Evaluation of Muscle Mass and Malnutrition in Patients with Colorectal Cancer Using the Global Leadership Initiative on Malnutrition Criteria and Comparing Bioelectrical Impedance Analysis and Computed Tomography Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Procedures
2.3. Anthropometric and BIA-Based Skeletal Muscle Mass
2.4. CT-Based Skeletal Muscle Mass Measurement
2.5. Biochemical Parameters
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and Malnutrition according to GLIM Criteria
3.2. Concordance Analysis between BIA-Defined and CT-Defined Low Muscle Mass
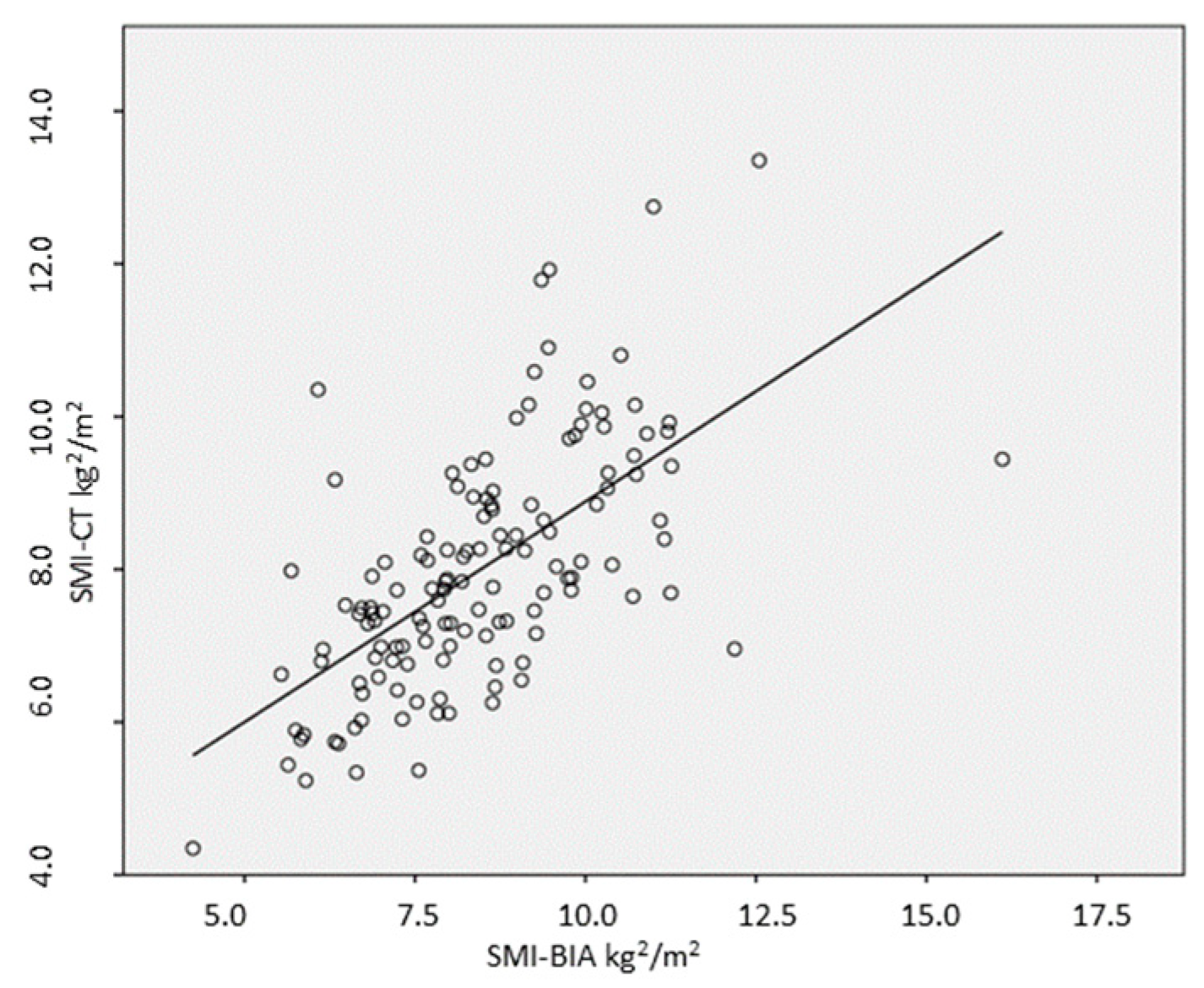
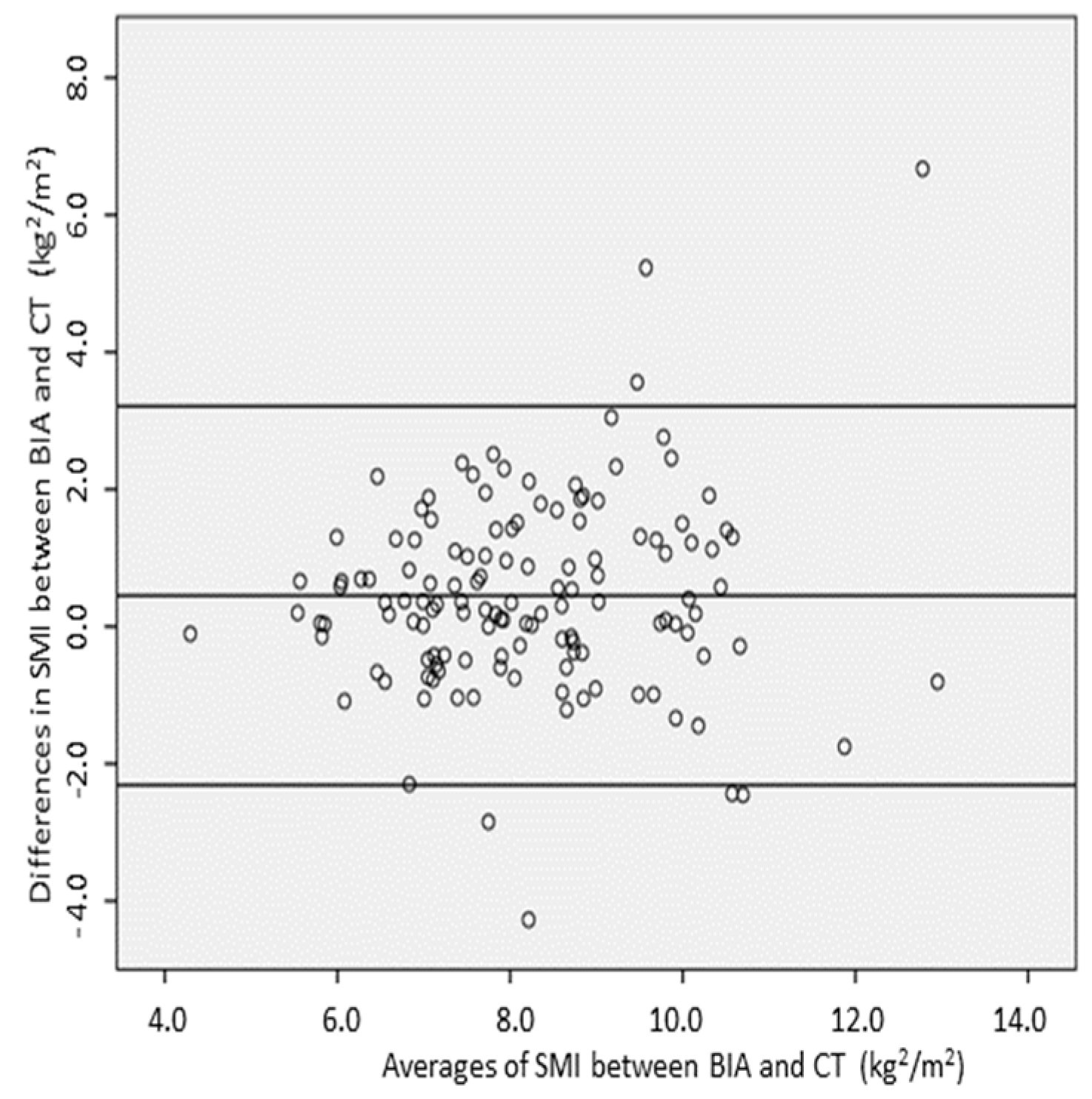
3.3. Relationship and Concordance between SMI Measurements Obtained by BIA and CT
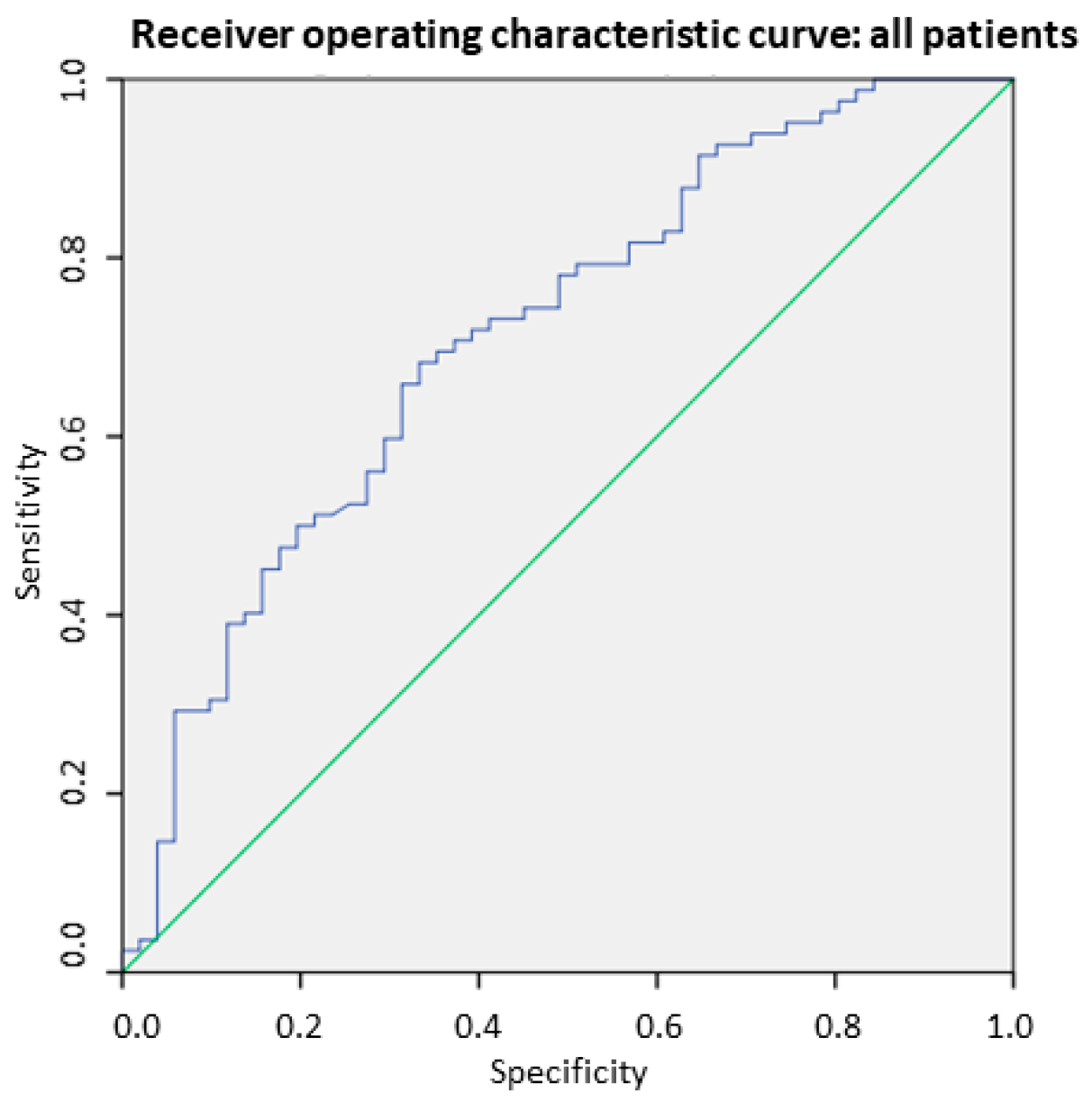
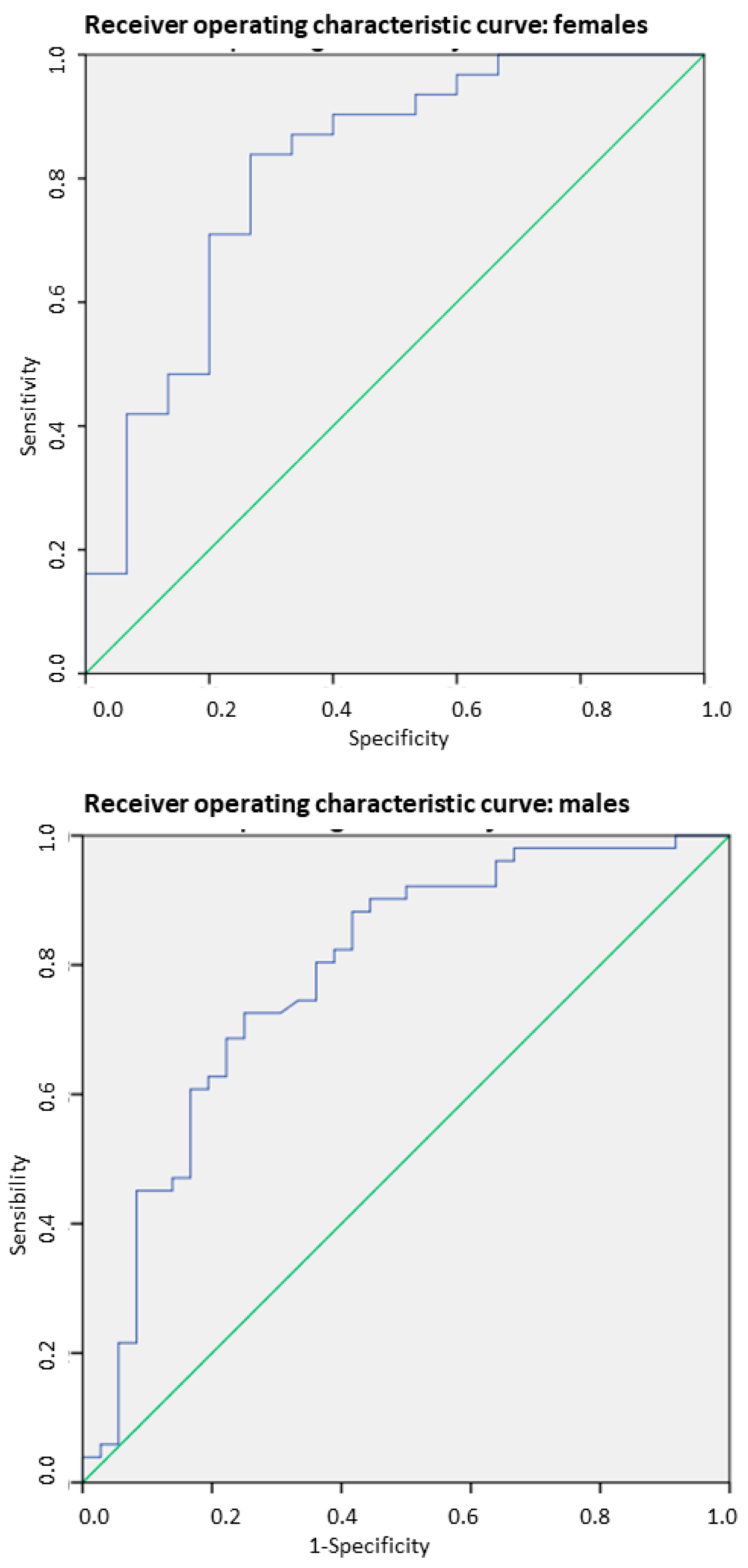
3.4. SMI-BIA for Detecting Low Muscle Mass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinninella, E.; Cintoni, M.; Raoul, P.; Pozzo, C.; Strippoli, A.; Bria, E.; Tortora, G.; Gasbarrini, A.; Mele, M.C. Muscle mass, assessed at diagnosis by L3-CT scan as a prognostic marker of clinical out-comes in patients with gastric cancer: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 2045–2054. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.T.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition [GLIM] diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo De Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef]
- Ceniccola, G.D.; Castro, M.G.; Piovacari, S.M.; Horie, L.M.; Corrêa, F.G.; Barrere, A.P.; Toledo, D.O. Current technologies in body composition assessment: Advantages and disadvantages. Nutrition 2019, 62, 25–31. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Haverkort, E.B.; Reijven, P.L.; Binnekade, J.M.; De Van Der Schueren, M.A.; Earthman, C.P.; Gouma, D.J.; De Haan, R.J. Bioelectrical impedance analysis to estimate body composition in surgical and oncological patients: A systematic review. Eur. J. Clin. Nutr. 2015, 69, 3–13. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Rock, C.D.; Edwards, J.; Mohamed, A.S.; Ruzensky, D.; Currie, A.; Rosemond, P.; Phan, J.; Gunn, G.B.; Frank, S.J.; et al. Bioelectrical impedance analysis as a quantitative measure of sarcopenia in head and neck cancer patients treated with radiotherapy. Radiother. Oncol. 2021, 159, 21–27. [Google Scholar] [CrossRef]
- Looijaard, W.G.; Stapel, S.N.; Dekker, I.M.; Rusticus, H.; Remmelzwaal, S.; Girbes, A.R.; Weijs, P.J.; Oudemans-van Straaten, H.M. Identifying critically ill patients with low muscle mass: Agreement between bioelectrical impedance analysis and computed tomography. Clin. Nutr. 2020, 39, 1809–1817. [Google Scholar] [CrossRef]
- van der Werf, A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Nurmohamed, S.A.; van der Pant, K.A.M.I.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef]
- Lukaski, H.; Johson, P.E. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J. Appl. Physiol. 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.S.; Shachar, S.S.; Muss, H.B.; Deal, A.M.; Popuri, K.; Yu, H.; Nyrop, K.A.; Alston, S.M.; Williams, G.R. Beyond sarcopenia: Characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J. 2018, 24, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yassaie, S.S.; Keane, C.; French, S.J.H.; Al-Herz, F.A.J.; Young, M.K.; Gordon, A.C. Decreased total psoas muscle area after neoadjuvant therapy is a predictor of increased mortality in patients undergoing oesophageal cancer resection. ANZ J. Surg. 2019, 89, 515–519. [Google Scholar] [CrossRef]
