Prevalence and Risk Factors of Vitamin D Deficiency in Patients Scheduled to Undergo Revision Arthroplasty of the Hip, Knee and Shoulder—Data from a Single-Centre Analysis
Abstract
1. Introduction
2. Patients and Methods
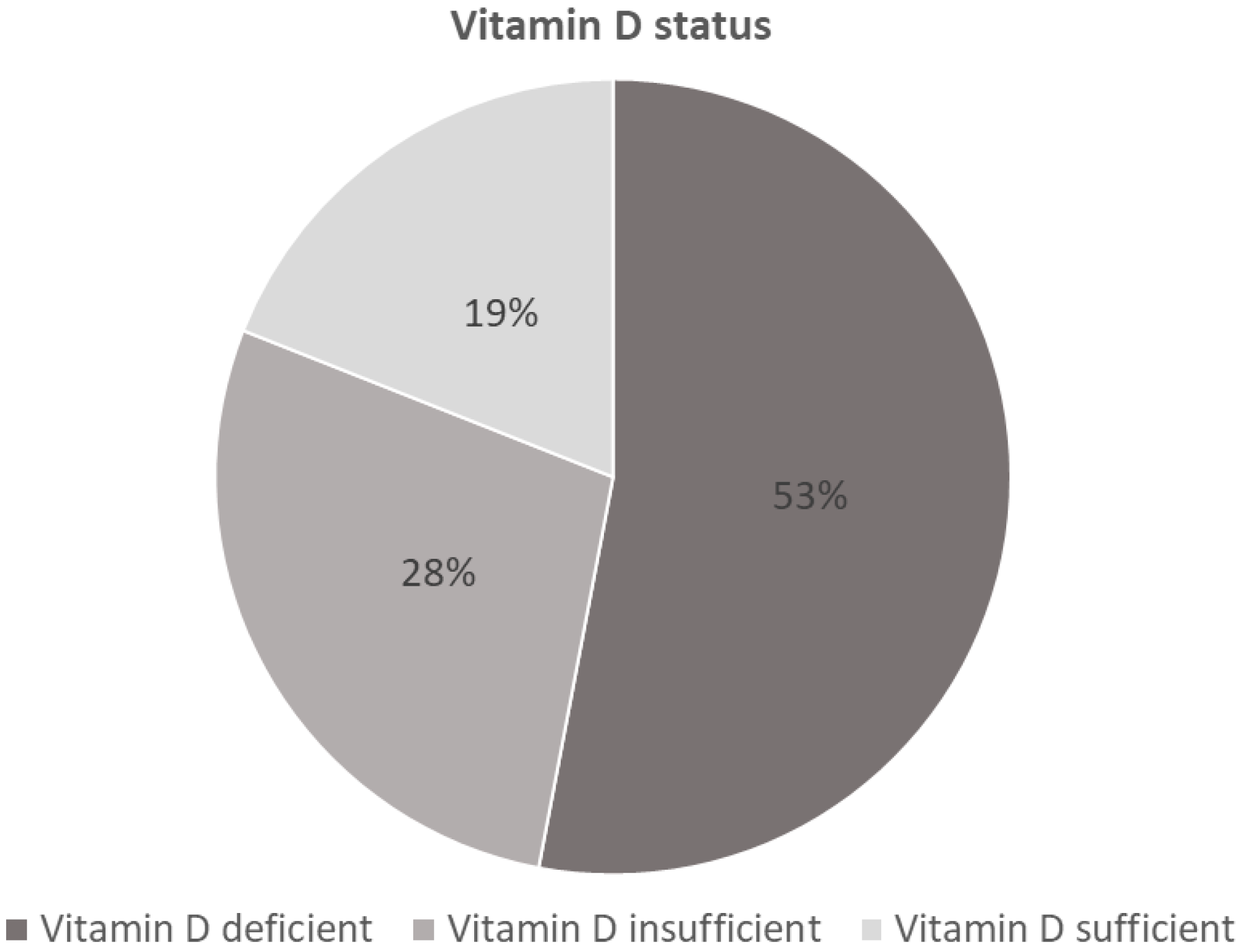
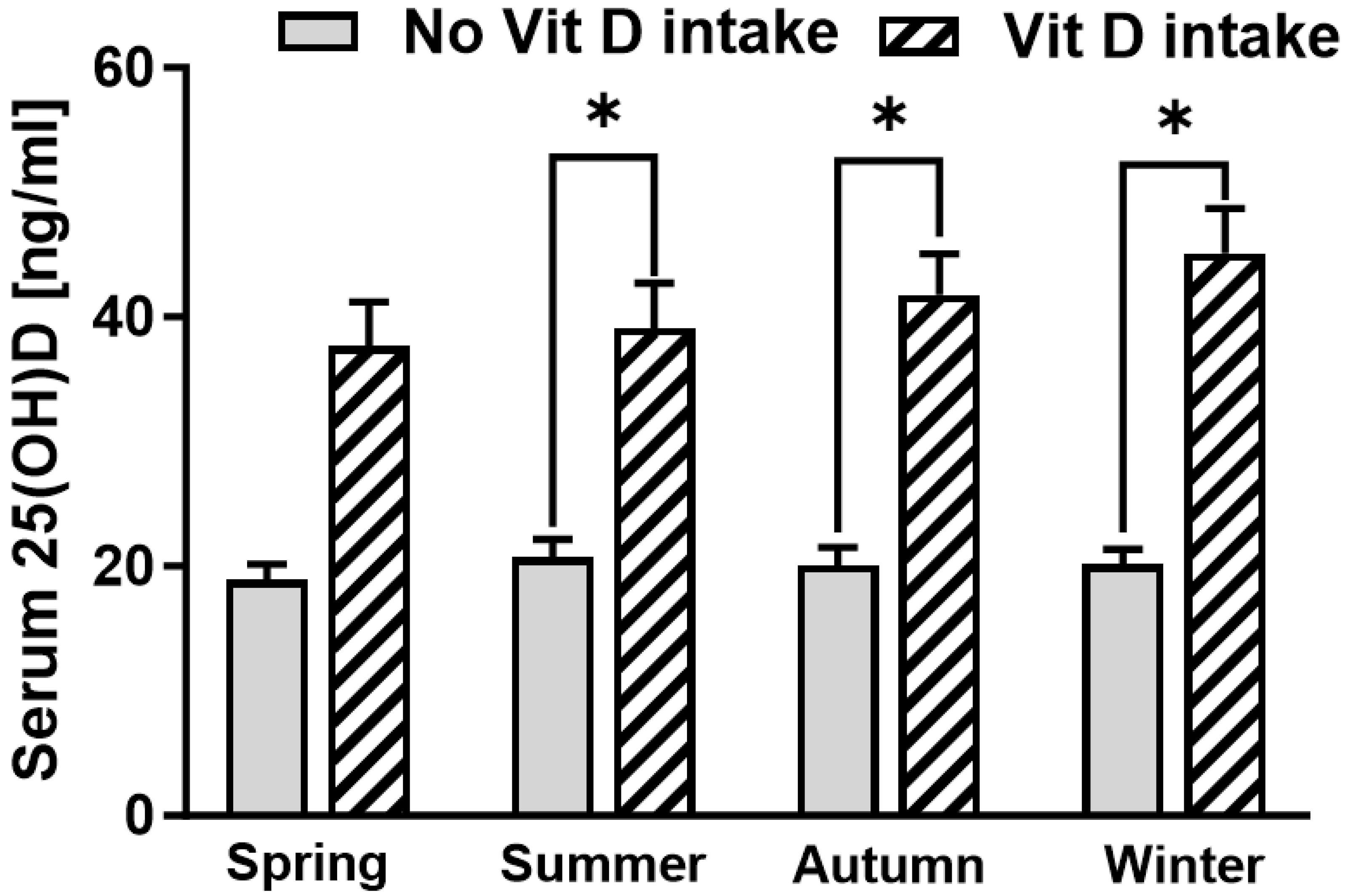
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rupp, M.; Lau, E.; Kurtz, S.M.; Alt, V. Projections of Primary TKA and THA in Germany from 2016 through 2040. Clin. Orthop. Relat. Res. 2020, 478, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040–2060. JB JS Open Access 2023, 8, e22.00112. [Google Scholar] [CrossRef] [PubMed]
- Shichman, I.; Askew, N.; Habibi, A.; Nherera, L.; Macaulay, W.; Seyler, T.; Schwarzkopf, R. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2040–2060. Arthroplast Today 2023, 21, 101152. [Google Scholar] [CrossRef] [PubMed]
- Heo, K.; Karzon, A.; Shah, J.; Ayeni, A.; Rodoni, B.; Erens, G.A.; Guild, G.N.; Premkumar, A. Trends in Costs and Professional Reimbursements for Revision Total Hip and Knee Arthroplasty. J. Arthroplast. 2024, 39, 612–618.e611. [Google Scholar] [CrossRef] [PubMed]
- Drees, P.; Eckardt, A.; Gay, R.E.; Gay, S.; Huber, L.C. Mechanisms of disease: Molecular insights into aseptic loosening of orthopedic implants. Nat. Clin. Pract. Rheumatol. 2007, 3, 165–171. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef]
- Horas, K.; van Herck, U.; Maier, G.S.; Maus, U.; Harrasser, N.; Jakob, F.; Weissenberger, M.; Arnholdt, J.; Holzapfel, B.M.; Rudert, M. Does vitamin D deficiency predict tumour malignancy in patients with bone tumours? Data from a multi-center cohort analysis. J. Bone Oncol. 2020, 25, 100329. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Maier, G.S.; Weissenberger, M.; Rudert, M.; Roth, K.E.; Horas, K. The role of vitamin D and vitamin D deficiency in orthopaedics and traumatology—A narrative overview of the literature. Ann. Transl. Med. 2021, 9, 942. [Google Scholar] [CrossRef]
- Birinci, M.; Hakyemez Ö, S.; Geçkalan, M.A.; Mutlu, M.; Yildiz, F.; Bilgen Ö, F.; Azboy, İ. Effect of Vitamin D Deficiency on Periprosthetic Joint Infection and Complications after Primary Total Joint Arthroplasty. J. Arthroplast. 2024, 39, S151–S157. [Google Scholar] [CrossRef]
