The Quality of Life in Elderly Patients in Comprehensive Conservative Management or Hemodialysis: A Case–Control Study in Analogous Basal Conditions
Abstract
1. Introduction
2. Materials and Methods
- -
- Age over 75 years;
- -
- eGFR < 12 mL/min/1.73m2;
- -
- 6 months of CCM or HD;
- -
- Availability of a 12-item Short-Form Health Survey at the end of the observational period.
- Type of intervention
- Demographic data as the gender and age.
- The Charlson Comorbidity Index (CCI) adjusted by the patient’s age (+3 in patients between 70 and 79 years old, +4 in patients over 80 years old) was detected at the beginning of HD or CM. Specifically, CCI evaluates the following conditions: diabetes, congestive heart failure, peripheral vascular disease, chronic pulmonary disease, liver disease, hemiplegia, renal disease, hematological or metastatic cancer, and acquired immunodeficiency syndrome.
- Blood examinations: creatinine, urea, sodium, potassium, calcium, phosphorus, parathyroid hormone (PTH), Vitamin D 25-OH, bicarbonate, albumin, cholesterol, and triglycerides. In all patients, venous blood samples were collected between 7 and 8 am at the end of the observational period. In HD patients, samples were collected in the long interval before the HD session.
- Urine output in 24 h.
- The median Visual Analog Scale (VAS) was calculated for pain assessment for each patient according to the scoring reported in the clinical notes in the observational period. To avoid differences in the number of observations between the case and control groups, we considered all outpatient visits in CCM patients and the first evaluation of the month in HD patients.
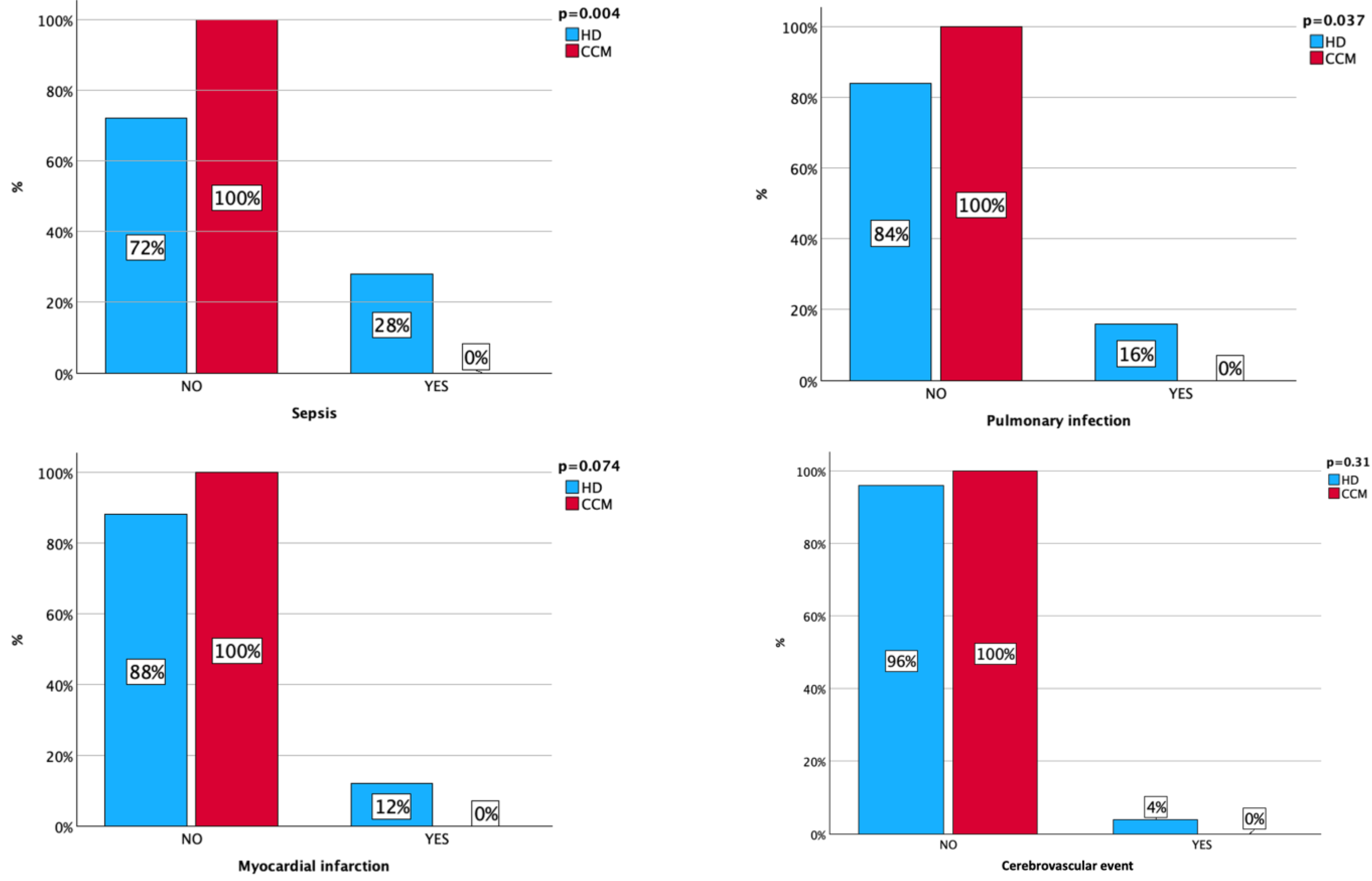
- Severe complications such as sepsis, pneumonia, myocardial infarction, and cerebrovascular events that required hospitalization were detected during the observational period.