- Tamura, T.; Sakurai, K.; Nambara, M.; Miki, Y.; Toyokawa, T.; Kubo, N.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Ohira, M. Adverse effects of preoperative sarcopenia on postoperative complications of patients with gastric cancer. Anticancer Res. 2019, 39, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ruiz-Vico, M.; González-Almendros, I.; Barrios, M.; Padín, S.; Alba, E.; Olveira, G. GLIM criteria using hand grip strength adequately predict six-month mortality in cancer inpatients. Nutrition 2019, 11, 2043. [Google Scholar] [CrossRef] [PubMed]
- Harter, J.; Orlandi, S.P.; Gonzalez, M.C. Nutritional and functional factors as prognostic of surgical cancer patients. Support. Care Cancer 2017, 25, 2525–2530. [Google Scholar] [CrossRef]
- Norman, K.; Stobaus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis-clinical relevance and applicability of imped- ance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Hansen, C.; Tobberup, R.; Rasmussen, H.H.; Delekta, A.M.; Holst, M. Measurement of body composition: Agreement between methods of measurement by bioimpedance and computed tomography in patients with non-small cell lung cancer. Clin. Nutr. ESPEN 2021, 44, 429–436. [Google Scholar] [CrossRef]
- Kim, D.; Sun, J.S.; Lee, Y.H.; Lee, J.H.; Hong, J.; Lee, J.M. Comparative assessment of skeletal muscle mass using computerized tomography and bioelectrical impedance analysis in critically ill patients. Clin. Nutr. 2019, 38, 2747–2755. [Google Scholar] [CrossRef]
- Zuo, J.; Zhou, D.; Zhang, L.; Zhou, X.; Gao, X.; Hou, W.; Wang, C.; Jiang, P.; Wang, X. Comparison of bioelectrical impedance analysis and computed tomography for the assessment of muscle mass in patients with gastric cancer. Nutrition 2024, 121, 112363. [Google Scholar] [CrossRef]
- Casirati, A.; Vandoni, G.; Della Valle, S.; Greco, G.; Platania, M.; Colatruglio, S.; Lalli, L.; Gavazzi, C. Nutritional status and body composition assessment in patients with a new diagnosis of advanced solid tumour: Exploratory comparison of computed tomography and bioelectrical impedance analysis. Clin. Nutr. 