- Vivek, K.; Kamal, R.; Perera, E.; Gupte, C.M. Vitamin D Deficiency Leads to Poorer Health Outcomes and Greater Length of Stay after Total Knee Arthroplasty and Supplementation Improves Outcomes: A Systematic Review and Meta-Analysis. JBJS Rev 2024, 12, e23. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.S.; Horas, K.; Seeger, J.B.; Roth, K.E.; Kurth, A.A.; Maus, U. Vitamin D insufficiency in the elderly orthopaedic patient: An epidemic phenomenon. Int. Orthop. 2014, 39, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Seyok, T.; Moye, S.; Sugita, L.; Eltouny, E.; Carrera, C.; Denagamage, P.; Charles, J.; Fitz, W.; Chen, A.F.; et al. High rates of vitamin D insufficiency among patients presenting for total knee arthroplasty. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2024, 42, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Cho, S.D.; Youm, Y.S.; Song, J.Y.; Lee, K.J.; Park, K.B. The Prevalence of Vitamin D Deficiency in Patients Undergoing Total Knee Arthroplasty: A Propensity Score Matching Analysis. Arch. Osteoporos. 2022, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Hurwitz, S.; Thornhill, T.S.; Kelly, M.; LeBoff, M.S. Osteoporosis and vitamin-D deficiency among postmenopausal women with osteoarthritis undergoing total hip arthroplasty. J. Bone Jt. Surg. Am. Vol. 2003, 85, 2371–2377. [Google Scholar] [CrossRef]
- Bogunovic, L.; Kim, A.D.; Beamer, B.S.; Nguyen, J.; Lane, J.M. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: A single-center analysis. J. Bone Jt. Surg. Am. Vol. 2010, 92, 2300–2304. [Google Scholar] [CrossRef]
- Emara, A.K.; Nageeb, E.; George, J.; Buttaro, M.A.; Higuera, C.; Piuzzi, N.S. Hypovitaminosis D in lower extremity Joint Arthroplasty: A systematic review and meta-analysis. J. Orthop. 2020, 21, 109–116. [Google Scholar] [CrossRef]
- Smith, J.M.; Cancienne, J.M.; Brockmeier, S.F.; Werner, B.C. Vitamin D deficiency and total shoulder arthroplasty complications. Shoulder Elb. 2021, 13, 99–105. [Google Scholar] [CrossRef]
- Inkrott, B.P.; Koberling, J.L.; Noel, C.R. Hypovitaminosis D in Patients Undergoing Shoulder Arthroplasty: A Single-Center Analysis. Orthopedics 2016, 39, e651–e656. [Google Scholar] [CrossRef]