2.1. Outcomes
2.2. Sample Size
2.3. Statistical Analysis
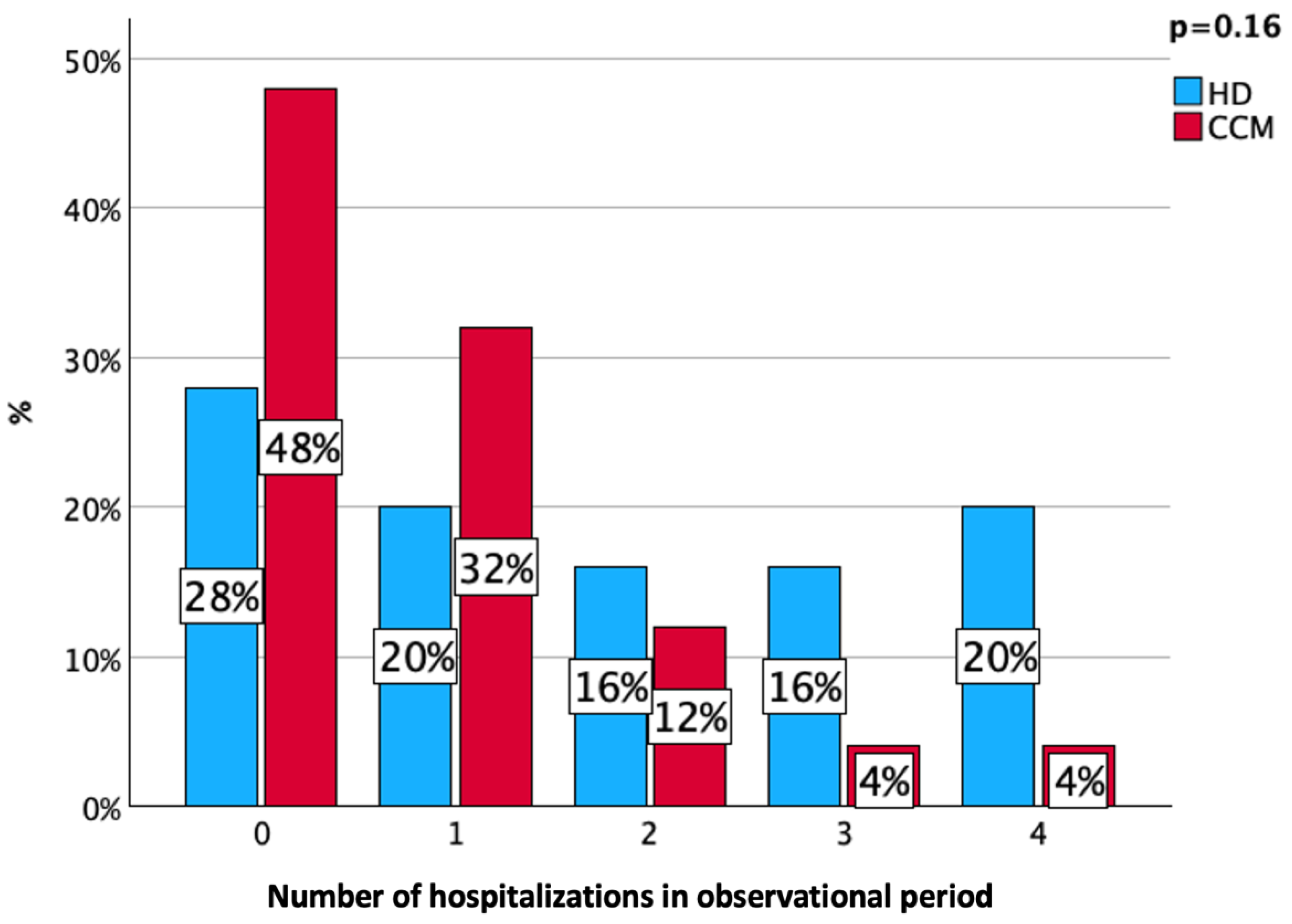
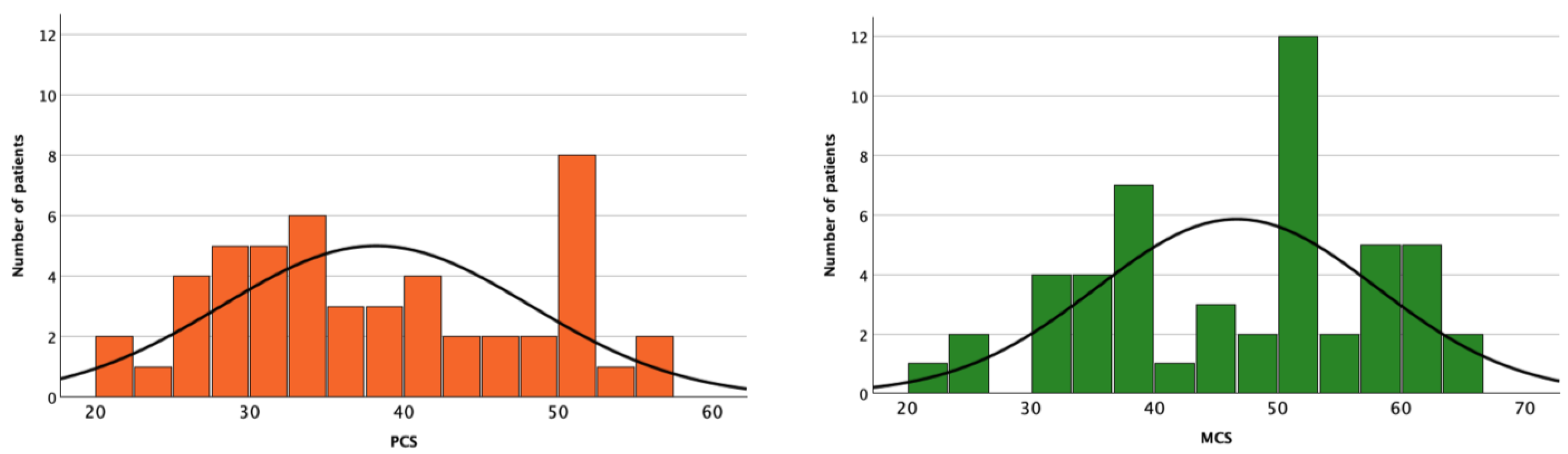
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- In general, would you say your health is:
- □1 Excellent □2 Very good □3 Good □4 Fair □5 Poor
- 2.
- Moderate activities such as moving a table, pushing a vacuum cleaner, bowling, or playing golf.
- □YES limited a lot □YES limited a little □NO, not limited at all
- 3.
- Climbing several flights of stairs.
- □YES limited a lot □YES limited a little □NO, not limited at all
- 4.
- Accomplished less than you would like. □YES □NO
- 5.
- Were limited in the kind of work or other activities. □YES □NO
- 6.
- Accomplished less than you would like. □YES □NO
- 7.
- Did work or activities less carefully than usual. □YES □NO
- 8.
- During the past 4 weeks, how much did pain interfere with your normal work (including work outside the home and housework)?
- □1 Not at all □2 A little bit □3 Moderately □4 Quite a bit □5 Extremely
- 9.
- Have you felt calm & peaceful? □All of the time □Most the bit of time □A good of the time □Some of the time □A little of the time □None of the time.
- 10.
- Did you have a lot of energy? □All of the time □Most the bit of time □A good of the time □Some of the time □A little of the time □None of the time.
- 11.
- Have you felt downhearted and Blue? □All of the time □Most the bit of time □A good of the time □Some of the time □A little of the time □None of the time.
- 12.
- During the past 4 weeks, how much of the time has your physical health or emotional problems interfered with your social activities (like visiting friends, relatives, etc.)?
