The Effect of a Caffeine and Nicotine Combination on Nicotine Withdrawal Syndrome in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Reagents and Equipment
2.3. Experimental Design
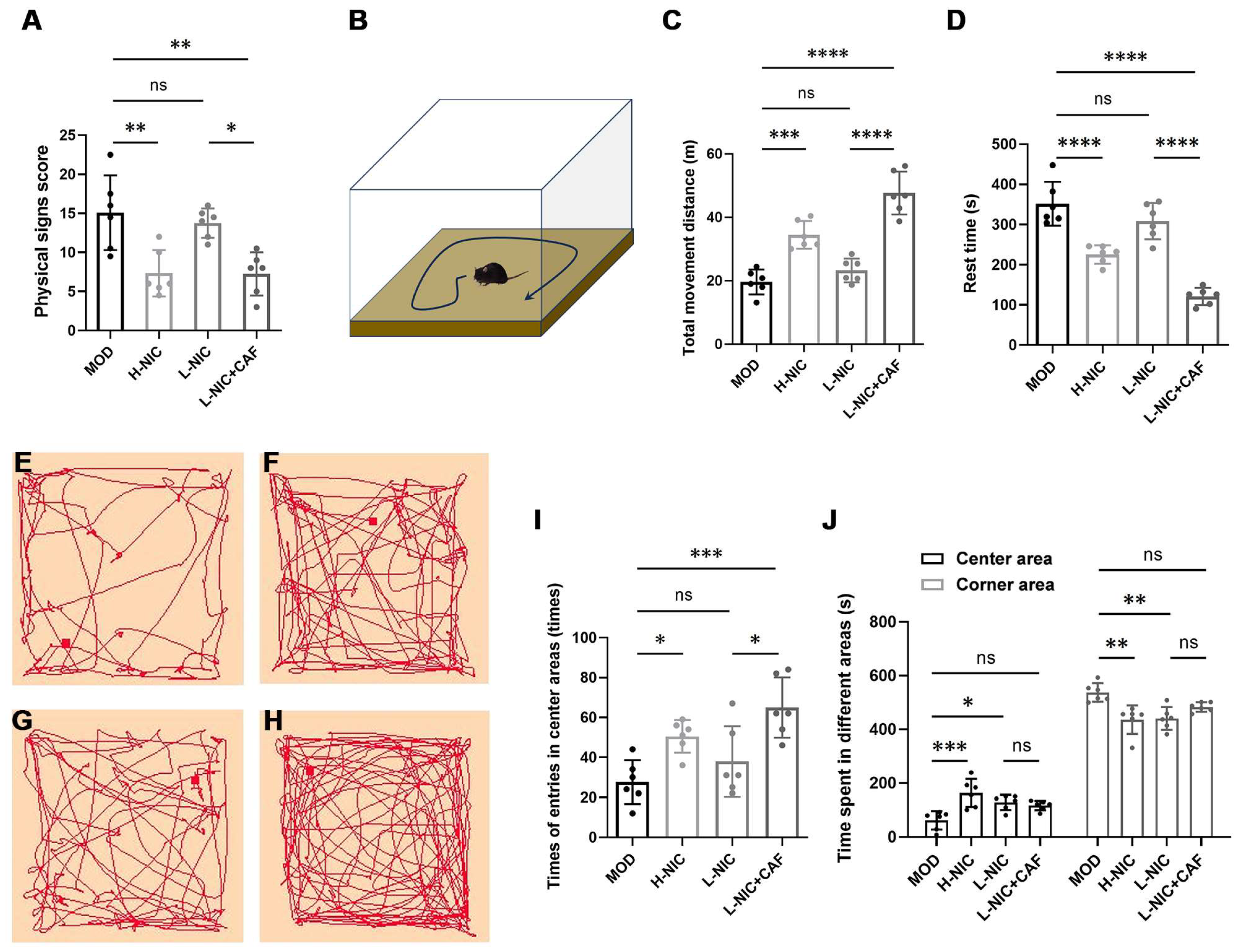
2.4. Assessment of Physical Signs
2.5. Open Field Test (OFT)
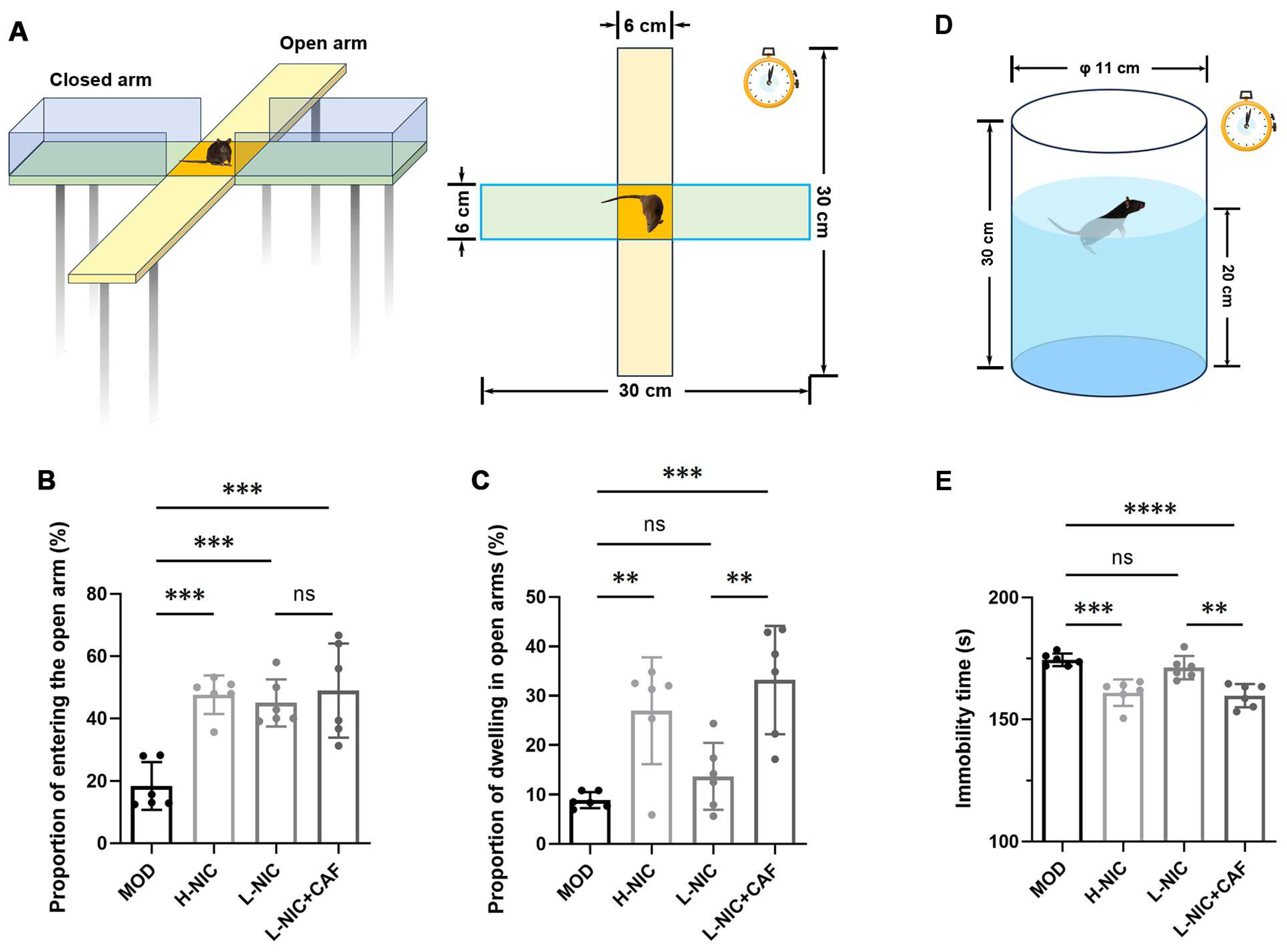
2.6. Elevated Plus Maze (EPM) Experiment
2.7. Forced Swimming Test (FST)
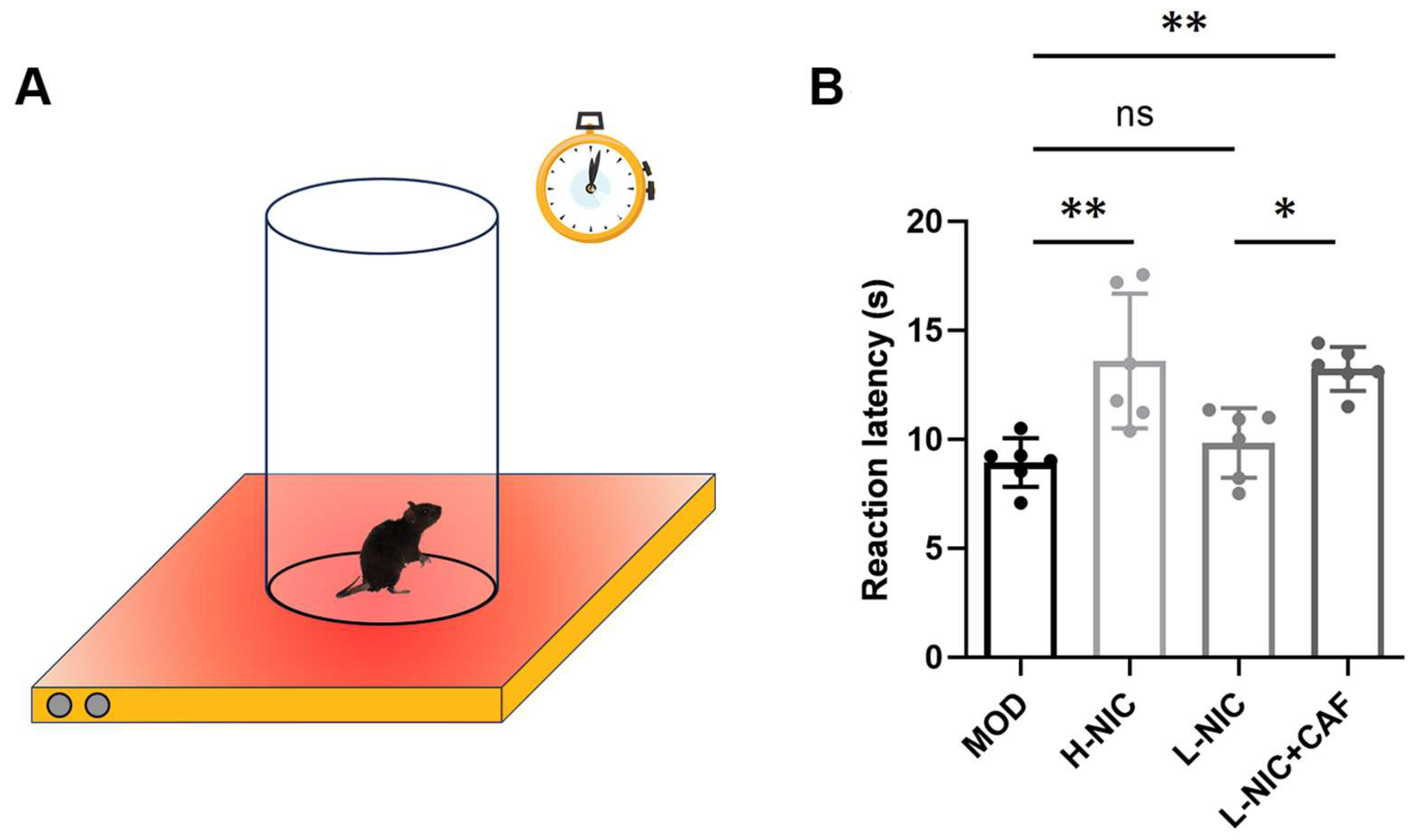
2.8. Hot Plate Test (HPT)
2.9. New Object Recognition Experiment (NOR)
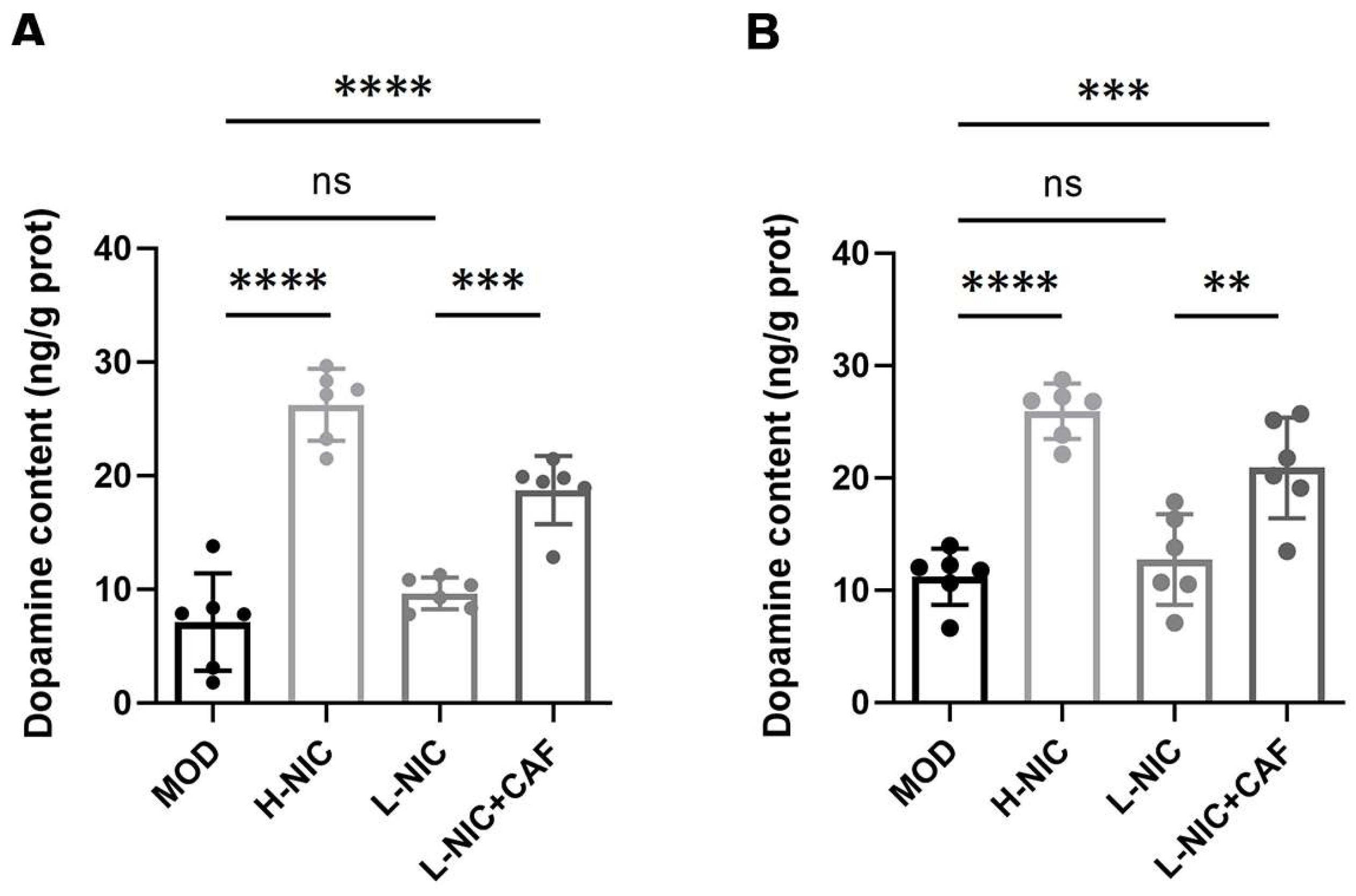
2.10. Determination of Dopamine Content
2.11. Statistical and Analysis
3. Results
3.1. Physical Signs and Behavior in OFT
3.2. Anxiety Behavior in EPM and Depressive Behavior during FST
3.3. Pain Latency in HPT
3.4. Cognitive Behavior in NOR
3.5. Dopamine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sansone, L.; Milani, F.; Fabrizi, R.; Belli, M.; Cristina, M.; Zaga, V.; de Iure, A.; Cicconi, L.; Bonassi, S.; Russo, P. Nicotine: From discovery to biological effects. Int. J. Mol. Sci. 2023, 24, 14570. [Google Scholar] [CrossRef] [PubMed]
- Muenstermann, C.; Clemens, K.J. Epigenetic mechanisms of nicotine dependence. Neurosci. Biobehav. Rev. 2024, 156, 105505. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.J.; Muldoon, P.P.; De Biasi, M.; Damaj, M.I. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology 2015, 96, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Begh, R.; Aveyard, P. Electronic cigarettes for smoking cessation. BMJ-Brit. Med. J. 2018, 360, j5543. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.B.; Warner, D.O. Safety and efficacy of nicotine replacement therapy in the perioperative period: A narrative review. Mayo Clin. Proc. 2015, 90, 1553–1561. [Google Scholar] [CrossRef]