- MacConnell, A.E.; Anderson, J.; Stanila, T.; Shivdasani, K.; Hand, R.; Boubekri, A.; Garbis, N.; Salazar, D. The effect of vitamin D insufficiency on outcomes and complication rates after shoulder arthroplasty: A single center retrospective examination. Semin. Arthroplast. JSES 2024, 34, 182–189. [Google Scholar] [CrossRef]
- Traven, S.A.; Chiaramonti, A.M.; Barfield, W.R.; Kirkland, P.A.; Demos, H.A.; Schutte, H.D.; Drew, J.M. Fewer Complications Following Revision Hip and Knee Arthroplasty in Patients with Normal Vitamin D Levels. J. Arthroplast. 2017, 32, S193–S196. [Google Scholar] [CrossRef] [PubMed]
- Duggan, J.L.; Fitz, W.; Lange, J.K.; Shah, V.M.; Olsen, A.; Iorio, R.; Chen, A.F. Postoperative Vitamin D Surveillance and Supplementation in Revision Total Knee Arthroplasty Patients: A Retrospective Cohort Analysis. Orthop. Clin. N. Am. 2024, 55, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Spinney, E. Vitamin D as a Preoperative Clinical Predictor for Prosthetic Joint Infection. JBJS J. Orthop. Physician Assist. 2020, 8, e0045. [Google Scholar] [CrossRef]
- Mouli, V.H.; Schudrowitz, N.; Carrera, C.X.; Uzosike, A.C.; Fitz, W.; Rajaee, S.S. High-Dose Vitamin D Supplementation Can Correct Hypovitaminosis D Prior to Total Knee Arthroplasty. J. Arthroplast. 2022, 37, 274–278. [Google Scholar] [CrossRef]
- Weintraub, M.T.; Guntin, J.; Yang, J.; DeBenedetti, A.; Karas, V.; Della Valle, C.J.; Nam, D. Vitamin D(3) Supplementation Prior to Total Knee Arthroplasty: A Randomized Controlled Trial. J. Arthroplast. 2023, 38, S114–S119. [Google Scholar] [CrossRef]
- Morrison, R.J.M.; Fishley, W.G.; Rankin, K.S.; Reed, M.R. The effect of vitamin D supplementation on outcomes following total hip or knee arthroplasty surgery: A rapid systematic review of current evidence. EFORT Open Rev. 2022, 7, 305–311. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Horas, K.; Maier, G.; Rudert, M.; Jakuscheit, A.; Weißenberger, M.; Stratos, I.; Heinz, T.; Rak, D.; Anderson, P.M.; Arnholdt, J. Vitamin D Deficiency Is Frequent in Patients with Rapidly Destructive Osteoarthritis-Data from a Single-Center Analysis. J. Clin. Med. 2024, 13, 1296. [Google Scholar] [CrossRef] [PubMed]
- Blankstein, M.; Lentine, B.; Nelms, N.J. The Use of Cement in Hip Arthroplasty: A Contemporary Perspective. J. Am. Acad. Orthop. Surg. 2020, 28, e586–e594. [Google Scholar] [CrossRef]