- □All of the time □Most of the time □Some of the time □A little of the time □None of the time
References
- Johansen, K.L.; Chertow, G.M.; Foley, R.N.; Gilbertson, D.T.; Herzog, C.A.; Ishani, A.; Israni, A.K.; Ku, E.; Tamura, M.K.; Li, S.; et al. US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2021, 77 (Suppl. S1), A7–A8. [Google Scholar] [CrossRef]
- Joseph, J.; Vellaisamy, M.; Subramanian, T.; Fernando, E.; Kaliaperumal, T.; Srinivasaprasad, N.D.; Surendran, S.; Annadurai, P.; Haridas, N. Frailty in Patients with Chronic Kidney Disease Stage Five. Cureus 2023, 15, e43787. [Google Scholar] [CrossRef] [PubMed]
- Soucie, J.M.; McClellan, W.M. Early death in dialysis patients: Risk factors and impact on incidence and mortality rates. J. Am. Soc. Nephrol. 1996, 7, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Couchoud, C.; Labeeuw, M.; Moranne, O.; Allot, V.; Esnault, V.; Frimat, L.; Stengel, B.; French Renal Epidemiology and Information Network (REIN) registry. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol. Dial. Transpl. 2009, 24, 1553–1561. [Google Scholar] [CrossRef]
- Cohen, L.M.; Ruthazer, R.; Moss, A.H.; Germain, M.J. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2010, 5, 72–79. [Google Scholar] [CrossRef]
- Miskulin, D.C.; Meyer, K.B.; Martin, A.A.; Fink, N.E.; Coresh, J.; Powe, N.R.; Klag, M.J.; Levey, A.S. Comorbidity and its change predict survival in incident dialysis patients. Am. J. Kidney Dis. 2003, 41, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.K.; Novara, G.; Nalesso, F.; Calò, L.A. Conservative Management in End-Stage Kidney Disease between the Dialysis Myth and Neglected Evidence-Based Medicine. J. Clin. Med. 2023, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Pun, P.H. The interplay between CKD, sudden cardiac death, and ventricular arrhythmias. Adv. Chronic Kidney Dis. 2014, 21, 480–488. [Google Scholar] [CrossRef]
- Izzo, C.; Secondulfo, C.; Bilancio, G.; Visco, V.; Virtuoso, N.; Migliarino, S.; Ciccarelli, M.; Di Pietro, P.; La Mura, L.; Damato, A.; et al. Chronic Kidney Disease with Mineral Bone Disorder and Vascular Calcification: An Overview. Life 2024, 14, 418. [Google Scholar] [CrossRef]
- Yamada, S.; Giachelli, C.M. Vascular calcification in CKD-MBD: Roles for phosphate, FGF23, and Klotho. Bone 2017, 100, 87–93. [Google Scholar] [CrossRef]
- Iseri, K.; Dai, L.; Chen, Z.; Qureshi, A.R.; Brismar, T.B.; Stenvinkel, P.; Lindholm, B. Bone mineral density and mortality in end-stage renal disease patients. Clin. Kidney J. 2020, 13, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fu, Z.; Wan, X.; Wu, X.; Ding, L. Correlation between geriatric nutritional risk index and intradialytic hypotension in elderly patients undergoing maintenance hemodialysis: A case-control study. J. Health Popul. Nutr. 2024, 43, 80. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Chen, Y.L.; Chen, C.S.; Yang, C.S.; Peng, S.J. Association of leptin with hemodialysis-related muscle cramps: A cross-sectional study. Blood Purif. 2009, 27, 159–164. [Google Scholar] [CrossRef]
- Havranek, E.P.; Masoudi, F.A.; Westfall, K.A.; Wolfe, P.; Ordin, D.L.; Krumholz, H.M. Spectrum of heart failure in older patients: Results from the National Heart Failure project. Am. Heart J. 2002, 143, 412–417. [Google Scholar] [CrossRef]
- Banerjee, D.; Ma, J.Z.; Collins, A.J.; Herzog, C.A. Long-term survival of incident hemodialysis patients who are hospitalized for congestive heart failure, pulmonary edema, or fluid overload. Clin. J. Am. Soc. Nephrol. 2007, 2, 1186–1190. [Google Scholar] [CrossRef]