2021, 40, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.J.; Lal, S.; Strauss, B.J.; Todd, C.; Pilling, M.; Burden, S.T. Measurement of Muscle Mass and Sarcopenia Using Anthropometry, Bioelectrical Impedance, and Computed Tomography in Surgical Patients with Colorectal Malignancy: Comparison of Agreement Between Methods. Nutr. Cancer. 2020, 72, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Meza-Valderrama, D.; Marco, E.; Dávalos-Yerovi, V.; Muns, M.D.; Tejero-Sánchez, M.; Duarte, E.; Sánchez-Rodríguez, D. Sarcopenia, malnutrition, and cachexia: Adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Aleixo, G.F.P.; Shachar, S.S.; Nyrop, K.A.; Muss, H.B.; Battaglini, C.L.; Williams, G.R. Bio- electrical impedance analysis for the assessment of sarcopenia in patients with cancer: A systematic review. Oncologist 2020, 25, 170–182. [Google Scholar] [CrossRef]
- Kikuchi, N.; Uojima, H.; Hidaka, H.; Iwasaki, S.; Wada, N.; Kubota, K.; Nakazawa, T.; Shibuya, A.; Kako, M.; Take, A.; et al. Evaluation of skeletal muscle mass in patients with chronic liver disease shows different results based on bioelectric impedance analysis and computed tomography. Ann. Nutr. Metab. 2022, 78, 336–344. [Google Scholar] [CrossRef]
- Hayashi, N.; Ando, Y.; Gyawali, B.; Shimokata, T.; Maeda, O.; Fukaya, M.; Goto, H.; Nagino, M.; Kodera, Y. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol. Rep. 2016, 35, 1727–1731. [Google Scholar] [CrossRef]
- Nishigori, T.; Tsunoda, S.; Obama, K.; Hisamori, S.; Hashimoto, K.; Itatani, Y.; Okada, K.; Sakai, Y. Optimal cutoff values of skeletal muscle index to define sarcopenia for prediction of survival in patients with advanced gastric cancer. Ann. Surg. Oncol. 2018, 25, 3596–3603. [Google Scholar] [CrossRef]
- Tegels, J.J.; Van Vugt, J.L.; Reisinger, K.W.; Hulsewé, K.W.; Hoofwijk, A.G.; Derikx, J.P.; Stoot, J.H. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J. Surg. Oncol. 2015, 112, 403–407. [Google Scholar] [CrossRef]