- Bernatz, J.T.; Brooks, A.E.; Squire, M.W.; Illgen, R.I., 2nd; Binkley, N.C.; Anderson, P.A. Osteoporosis Is Common and Undertreated Prior to Total Joint Arthroplasty. J. Arthroplast. 2019, 34, 1347–1353. [Google Scholar] [CrossRef]
- Agarwal, A.R.; Malyavko, A.; Gu, A.; Harris, A.B.; Rao, S.; Sterling, R.; Golladay, G.J.; Thakkar, S.C. Can Hip and Knee Arthroplasty Surgeons Help Address the Osteoporosis Epidemic? Clin. Orthop. Relat. Res. 2023, 481, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.L.; Hsu, C.J.; Ma, Y.G.; Liu, D.; Peng, R.; Xu, X.H.; Lu, H.D. Prevalence and treatment rate of osteoporosis in patients undergoing total knee and hip arthroplasty: A systematic review and meta-analysis. Arch. Osteoporos. 2022, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Levin, J.E.; Amen, T.B.; Arzani, A.; Manzi, J.E.; Lane, J.M. Total Joint Arthroplasty and Osteoporosis: Looking beyond the Joint to Bone Health. J. Arthroplast. 2022, 37, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, H.; Liu, K.; Wang, Y.; Liu, Q.; Sun, T.; Chen, S.; Ren, L. Smoking behavior and circulating vitamin D levels in adults: A meta-analysis. Food Sci Nutr. 2021, 9, 5820–5832. [Google Scholar] [CrossRef] [PubMed]
- Alzohily, B.; AlMenhali, A.; Gariballa, S.; Munawar, N.; Yasin, J.; Shah, I. Unraveling the complex interplay between obesity and vitamin D metabolism. Sci. Rep. 2024, 14, 7583. [Google Scholar] [CrossRef]
- Maier, G.S.; Kolbow, K.; Lazovic, D.; Maus, U. The Importance of Bone Mineral Density in Hip Arthroplasty: Results of a Survey Asking Orthopaedic Surgeons about Their Opinions and Attitudes Concerning Osteoporosis and Hip Arthroplasty. Adv. Orthop. 2016, 2016, 8079354. [Google Scholar] [CrossRef]


| Number of Patients | ||||
|---|---|---|---|---|
| Vit D Suppl. (Total Number; Percentage) | ||||
| Total (249; 100%) | No. (191; 77%) | Yes (58; 23%) | ||
| Gender | Female | 142 (57%) | 93 (65%) | 49 (35%) |
| Male | 107 (43%) | 98 (92%) | 9 (8%) | |
| Revision | Hip | 118 | 87 (74%) | 31 (26%) |
| Knee | 116 | 92 (79%) | 24 (21%) | |
| Shoulder | 15 | 12 (80%) | 3 (20%) | |
| Reason | Infection | 84 (34%) | 69 (82%) | 15 (18%) |
| As. Loos. | 160 (64%) | 117 (73%) | 43 (27%) | |
| Fracture | 5 (2%) | 5 (100%) | 0 | |
| Age | Range: 26–96 years | Mean: 68.29 years | ||
| (A) Patients without oral supplementation | ||||||