- Wang, L.; Jia, L.; Jiang, A. Pathology of catheter-related complications: What we need to know and what should be discovered. J. Int. Med. Res. 2022, 50, 3000605221127890. [Google Scholar] [CrossRef] [PubMed]
- Einbinder, Y.; Rozenberg, I.; Benchetrit, S.; Raigorodetsky, M.; Cohen-Hagai, K. Age-based comparison of vascular access outcomes in maintenance hemodialysis population. J. Vasc. Access. 2024, 25, 280–286. [Google Scholar] [CrossRef]
- Chewcharat, A.; Takkavatakarn, K.; Wongrattanagorn, S.; Panrong, K.; Kittiskulnam, P.; Eiam-Ong, S.; Susantitaphong, P. The Effects of Restricted Protein Diet Supplemented with Ketoanalogue on Renal Function, Blood Pressure, Nutritional Status, and Chronic Kidney Disease-Mineral and Bone Disorder in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. J Ren. Nutr. 2020, 30, 189–199. [Google Scholar] [CrossRef]
- Liu, Z.; Su, G.; Guo, X.; Wu, Y.; Liu, X.; Zou, C.; Zhang, L.; Yang, Q.; Xu, Y.; Ma, W. Dietary interventions for mineral and bone disorder in people with chronic kidney disease. Cochrane Database Syst. Rev. 2015, 2015, CD010350. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Yang, L.; Li, Z.; Qin, W. Effect of restricted protein diet supplemented with keto analogues in chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2016, 48, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.K.; Murtagh, F.E.M.; McGeechan, K.; Crail, S.; Burns, A.; Tran, A.D.; Morton, R.L. Health-related quality of life and well-being in people over 75 years of age with end-stage kidney disease managed with dialysis or comprehensive conservative care: A cross-sectional study in the UK and Australia. BMJ Open. 2019, 9, e027776. [Google Scholar] [CrossRef]
- Verberne, W.R.; Dijkers, J.; Kelder, J.C.; Geers, A.B.M.; Jellema, W.T.; Vincent, H.H.; van Delden, J.J.M.; Bos, W.J.W. Value-based evaluation of dialysis versus conservative care in older patients with advanced chronic kidney disease: A cohort study. BMC Nephrol. 2018, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Iyasere, O.; Brown, E.A.; Johansson, L.; Davenport, A.; Farrington, K.; Maxwell, A.P.; Collinson, H.; Fan, S.; Habib, A.-M.; Stoves, J.; et al. Quality of life with conservative care compared with assisted peritoneal dialysis and haemodialysis. Clin. Kidney J. 2018, 12, 262–268. [Google Scholar] [CrossRef] [PubMed]
- van Loon, I.N.; Goto, N.A.; Boereboom, F.T.J.; Verhaar, M.C.; Bots, M.L.; Hamaker, M.E. Quality of life after the initiation of dialysis or maximal conservative management in elderly patients: A longitudinal analysis of the Geriatric assessment in OLder patients starting Dialysis (GOLD) study. BMC Nephrol. 2019, 20, 108. [Google Scholar] [CrossRef]
- Seow, Y.Y.; Cheung, Y.B.; Qu, L.M.; Yee, A.C. Trajectory of quality of life for poor prognosis stage 5D chronic kidney disease with and without dialysis. Am. J. Nephrol. 2013, 37, 231–238. [Google Scholar] [CrossRef]
- So, S.; Li, K.; Hoffman, A.T.; Josland, E.; Brown, M.A. Quality of Life in Patients with Chronic Kidney Disease Managed with or without Dialysis: An Observational Study. Kidney360 2022, 3, 1890–1898. [Google Scholar] [CrossRef]
- So, S.; Brown, M.A.; Li, K. Factors associated with quality of life in patients with kidney failure managed conservatively and with dialysis: A cross-sectional study. BMC Nephrol. 2023, 24, 322. [Google Scholar] [CrossRef]