- Mcgehee, D.S.; Heath, M.J.S.; Gelber, S.; Devay, P.; Role, L.W. Nicotine enhancement of fast excitatory synaptic transmission in Cns by presynaptic receptors. Science 1995, 269, 1692–1696. [Google Scholar] [CrossRef]
- Le Foll, B.; Chefer, S.I.; Kimes, A.S.; Stein, E.A.; Goldberg, S.R.; Mukhin, A.G. Impact of short access nicotine self-administration on expression of α4β2*nicotinic acetylcholine receptors in non-human primates. Psychopharmacology 2016, 233, 1829–1835. [Google Scholar] [CrossRef]
- Wills, L.; Ables, J.L.; Braunscheidel, K.M.; Caligiuri, S.P.; Elayouby, K.S.; Fillinger, C.; Ishikawa, M.; Moen, J.K.; Kenny, P.J. Neurobiological mechanisms of nicotine reward and aversion. Pharmacol. Rev. 2022, 74, 271–310. [Google Scholar] [CrossRef]
- Markou, A. Neurobiology of nicotine dependence. Philos. Trans. R. Soc. B 2008, 363, 3159–3168. [Google Scholar] [CrossRef]
- Wittenberg, R.E.; Wolfman, S.L.; De Biasi, M.; Dani, J.A. Nicotinic acetylcholine receptors and nicotine addiction: A brief introduction. Neuropharmacology 2020, 177, 108256. [Google Scholar] [CrossRef]
- Varani, A.P.; Pedrón, V.T.; Aon, A.J.; Canero, E.M.; Balerio, G.N. GABAB receptors blockage modulates somatic and aversive manifestations induced by nicotine withdrawal. Biomed. Pharmacother. 2021, 140, 111786. [Google Scholar] [CrossRef]
- Sebastianutto, I.; Goyet, E.; Andreoli, L.; Font-Ingles, J.; Moreno-Delgado, D.; Bouquier, N.; Jahannault-Talignani, C.; Moutin, E.; Di Menna, L.; Maslava, N.; et al. D1-mGlu5 heteromers mediate noncanonical dopamine signaling in Parkinson’s disease. J. Clin. Investig. 2020, 130, 1168–1184. [Google Scholar] [CrossRef] [PubMed]
- Lorist, M.M.; Tops, M. Caffeine, fatigue, and cognition. Brain Cogn. 2003, 53, 82–94. [Google Scholar] [CrossRef]