- Palmela, C.; Velho, S.; Agostinho, L.; Branco, F.; Santos, M.; Santos, M.P.; Oliveira, M.H.; Strecht, J.; Maio, R.; Cravo, M.; et al. Body composition as a prognostic factor of neoadjuvant chemotherapy toxicity and outcome in patients with locally advanced gastric cancer. J. Gastric Cancer 2017, 17, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, G.; Gallo, G.; Vescio, G.; Picciariello, A.; De Paola, G.; Trompetto, M.; Currò, G.; Ammendola, M. Mast Cells, microRNAs and Others: The Role of Translational Research on Colorectal Cancer in the Forthcoming Era of Precision Medicine. J. Clin. Med. 2020, 9, 2852. [Google Scholar] [CrossRef] [PubMed]
- Abedin, K.; Lean, Q.Y.; Wheelwright, S. Nutritional self-management in colorectal cancer patients and survivors: A scoping review. Colorectal Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Soria-Utrilla, V.; Sánchez-Torralvo, F.J.; Palmas-Candia, F.X.; Fernández-Jiménez, R.; Mucarzel-Suarez-Arana, F.; Guirado-Peláez, P.; Olveira, G.; García-Almeida, J.M.; Burgos-Peláez, R. AI-Assisted Body Composition Assessment Using CT Imaging in Colorectal Cancer Patients: Predictive Capacity for Sarcopenia and Malnutrition Diagnosis. Nutrients 2024, 16, 1869. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.B.; Alavi, D.T.; Beichmann, B.; Pesonen, M.; Henriksen, C.; Paur, I.; Bøhn, S.K.; Lauritzen, P.M.; Blomhoff, R.; Henriksen, H.B. The choice of diagnostic modality influences the proportion of low muscle strength, low muscle mass, and sarcopenia in colorectal cancer patients. Clin. Nutr. ESPEN 2024, 60, 17–23. [Google Scholar] [CrossRef]
- Degiuli, M.; Elmore, U.; De Luca, R.; De Nardi, P.; Tomatis, M.; Biondi, A.; Persiani, R.; Solaini, L.; Rizzo, G.; Soriero, D.; et al. Risk factors for anastomotic leakage after anterior resection for rectal cancer (RALAR study): A nationwide retrospective study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Colorectal Dis. 2022, 24, 264–276. [Google Scholar]