- Deng, F.; Di, W.; Ma, Y.; Li, Y.; Liao, T.; Mou, J.; Hong, D.; Wang, W.; Chen, J.; Jiang, P.; et al. The relationship between prescription of ultrafiltration and intradialytic hypotension in Chinese hemodialysis patients. Ann. Palliat. Med. 2021, 10, 5316–5321. [Google Scholar] [CrossRef]
- Álvarez Nadal, M.; Viera Ramírez, E.R.; Martín Capón, I.; Fernández Lucas, M. Absolute blood volume variations and vascular refilling in hemodialysis patients. Semin. Dial. 2021, 34, 229–234. [Google Scholar] [CrossRef]
- Yu, J.; Chen, X.; Li, Y.; Wang, Y.; Liu, Z.; Shen, B.; Teng, J.; Zou, J.; Ding, X. High ultrafiltration rate induced intradialytic hypotension is a predictor for cardiac remodeling: A 5-year cohort study. Ren. Fail. 2021, 43, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Moloney, B.M.; Chertow, G.M.; Mc Causland, F.R. Association of Diabetes with Changes in Blood Pressure during Hemodialysis: A Secondary Analysis of the Frequent Hemodialysis Network Daily Trial. Am. J. Nephrol. 2024, 55, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.A.; Waikar, S.S.; Mc Causland, F.R. Higher NT-proBNP levels and the risk of intradialytic hypotension at hemodialysis initiation. Hemodial. Int. 2024, 28, 77–84. [Google Scholar] [CrossRef]
- Crepaldi, C.; Rosner, M.; Teixeira, C.; Martos, L.B.; Martino, F.K.; Rodighiero, M.P.; Ronco, C. Is brain natriuretic peptide a reliable biomarker of hydration status in all peritoneal dialysis patients? Blood Purif. 2014, 37, 238–242. [Google Scholar] [CrossRef]
- Odudu, A.; McIntyre, C.W. An Update on Intradialytic Cardiac Dysfunction. Semin. Dial. 2016, 29, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, J.; Oosterhuis, J.K.; Paans, W.; Krijnen, W.P.; Gaillard, C.A.J.M.; Westerhuis, R.; Franssen, C.F.M. Association between quality of life and various aspects of intradialytic hypotension including patient-reported intradialytic symptom score. BMC Nephrol. 2019, 20, 164. [Google Scholar] [CrossRef]
- Wang, J.; Yao, J.; Zhu, X.; Wang, T.; Lu, J.; Wei, Q.; Xue, J.; Wu, Y.; You, L. Impact of frequent intradialytic hypotension on quality of life in patients undergoing hemodialysis. BMC Nephrol. 2023, 24, 209. [Google Scholar] [CrossRef]
- Chou, J.A.; Streja, E.; Nguyen, D.V.; Rhee, C.M.; Obi, Y.; Inrig, J.K.; Amin, A.; Kovesdy, C.P.; Sim, J.J.; Kalantar-Zadeh, K. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol. Dial. Transpl. 2018, 33, 149–159. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, J.; Wang, T.; Zhu, X.; Xue, J.; You, L. Association of frequent intradialytic hypotension with the clinical outcomes of patients on hemodialysis: A prospective cohort study. Ren. Fail. 2024, 46, 2296612. [Google Scholar] [CrossRef]
- Caprara, C.; Kinsey, G.R.; Corradi, V.; Xin, W.; Ma, J.Z.; Scalzotto, E.; Martino, F.K.; Okusa, M.D.; Nalesso, F.; Ferrari, F.; et al. The Influence of Hemodialysis on T Regulatory Cells: A Meta-Analysis and Systematic Review. Blood Purif. 2016, 42, 307–313. [Google Scholar] [CrossRef]
- Xiaoyan, J.; Rongyi, C.; Xuesen, C.; Jianzhou, Z.; Jun, J.; Xiaoqiang, D.; Xiaofang, Y. The difference of T cell phenotypes in end stage renal disease patients under different dialysis modality. BMC Nephrol. 2019, 20, 301. [Google Scholar] [CrossRef]