| Predictor | Coefficient | n | Std. Error | T-Statistic | p-Value | 95% CI |
| Sex Male vs. Female | 1.66 | 98/98 | 1.47 | 1.12 | 0.26 | −1.26; 0.47 |
| Age | −0.02 | 196 | 0.08 | −0.23 | 0.82 | −0.17; 0.14 |
| BMI | −0.36 | 196 | 0.12 | −2.98 | <0.01 | −0.12; −2.98 |
| Nicotine abuse | −7.51 | 21 | 2.47 | −3.04 | <0.01 | −12.38; −2.64 |
| Season | 0.04 | 196 | 0.61 | 0.07 | 0.95 | −1.17; 1.25 |
| (B) Patients with oral supplementation | ||||||
| Predictor | Coefficient | n | Std. Error | T-Statistic | p-Value | 95% CI |
| Sex Male vs. Female | 2.65 | 9/48 | 4.98 | 0.53 | 0.59 | −13.27; 10.62 |
| Age | 0.28 | 57 | 0.26 | 1.09 | 0.28 | −0.24; 0.81 |
| BMI | −0.01 | 57 | 0.03 | −0.01 | 0.99 | −0.62; 0.62 |
| Nicotine abuse | −1.33 | 6 | 5.91 | −0.22 | 0.82 | −13.27; 10.62 |
| Season | 3.97 | 57 | 1.74 | 2.28 | 0.03 | 0.45; 7.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horas, K.; Hoxha, M.; Heinz, T.; Jakuscheit, A.; List, K.; Maier, G.S.; Weißenberger, M.; Rudert, M. Prevalence and Risk Factors of Vitamin D Deficiency in Patients Scheduled to Undergo Revision Arthroplasty of the Hip, Knee and Shoulder—Data from a Single-Centre Analysis. Nutrients 2024, 16, 3060. https://doi.org/10.3390/nu16183060
Horas K, Hoxha M, Heinz T, Jakuscheit A, List K, Maier GS, Weißenberger M, Rudert M. Prevalence and Risk Factors of Vitamin D Deficiency in Patients Scheduled to Undergo Revision Arthroplasty of the Hip, Knee and Shoulder—Data from a Single-Centre Analysis. Nutrients. 2024; 16(18):3060. https://doi.org/10.3390/nu16183060
Chicago/Turabian StyleHoras, Konstantin, Miledi Hoxha, Tizian Heinz, Axel Jakuscheit, Kilian List, Gerrit S. Maier, Manuel Weißenberger, and Maximilian Rudert. 2024. "Prevalence and Risk Factors of Vitamin D Deficiency in Patients Scheduled to Undergo Revision Arthroplasty of the Hip, Knee and Shoulder—Data from a Single-Centre Analysis" Nutrients 16, no. 18: 3060. https://doi.org/10.3390/nu16183060
APA StyleHoras, K., Hoxha, M., Heinz, T., Jakuscheit, A., List, K., Maier, G. S., Weißenberger, M., & Rudert, M. (2024). Prevalence and Risk Factors of Vitamin D Deficiency in Patients Scheduled to Undergo Revision Arthroplasty of the Hip, Knee and Shoulder—Data from a Single-Centre Analysis. Nutrients, 16(18), 3060. https://doi.org/10.3390/nu16183060