- Vazquez, J.C.; de la Torre, O.M.; Palome, J.L.; Redolar-Ripoll, D. Effects of caffeine consumption on attention deficit hyperactivity disorder (ADHD) treatment: A Systematic Review of Animal Studies. Nutrients 2022, 14, 739. [Google Scholar] [CrossRef]
- Acquas, E.; Tanda, G.; Di Chiara, G. Differential effects of caffeine on dopamine and acetylcholine transmission in brain areas of drug-naive and caffeine-pretreated rats. Neuropsychopharmacology 2002, 27, 182–193. [Google Scholar] [CrossRef]
- Cauli, O.; Pinna, A.; Valentini, V.; Morelli, M. Subchronic caffeine exposure induces sensitization to caffeine and cross-sensitization to amphetamine ipsilateral turning behavior independent from dopamine release. Neuropsychopharmacology 2003, 28, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.N.; Dong, W.J.; Tang, Y.M.; Hu, R.S.; Yu, X.X.; Chen, X.A. Characterization of the volatile flavour compounds in Yunnan Arabica coffee prepared by different primary processing methods using HS-SPME/GC-MS and HS-GC-IMS. LWT-Food Sci. Technol. 2024, 192, 115717. [Google Scholar] [CrossRef]
- Freitas, V.V.; Borges, L.L.R.; Vidigal, M.C.T.R.; dos Santos, M.H.; Stringheta, P.C. Coffee: A comprehensive overview of origin, market, and the quality process. Trends Food Sci. Technol. 2024, 146, 104411. [Google Scholar] [CrossRef]
- Liu, X.; Jernigan, C. Effects of caffeine on persistence and reinstatement of nicotine-seeking behavior in rats: Interaction with nicotine-associated cues. Psychopharmacology 2012, 220, 541–550. [Google Scholar] [CrossRef]
- Kowianski, P.; Lietzau, G.; Steliga, A.; Czuba, E.; Ludkiewicz, B.; Waskow, M.; Spodnik, J.H.; Morys, J. Nicotine-induced CREB and DeltaFosB activity is modified by caffeine in the brain reward system of the rat. J. Chem. Neuroanat. 2018, 88, 1–12. [Google Scholar] [CrossRef]