- Apetrii, M.; Timofte, D.; Voroneanu, L.; Covic, A. Nutrition in Chronic Kidney Disease-The Role of Proteins and Specific Diets. Nutrients 2021, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.K.; Zattarin, A.; Cinquini, C.; Toniazzo, S.; Pesenti, F.F.; Stefanelli, L.F.; Cacciapuoti, M.; Bettin, E.; Calò, L.A.; Spinella, P. Low-Protein Diet in Elderly Patients with Chronic Kidney Disease Stage 4 and 5 in Conservative Management: Focus on Sarcopenia Development. Nutrients 2024, 16, 1498. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, V.; Cupisti, A.; Locatelli, F.; Bolasco, P.; Brunori, G.; Cancarini, G.; Caria, S.; De Nicola, L.; Di Iorio, B.R.; Di Micco, L.; et al. Low-protein diets for chronic kidney disease patients: The Italian experience. BMC Nephrol. 2016, 17, 77. [Google Scholar] [CrossRef]
- He, X.; Song, Y.; Cao, Y.; Miao, L.; Zhu, B. Post intensive care syndrome: A review of clinical symptoms, evaluation, intervention. Heliyon 2024, 10, e31278. [Google Scholar] [CrossRef] [PubMed]
- Estrup, S.; Kjer, C.K.W.; Vilhelmsen, F.; Ahmed, N.; Poulsen, L.M.; Gøgenur, I.; Mathiesen, O. Health-related quality of life, anxiety and depression and physical recovery after critical illness—A prospective cohort study. Acta Anaesthesiol. Scand. 2022, 66, 85–93. [Google Scholar] [CrossRef]
- Jhamb, M.; Tucker, L.; Liebschutz, J. When ESKD complicates the management of pain. Semin. Dial. 2020, 33, 286–296. [Google Scholar] [CrossRef]
- Carlsson, A.M. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983, 16, 87–101. [Google Scholar] [CrossRef]
- Morton, R.L.; Turner, R.M.; Howard, K.; Snelling, P.; Webster, A.C. Patients who plan for conservative care rather than dialysis: A national observational study in Australia. Am. J. Kidney Dis. 2012, 59, 419–427. [Google Scholar] [CrossRef]
| Related to Lack of HD Complications | Related to CCM |
|---|---|
| Avoiding intradialytic hypotension | Slow CKD progression |
| Avoiding intradialytic cramps | Adequate control of urea retention |
| Limiting hospital facility access | Adequate control of anemia |
| Avoiding vascular access thrombosis | Adequate control of calcium–phosphate metabolism |
| Avoiding vascular access infection | Adequate control of metabolic acidosis |
| Reducing hospitalization rate | Positive impact of blood pressure control |
| Entire Population | HD | CCM | p-Value | |
|---|---|---|---|---|
| Age (years) * | 81.5 (±4.5) | 80.6 (±4.7) | 82.5 (±4.3) | 0.15 |
| Female (%) | 40 | 24 | 56 | 0.021 |
| CCI 9 10 11 12 13 14 | 14 28 12 18 12 6 | 12 28 24 20 12 4 | 16 28 20 16 12 8 | 0.98 |
| eGFR * (mL/min) | 8.22 (±2.1) | 7.95 (±1.7) | 8.45 (±2.4) | 0.37 |
| Urea * (mmol/L) | 28.5 (±9.5) | 30.9 (±9.5) | 26.1 (±9) | 0.07 |
| Hemoglobin * (g/dL) | 10.7 (±1.6) | 10.6 (±1.3) | 11 (±1.6) | 0.002 |
| BMI * (Kg2/m2) | 24.4 (± 3.6) | 24.7 (±4.06) | 24.1 (±3) | 0.51 |
| Albumin * (g/L) | 3.49 (±0.5) | 3.4 (±0.54) | 3.6 (±0.54) | 0.14 |
| Sodium * (mmol/L) | 138 (±4.6) | 137.4 (±5.6) | 139.8 (±2.9) | 0.064 |
| Potassium * (mmol/L) | 4.2 (±0.79) | 4.2 (± 1.08) | 4.3 (±0.5) | 0.73 |
| Calcium * (mmol/L) | 2.23(±0.18) | 2.21 (±0.23) | 2.25 (±0.13) | 0.45 |
| Phosphate ^ (mmol/L) | 1.56 [1.3–1.8] | 1.6 [1.4–1.8] | 1.48 [1.2–1.7] | 0.28 |
| Entire Population | HD | CCM | p-Value | |
|---|---|---|---|---|
| Urine output * (cc/d) | 1.34 (±0.50) | 1.09 (±0.49) | 1.6 (±0.37) | <0.001 |
| BMI * (Kg/m2) | 24.3 (±3.2) | 24.5 (±3.7) | 24.2 (±2.9) | 0.84 |
| Urea * (mmol/L) | 24.6 (±7.7) | 27 (±8.2) | 21.9 (±6.1) | 0,01 |
| Albumin *(g/L) | 3.7 (±0.5) | 3.7 (±0–4) | 3.6 (±0.6) | 0.54 |
| Hemoglobin (g/dL) | 10.9(±1.4) | 10.8(±1.5) | 11 (±1.2) | 0.61 |
| Sodium *(mmol/L) | 137.7 (±3.5) | 136.8 (±3.5) | 138.6 (±3.4) | 0.08 |
| Potassium (mmol/L) | 4.5 (±0.8) | 4.7 (±0.8) | 4.4 (±0.7) | 0.01 |