- Park, I.K.; Yang, S.S.; Chung, E.; Cho, E.S.; Lee, H.S.; Shin, S.J.; Im, Y.C.; Park, E.J.; Baik, S.H.; Lee, K.Y.; et al. Skeletal muscle gauge as a prognostic factor in patients with colorectal cancer. Cancer Med. 2021, 10, 8451–8461. [Google Scholar] [CrossRef]
- Monaco-Ferreira, D.V.; Magro, D.O.; Coy, C.S.R. Evaluation of different tools for body composition assessment in colorectal cancer—A systematic review. Arq. Gastroenterol. 2022, 59, 296–303. [Google Scholar] [CrossRef]
| Parameters | All | Male | Female | p-Value |
|---|---|---|---|---|
| Age, years | 69.8 ± 9.5 | 69.3 ± 9.4 | 70.6 ± 9.8 | 0.36 |
| Gender (female/male) | 49/88 | 88 | 49 | 0.03 |
| Albumin, g/L | 4.3 ± 0.4 | 4.4 ± 0.3 | 4.3 ± 0.5 | 0.41 |
| Prealbumin, mg/dL | 25.2 ± 7.5 | 26.7 ± 7.5 | 23.3 ± 7.1 | 0.38 |
| L3-SMI, cm2/m2 | 40.3 ± 8.9 | 43.7 ± 8.4 | 34.6 ± 7.5 | 0.02 |
| SMI-BIA, kg2/m2 | 8.5 ± 1.7 | 9.1 ± 1.7 | 7.3 ± 1.1 | 0.03 |
| SMI-CT, kg2/m2 | 7.9 ± 1.5 | 8.4 ± 1.5 | 7.1 ± 1.1 | 0.02 |
| SMG, AU | 1511.5 ± 588.5 | 1689.2 ± 597.5 | 1189.6 ± 425.5 | 0.01 |
| GLIM-BIA malnourished, % | 19.0% | 18.2% | 20.8% | 0.56 |
| GLIM-CT malnourished, % | 36.5% * | 36.4% | 38.3% | 0.49 |
| CT-defined low muscle mass, % | 38.0% | 40.9 | 32.7 | 0.23 |
| BIA-defined low muscle mass, % | 13.1% ** | 16.1% | 8.7% | 0.15 |
| Parameters | GLIM-BIA Well-Nourished | GLIM-BIA Malnutrition | p-Value | GLIM-CT Well-Nourished | GLIM-CT Malnutrition | p-Value |
|---|---|---|---|---|---|---|
| Age, years | 68.6 ± 9.4 | 74.6 ± 8.9 | 0.01 | 68.4 ± 4.2 | 70.2 ± 9.7 | 0.20 |
| Gender (female/male) | 38/72 | 11/16 | 0.43 | 34/53 | 15/35 | 0.38 |
| Albumin, g/L | 4.4 ± 0.3 | 3.9 ± 0.2 | 0.01 | 4.3 ± 0.3 | 4.1 ± 0.4 | 0.04 |
