternative treatment for premature atherosclerosis? Int. J. Cardiol. 2007, 116, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Singer, A.H.; Tsao, P.; Zera, P.; Rowan, R.A.; Billingham, M.E. Antiatherogenic effects of L-arginine in the hypercholesterolemic rabbit. J. Clin. Investig. 1992, 90, 1168–1172. [Google Scholar] [CrossRef]
- Aji, W.; Ravalli, S.; Szabolcs, M.; Jiang, X.C.; Sciacca, R.R.; Michler, R.E.; Cannon, P.J. L-arginine prevents xanthoma development and inhibits atherosclerosis in LDL receptor knockout mice. Circulation 1997, 95, 430–437. [Google Scholar] [CrossRef]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef]
- Molinari, C.; Uberti, F.; Grossini, E.; Vacca, G.; Carda, S.; Invernizzi, M.; Cisari, C. 1α,25-dihydroxycholecalciferol induces nitric oxide production in cultured endothelial cells. Cell Physiol. Biochem. 2011, 27, 661–668. [Google Scholar] [CrossRef]
- Mortensen, A.; Lykkesfeldt, J. Does vitamin C enhance nitric oxide bioavailability in a tetrahydrobiopterin-dependent manner? In vitro, in vivo and clinical studies. Nitric. Oxide 2014, 36, 51–57. [Google Scholar] [CrossRef]