- O’Dell, L.E.; Khroyan, T.V. Rodent models of nicotine reward: What do they tell us about tobacco abuse in humans? Pharmacol. Biochem. Behav. 2009, 91, 481–488. [Google Scholar] [CrossRef]
- Elhassan, S.; Bagdas, D.; Damaj, M.I. Effects of nicotine metabolites on nicotine withdrawal behaviors in Mice. Nicotine Tob. Res. 2017, 19, 763–766. [Google Scholar] [CrossRef]
- Helal, M.G.; Ayoub, S.E.; Elkashefand, W.F.; Ibrahim, T.M. Caffeine affects HFD-induced hepatic steatosis by multifactorial intervention. Hum. Exp. Toxicol. 2018, 37, 983–990. [Google Scholar] [CrossRef]
- Klenowski, P.M.; Zhao-Shea, R.; Freels, T.G.; Molas, S.; Tapper, A.R. Dynamic activity of interpeduncular nucleus GABAergic neurons controls expression of nicotine withdrawal in male mice. Neuropsychopharmacology 2022, 47, 641–651. [Google Scholar] [CrossRef]
- Castañé, A.; Valjent, E.; Ledent, C.; Parmentier, M.; Maldonado, R.; Valverde, O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology 2002, 43, 857–867. [Google Scholar] [CrossRef]
- Ueno, H.; Shimada, A.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. Anti-depressive-like effect of 2-phenylethanol inhalation in mice. Biomed. Pharmacother. 2019, 111, 1499–1506. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim Test. JoVE-J. Vis. Exp. 2012, 59, 3638. [Google Scholar] [CrossRef]
- Alijanpour, S.; Ghasemzadeh, Z.; Ebrahimi-Ghiri, M.; Zarrindast, M.R. Basolateral amygdala cannabinoid CB1 receptors mediate the antinociceptive activity of harmaline in adolescent male mice. Physiol. Behav. 2022, 254, 113886. [Google Scholar] [CrossRef]
- Chang, P.K.; Chu, J.; Tsai, Y.T.; Lai, Y.H.; Chen, J.C. Dopamine D3 receptor and GSK3β signaling mediate deficits in novel object recognition memory within dopamine transporter knockdown mice. J. Biomed. Sci. 2020, 27, 16. [Google Scholar] [CrossRef]