| Prealbumin, mg/dL | 26.4 ± 3.1 | 20.0 ± 3.0 | 0.02 | 26.3 ± 2.9 | 24.6 ± 2.6 | 0.04 |
| L3-SMI, cm2/m2 | 41.9 ± 8.6 | 33.6 ± 7.6 | 0.01 | 43.7 ± 8.4 | 34.6 ± 7.5 | 0.02 |
| SMI-BIA, kg2/m2 | 8.9 ± 1.5 | 6.7 ± 1.1 | 0.02 | 9.1 ± 1.6 | 7.5 ± 1.4 | 0.02 |
| SMI-CT, kg2/m2 | 8.2 ± 1.4 | 6.6 ± 1.1 | 0.02 | 8.8 ± 1.5 | 6.9 ± 1.1 | 0.01 |
| SMG, AU | 1596.3 ± 581.5 | 1158.9 ± 484.5 | 0.01 | 1700.8 ± 517.5 | 1244.9 ± 424.5 | 0.01 |
| CT-defined poor muscle mass (%) | 29.1% | 76.9% | 0.001 | 0% | 86.7% | 0.001 |
| BIA-defined poor muscle mass (%) | 0% | 69.2% | 0.001 | 4.1% | 25.9% | 0.001 |
| Parameters | Low SMI-CT | Normal SMI-CT | p-Value | Low SMI-BIA | Normal SMI-BIA | p-Value |
|---|---|---|---|---|---|---|
| Age | 69.4 ± 9.4 | 70.2 ± 9.3 | 0.48 | 76.3 ± 6.5 | 68.7 ± 5.8 | 0.26 |
| Gender (female/male) | 16/36 | 33/52 | 0.29 | 7/15 | 42/73 | 0.03 |
| Albumin, g/L | 4.2 ± 0.4 | 4.3 ± 0.4 | 0.48 | 3.9 ± 0.4 | 4.4 ± 0.4 | 0.02 |
| Prealbumin, mg/dL | 24.7 ± 6.6 | 26.1 ± 6.9 | 0.37 | 20.3 ± 7.4 | 26.1 ± 7.2 | 0.01 |
| L3-SMI, cm2/m2 | 34.5 ± 6.4 | 43.7 ± 5.5 | 0.02 | 33.9 ± 7.8 | 41.4 ± 8.1 | 0.01 |
| SMI-BIA, kg2/m2 | 7.5 ± 1.4 | 9.0 ± 1.2 | 0.03 | 6.5 ± 0.9 | 8.7 ± 1.0 | 0.02 |
| SMI-CT, kg2/m2 | 6.9 ± 1.1 | 8.6 ± 1.5 | 0.01 | 6.8 ± 1.3 | 8.2 ± 1.1 | 0.02 |
| SMG, AU | 1689.8 ± 599.5 | 1171.8 ± 383.5 | 0.01 | 1579.1 ± 424.1 | 1203.1 ± 510.8 | 0.01 |
| GLIM-BIA malnourished (%) | 38.5% | 7.1% | 0.02 | 100% | 7.0% | 0.001 |
| GLIM-CT malnourished (%) | 100% | 9.6% | 0.001 | 25.9% | 74.1% | 0.02 |
| Group | n | SMIBIA (kg/m2) | SMICT (kg/m2) | p-Value | r | p-Value |
|---|---|---|---|---|---|---|
| All | 137 | 7.9 ± 1.5 | 8.4 ± 1.7 | 0.001 | 0.63 | 0.001 |
| Female | 49 | 7.3 ± 1.1 | 7.1 ± 1.1 | 0.001 | 0.57 | 0.001 |
| Male | 88 | 9.0 ± 1.7 | 8.5 ± 1.6 | 0.001 | 0.54 | 0.001 |
| <65 y | 41 | 9.4 ± 1.6 | 8.5 ± 1.8 | 0.001 | 0.64 | 0.001 |
| >65 y | 96 | 8.1 ± 1.6 | 7.6 ± 1.3 | 0.001 | 0.61 | 0.001 |