- Green, D.; O’Driscoll, G.; Rankin, J.M.; Maiorana, A.J.; Taylor, R.R. Beneficial effect of vitamin E administration on nitric oxide function in subjects with hypercholesterolaemia. Clin. Sci. 1998, 95, 361–367. [Google Scholar] [CrossRef]
- Oppert, J.M.; Ciangura, C.; Bellicha, A. Physical activity and exercise for weight loss and maintenance in people living with obesity. Rev. Endocr. Metab. Disord. 2013, 24, 937–949. [Google Scholar] [CrossRef] [PubMed]

| Healthy (n = 6) | OBESE | ||||
|---|---|---|---|---|---|
| SED (n = 6) | ET (n = 6) | CH (n = 6) | ET+CH (n = 6) | ||
| Body weight (g) | 481.8 ± 26.3 | 580.7 ± 36.6 * | 490.6 ± 35.3 † | 566.7 ± 25.1 *§ | 570.0 ± 33.0 *§ |
| Epididymal fat mass (g) | 7.35 ± 1.50 | 11.01 ± 0.70 * | 7.33 ± 1.94 † | 9.12 ± 2.86 | 7.63 ± 1.16 † |
| Soleus muscle mass (g) | 0.42 ± 0.05 | 0.37 ± 0.07 | 0.44 ± 0.05 †‡ | 0.38 ± 0.05 | 0.36 ± 0.03 *§ |
| Soleus CS enzyme activity (µmol/g/min) | 13.19 ± 9.43 | 11.93 ± 2.39 | 22.40 ± 10.57 *† | 15.68 ± 8.68 | 24.65 ± 3.03 *† |