| Calcium * (mmol/L) | 2.3 (±0.29) | 2.2 (±0.19) | 2.24 (±0.4) | 0.28 |
| Phosphate ^ (mmol/L) | 1.37 [1.3–1.7] | 1.6 [1.3–1.8] | 1.3 [1.1–1.55] | 0.03 |
| PTH ^ (ng/L) | 286 [172–370] | 274 [167.5–399] | 288 [178–348] | 0.82 |
| Bicarbonate * (mmol/L) | 23.6 (±3.2) | 25.9 (±0.8) | 23.5 (±3.2) | 0.31 |
| VAS ^ score | 4 [2–6] | 6 [2–6] | 4 [2–6] | 0.09 |
| Physical Composite Score | Mental Composite Score | |||||
|---|---|---|---|---|---|---|
| B | p | 95% CI | B | p | 95% CI | |
| Age (^) | 0.003 | 0.72 | −0.14 0.02 | 0.001 | 0.98 | −0.1 0.017 |
| CCM | 0.24 | 0.001 | 0.1 0.38 | 0.15 | 0.048 | 0.001–0.3 |
| Female | 0.052 | 0.51 | −0.1 0.21 | −0.01 | 0.91 | −0.02 0.13 |
| Protein intake | −0.004 | 0.008 | −0.007 −0.00 | −0.002 | 0.26 | −0.005 0.001 |
| Urine output | 0.25 | 0.001 | 0.1 0.4 | 0.18 | 0.02 | 0.028 0.33 |
| BMI | 0.01 | 0.43 | −0.02 0.04 | 0.004 | 0.77 | −0.23 0.3 |
| Hemoglobin | −0.008 | 0.78 | −0.06 0.05 | −0.31 | 0.26 | −0.09 0.024 |
| Albumin | −0.06 | 0.47 | −0.21 0.1 | 0.003 | 0.96 | −0.15 0.16 |
| Urea | −0.009 | 0.09 | −0.02 0.001 | −0.006 | 0.26 | −0.02 0.004 |
| Sodium | 0.009 | 0.4 | −0.01 0.03 | 0.013 | 0.24 | −0.01 0.035 |
| Potassium | 0.02 | 0.67 | −0.08 0.12 | 0.004 | 0.93 | −0.97 0.105 |
| Calcium | 0.18 | 0.16 | −0.07 0.45 | 0.024 | 0.86 | −0.24 0.29 |
| Phosphate (^) | −0.14 | 0.43 | −0.49 0.22 | −0.31 | 0.083 | −0.65 0.41 |
| PTH (^) | −0.08 | 0.24 | −0.22 0.06 | −0.27 | 0.7 | −0.17 0.114 |
| Bicarbonate | 0.008 | 0.66 | −0.3 0.04 | 0.01 | 0.58 | −0.25 0.044 |
| Hospitalization | −0.18 | 0.024 | −0.33 −0.25 | −0.1 | 0.21 | −0.26 0.06 |
| VAS score (^) | −0.26 | <0.001 | −0.4 −0.14 | −0.14 | 0.048 | −0.28 −0.001 |
| Physical Composite Score | Metal Composite Score | |||||
|---|---|---|---|---|---|---|
| B | p | 95% CI | B | p | 95% CI | |
| CCM | 0.19 | 0.003 | 0.07 0.32 | 0.16 | 0.03 | 0.16 0.3 |
| VAS score ^ | −0.24 | <0.001 | −0.36 −0.12 | −0.157 | 0.024 | −0.28 −0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, F.K.; Campo, D.; Stefanelli, L.F.; Zattarin, A.; Piccolo, D.; Cacciapuoti, M.; Bogo, M.; Del Prete, D.; Nalesso, F.; Calò, L.A. The Quality of Life in Elderly Patients in Comprehensive Conservative Management or Hemodialysis: A Case–Control Study in Analogous Basal Conditions. Nutrients 2024, 16, 3037. https://doi.org/10.3390/nu16173037
Martino FK, Campo D, Stefanelli LF, Zattarin A, Piccolo D, Cacciapuoti M, Bogo M, Del Prete D, Nalesso F, Calò LA. The Quality of Life in Elderly Patients in Comprehensive Conservative Management or Hemodialysis: A Case–Control Study in Analogous Basal Conditions. Nutrients. 2024; 16(17):3037. https://doi.org/10.3390/nu16173037
Chicago/Turabian StyleMartino, Francesca K., Daniela Campo, Lucia Federica Stefanelli, Alessandra Zattarin, Daria Piccolo, Martina Cacciapuoti, Marco Bogo, Dorella Del Prete, Federico Nalesso, and Lorenzo A. Calò. 2024. "The Quality of Life in Elderly Patients in Comprehensive Conservative Management or Hemodialysis: A Case–Control Study in Analogous Basal Conditions" Nutrients 16, no. 17: 3037. https://doi.org/10.3390/nu16173037
APA StyleMartino, F. K., Campo, D., Stefanelli, L. F., Zattarin, A., Piccolo, D., Cacciapuoti, M., Bogo, M., Del Prete, D., Nalesso, F., & Calò, L. A. (2024). The Quality of Life in Elderly Patients in Comprehensive Conservative Management or Hemodialysis: A Case–Control Study in Analogous Basal Conditions. Nutrients, 16(17), 3037. https://doi.org/10.3390/nu16173037