- Negida, A.; Elfil, M.; Attia, A.; Farahat, E.; Gabr, M.; Essam, A.; Attia, D.; Ahmed, H. Caffeine; the forgotten potential for Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2017, 16, 652–657. [Google Scholar] [CrossRef]
- Ferré, S. Mechanisms of the psychostimulant effects of caffeine: Implications for substance use disorders. Psychopharmacology 2016, 233, 1963–1979. [Google Scholar] [CrossRef]
- Rud, M.A.; Do, T.N.; Siegel, J.A. Effects of early adolescent methamphetamine exposure on anxiety-like behavior and corticosterone levels in mice. Neurosci. Lett. 2016, 633, 257–261. [Google Scholar] [CrossRef]
- Anchan, D.; Clark, S.; Pollard, K.; Vasudevan, N. GPR30 activation decreases anxiety in the open field test but not in the elevated plus maze test in female mice. Brain Behav. 2014, 4, 51–59. [Google Scholar] [CrossRef]
- Himanshu, D.; Deepa Sarkar, N. A review of behavioral tests to evaluate different types of anxiety and anti-anxiety effects. Clin. Psychopharmacol. Neurosci. 2020, 18, 341–351. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaça, M.V.; Aguiar, D.C.; Guimaraes, F.S. Animal models of anxiety disorders and stress. Rev. Bras. Psiquiatr. 2013, 35, S101–S111. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, L.; Feng, L.Y.; Yao, L. The anxiolytic effect of essential oil of Cananga odorata exposure on mice and determination of its major active constituents. Phytomedicine 2016, 23, 1727–1734. [Google Scholar] [CrossRef]