| Fasting blood glucose levels (mmol/L) | 5.71 ± 0.51 | 19.19 ± 2.03 * | 8.46 ± 1.67 *†‡ | 16.11 ± 1.91 *† | 6.90 ± 0.47 †‡ |
| HOMA-IR score | 0.26 ± 0.20 | 11.30 ± 1.50 * | 3.86 ± 0.66 *† | 7.68 ± 0.68 *† | 2.60 ± 0.24 *†‡§ |
| HR (beats/min) | 349.7 ± 24.7 | 344.8 ± 4.8 | 342.0 ± 20.1 | 330.3 ± 11.9 | 328.7 ± 49.1 |
| SBP (mmHg) | 101.0 ± 14.2 | 108.2 ± 11.3 | 99.1 ± 4.6 | 105.3 ± 8.5 | 110.0 ± 10.0 |
| DBP (mmHg) | 73.67 ± 12.74 | 79.40 ± 6.47 | 77.14 ± 5.37 | 82.67 ± 14.19 | 78.30 ± 5.77 |
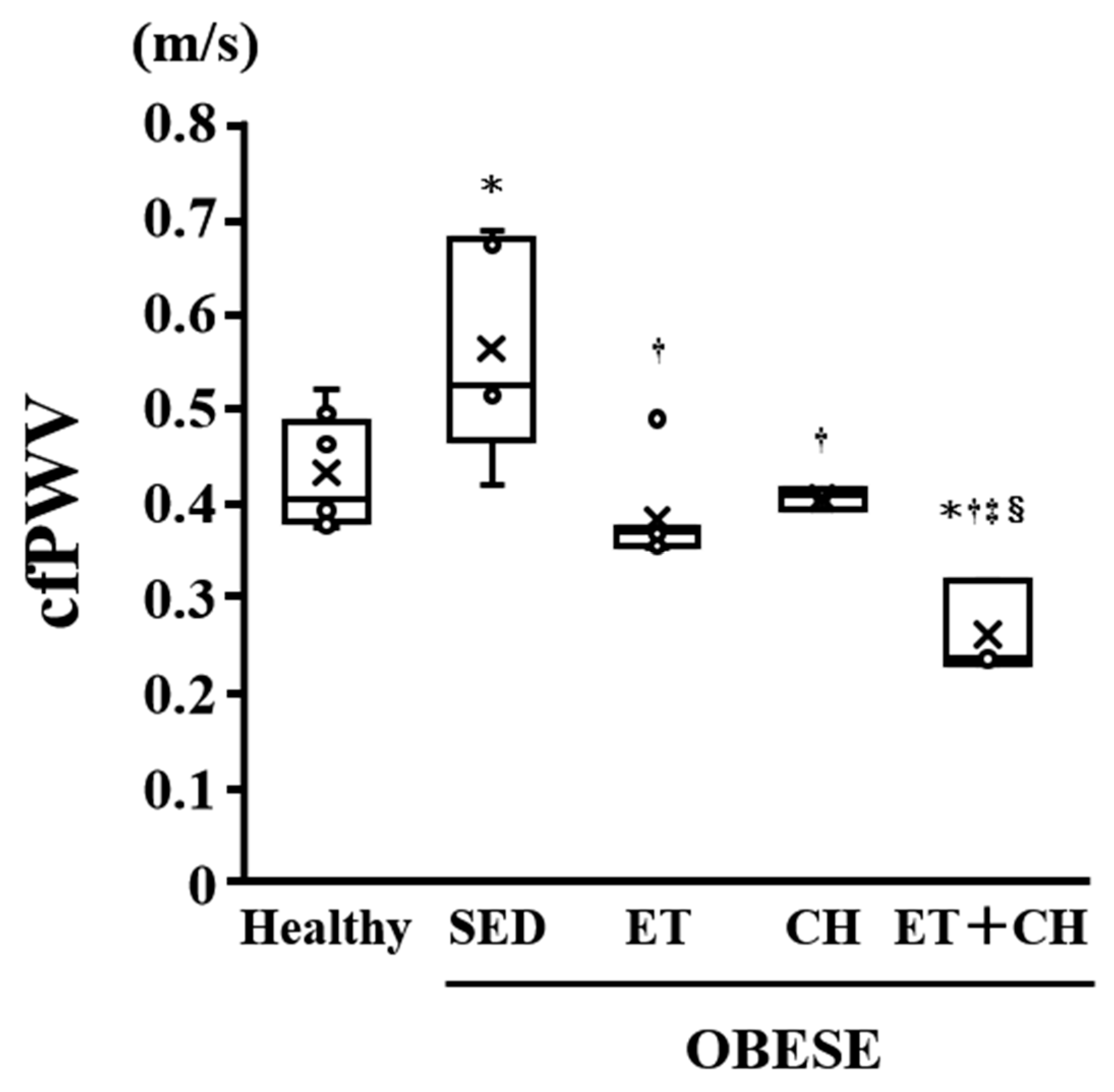
| cfPWV (m/s) | 0.43 ± 0.02 | 0.56 ± 0.05 * | 0.38 ± 0.02 † | 0.40 ± 0.01 † | 0.26 ± 0.03 *†‡§ |
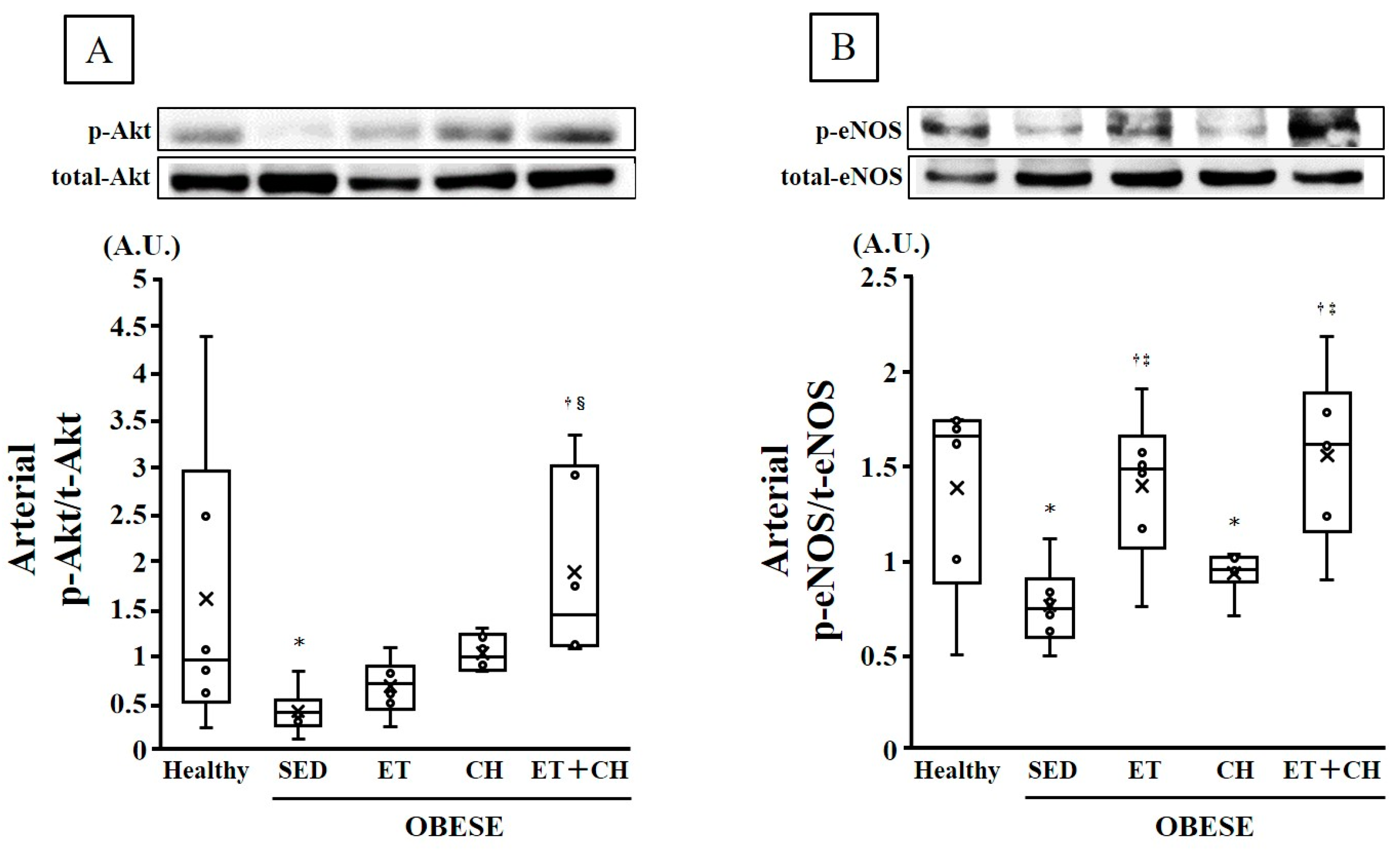
| p-Akt/t-Akt (A.U.) | 1.59 ± 0.64 | 0.40 ± 0.10 * | 0.67 ± 0.12 | 1.02 ± 0.08 | 1.88 ± 0.41 †§ |
| p-eNOS/t-eNOS (A.U.) | 1.38 ± 0.21 | 0.76 ± 0.09 * | 1.39 ± 0.16 †‡ | 0.93 ± 0.05 * | 1.55 ± 0.18 †‡ |
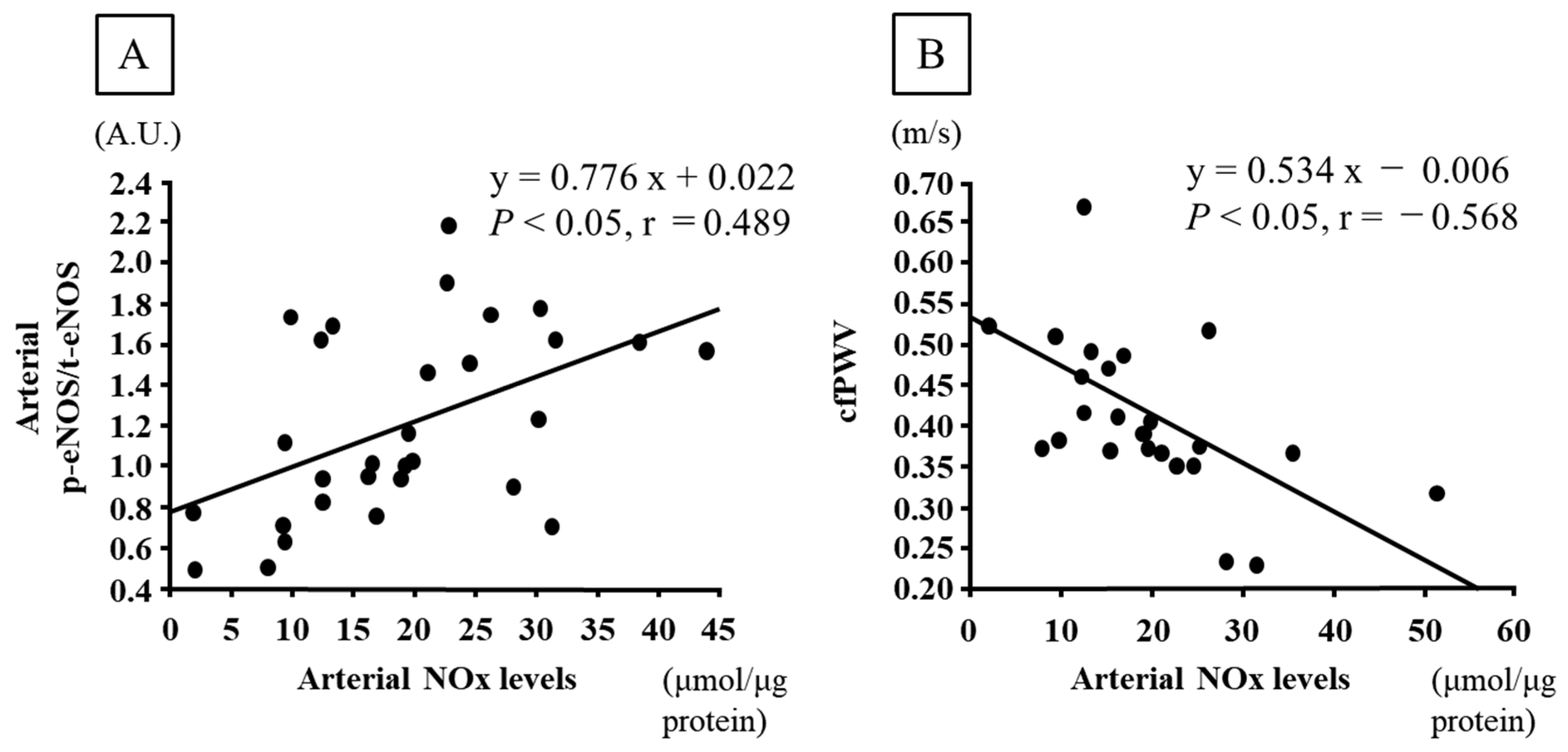
| Arterial NOx levels (μmol/μg protein) | 14.8 ± 2.8 | 7.4 ± 1.8 * | 24.8 ± 4.0 *† | 19.2 ± 2.6 † | 30.2 ± 2.1 *†‡§ |
| Average food intake (g/d) | 20.30 ± 0.19 | 26.97 ± 0.56 *§ | 20.70 ± 0.40 | 25.80 ± 0.29 *§ | 26.42 ± 0.37 *§ |