- Bagosi, Z.; Palotai, M.; Simon, B.; Bokor, P.; Buzás, A.; Balangó, B.; Pintér, D.; Jászberényi, M.; Csabafi, K.; Szabó, G. Selective CRF2 receptor agonists ameliorate the anxiety- and depression-like state developed during chronic nicotine treatment and consequent acute withdrawal in mice. Brain Res. 2016, 1652, 21–29. [Google Scholar] [CrossRef]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Mannucci, C.; Tedesco, M.; Bellomo, M.; CapUti, A.P.; Calapai, G. Long-term effects of nicotine on the forced swimming test in mice: An experimental model for the study of depression caused by smoke. Neurochem. Int. 2006, 49, 481–486. [Google Scholar] [CrossRef]
- Shen, L.; Qiu, H.B.; Xu, H.H.; Wei, K.; Zhao, L.; Zhu, C.C.; Li, C.J.; Lu, Z.J. Nicotine withdrawal induces hyperalgesia via downregulation of descending serotonergic pathway in the nucleus raphe magnus. Neuropharmacology 2021, 189, 108515. [Google Scholar] [CrossRef]
- Ditre, J.W.; Zale, E.L.; LaRowe, L.R.; Kosiba, J.D.; De Vita, M.J. Nicotine deprivation increases pain intensity, neurogenic inflammation, and mechanical hyperalgesia among daily tobacco smokers. J. Abnorm. Psychol. 2018, 127, 578–589. [Google Scholar] [CrossRef]
- Nastase, A.; Ioan, S.; Braga, R.I.; Zagrean, L.; Moldovan, M. Coffee drinking enhances the analgesic effect of cigarette smoking. Neuroreport 2007, 18, 921–924. [Google Scholar] [CrossRef]
- Sawynok, J. Adenosine Receptor targets for pain. Neuroscience 2016, 338, 1–18. [Google Scholar] [CrossRef]
- Gloyer, L.; Golumba-Nagy, V.; Meyer, A.; Yan, S.; Schiller, J.; Breuninger, M.; Jochimsen, D.; Kofler, D.M. Adenosine receptor A2a blockade by caffeine increases IFN-gamma production in Th1 cells from patients with rheumatoid arthritis. Scand. J. Rheumatol. 2022, 51, 279–283. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and cognitive control in prefrontal cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Pittolo, S.; Yokoyama, S.; Willoughby, D.D.; Taylor, C.R.; Reitman, M.E.; Tse, V.; Wu, Z.F.; Etchenique, R.; Li, Y.L.; Poskanzer, K.E. Dopamine activates astrocytes in prefrontal cortex via receptors. Cell Rep. 2022, 40, 111426. [Google Scholar] [CrossRef]