| Healthy (n = 6) | OBESE | |||
|---|---|---|---|---|
| ET (n = 6) | CH (n = 6) | ET+CH (n = 6) | ||
| p-Value | p-Value | p-Value | p-Value | |
| Body weight (g) | 0.0003 | 0.0015 | 0.4577 | 0.5962 |
| Epididymal fat mass (g) | 0.0003 | 0.0014 | 0.1449 | 0.0001 |
| Soleus muscle mass (g) | 0.1749 | 0.0917 | 0.8448 | 0.6941 |
| Soleus CS enzyme activity (µmol/g/min) | 0.7576 | 0.0395 | 0.3280 | 0.0001 |
| Fasting blood glucose levels (mmol/L) | 0.0001 | 0.0001 | 0.0221 | 0.0001 |
| HOMA-IR score | 0.0001 | 0.0001 | 0.0003 | 0.0001 |
| HR (beats/min) | 0.8203 | 0.1492 | 0.4134 | 0.3242 |
| SBP (mmHg) | 0.3203 | 0.1864 | 0.3847 | 0.7949 |
| DBP (mmHg) | 0.2223 | 0.6877 | 0.6458 | 0.7602 |
| cfPWV (m/s) | 0.3370 | 0.8475 | 0.0463 | 0.0119 |
| p-Akt/t-Akt (A.U.) | 0.0954 | 0.1204 | 0.0006 | 0.0057 |
| p-eNOS/t-eNOS (A.U.) | 0.0206 | 0.0059 | 0.1105 | 0.0027 |
| Arterial NOx levels (μmol/μg protein) | 0.0481 | 0.0026 | 0.0039 | 0.0001 |
| Average food intake (g/d) | 0.0001 | 0.0001 | 0.0936 | 0.4317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamazaki, H.; Fujie, S.; Inoue, K.; Uchida, M.; Iemitsu, M. Combined Aerobic Exercise Training and Chlorella Intake Reduces Arterial Stiffness through Enhanced Arterial Nitric Oxide Production in Obese Rats. Nutrients 2024, 16, 3080. https://doi.org/10.3390/nu16183080
Yamazaki H, Fujie S, Inoue K, Uchida M, Iemitsu M. Combined Aerobic Exercise Training and Chlorella Intake Reduces Arterial Stiffness through Enhanced Arterial Nitric Oxide Production in Obese Rats. Nutrients. 2024; 16(18):3080. https://doi.org/10.3390/nu16183080
Chicago/Turabian StyleYamazaki, Henry, Shumpei Fujie, Kenichiro Inoue, Masataka Uchida, and Motoyuki Iemitsu. 2024. "Combined Aerobic Exercise Training and Chlorella Intake Reduces Arterial Stiffness through Enhanced Arterial Nitric Oxide Production in Obese Rats" Nutrients 16, no. 18: 3080. https://doi.org/10.3390/nu16183080
APA StyleYamazaki, H., Fujie, S., Inoue, K., Uchida, M., & Iemitsu, M. (2024). Combined Aerobic Exercise Training and Chlorella Intake Reduces Arterial Stiffness through Enhanced Arterial Nitric Oxide Production in Obese Rats. Nutrients, 16(18), 3080. https://doi.org/10.3390/nu16183080