- Zhong, P.; Qin, L.Y.; Yan, Z. Dopamine differentially regulates response dynamics of prefrontal cortical principal neurons and interneurons to optogenetic stimulation of inputs from ventral tegmental area. Cereb. Cortex 2020, 30, 4402–4409. [Google Scholar] [CrossRef]
- Livingstone, P.D.; Srinivasan, J.; Kew, J.N.; Dawson, L.A.; Gotti, C.; Moretti, M.; Shoaib, M.; Wonnacott, S. α7 and non-α7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur. J. Neurosci. 2009, 29, 539–550. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Lu, N.; Li, X.; Liu, Q.; Li, Y.; Li, X.; Yu, X.; Zhao, H.; Liu, C.; Tang, X.; et al. The Effect of a Caffeine and Nicotine Combination on Nicotine Withdrawal Syndrome in Mice. Nutrients 2024, 16, 3048. https://doi.org/10.3390/nu16183048
Chen Z, Lu N, Li X, Liu Q, Li Y, Li X, Yu X, Zhao H, Liu C, Tang X, et al. The Effect of a Caffeine and Nicotine Combination on Nicotine Withdrawal Syndrome in Mice. Nutrients. 2024; 16(18):3048. https://doi.org/10.3390/nu16183048
Chicago/Turabian StyleChen, Zhe, Naiyan Lu, Xu Li, Qingrun Liu, Yujie Li, Xiyue Li, Ximiao Yu, Haotian Zhao, Chang Liu, Xue Tang, and et al. 2024. "The Effect of a Caffeine and Nicotine Combination on Nicotine Withdrawal Syndrome in Mice" Nutrients 16, no. 18: 3048. https://doi.org/10.3390/nu16183048
APA StyleChen, Z., Lu, N., Li, X., Liu, Q., Li, Y., Li, X., Yu, X., Zhao, H., Liu, C., Tang, X., Wang, X., & Huang, W. (2024). The Effect of a Caffeine and Nicotine Combination on Nicotine Withdrawal Syndrome in Mice. Nutrients, 16(18), 3048. https://doi.org/10.3390/nu16183048