| BMI < 25 | 34 | 7.2 ± 1.5 | 6.9 ± 1.0 | 0.001 | 0.57 | 0.001 |
| BMI > 25 | 103 | 9.0 ± 1.6 | 8.3 ± 1.5 | 0.001 | 0.59 | 0.001 |
| GLIM-CT well-nourished | 84 | 9.0 ± 1.7 | 8.6 ± 1.5 | 0.001 | 0.53 | 0.001 |
| GLIM-CT malnourished | 53 | 7.5 ± 1.4 | 6.9 ± 1.1 | 0.001 | 0.59 | 0.001 |
| GLIM-BIA well-nourished | 106 | 8.9 ± 1.6 | 8.3 ± 1.5 | 0.001 | 0.57 | 0.001 |
| GLIM-BIA malnourished | 31 | 6.9 ± 1.3 | 6.8 ± 1.5 | 0.23 | 0.51 | 0.009 |
| CT-defined normal muscle mass (%) | 82 | 8.9 ± 1.7 | 8.8 ± 1.5 | 0.39 | 0.58 | 0.001 |
| CT-defined low muscle mass (%) | 55 | 7.7 ± 1.5 | 6.7 ± 0.9 | 0.001 | 0.60 | 0.001 |
| BIA-defined normal muscle mass (%) | 115 | 8.7 ± 1.6 | 8.2 ± 1.5 | 0.001 | 0.61 | 0.001 |
| BIA-defined low muscle mass (%) | 22 | 6.8 ± 1.3 | 6.4 ± 0.9 | 0.21 | 0.45 | 0.049 |
| Group | n | SMIBIA-CT (kg/m2) | p * | 95% LOA |
|---|---|---|---|---|
| All | 137 | 0.45 ± 1.4 | - | 0.21–0.65 |
| Female | 49 | 0.23 ± 1.0 | - | −0.06–0.52 |
| Male | 88 | 0.60 ± 1.6 | 0.19 | 0.23–0.90 |
| <65 y | 41 | 0.85 ± 1.4 | - | 0.39–1.30 |
| >65 y | 96 | 0.27 ± 1.4 | 0.12 | 0.008–0.55 |
| BMI < 25 | 34 | 0.24 ± 1.3 | - | 0.68–0.20 |
| BMI > 25 | 103 | 0.69 ± 1.4 | 0.15 | 0.41–0.97 |
| GLIM-CT well-nourished | 84 | 0.39 ± 1.1 | - | 0.06–0.72 |
| GLIM-CT malnourished | 53 | 0.58 ± 0.9 | 0.01 | 0.24–0.92 |
| GLIM-BIA well-nourished | 106 | −0.06 ± 1.3 | - | −0.56–0.44 |
| GLIM-BIA malnourished | 31 | 0.59 ± 1.4 | 0.03 | 0.31–0.87 |
| CT-defined normal muscle mass (%) | 82 | 0.13 ± 1.4 | - | −0.17–0.44 |
| CT-defined low muscle mass (%) | 55 | 0.96 ± 1.3 | 0.001 | 0.61–1.31 |
| BIA-defined normal muscle mass (%) | 115 | 0.58 ± 1.4 | - | 0.33–0.84 |
| BIA-defined low muscle mass (%) | 22 | −0.39 ± 1.3 | 0.006 | −1.02–0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Luis Roman, D.; López Gómez, J.J.; Muñoz, M.; Primo, D.; Izaola, O.; Sánchez, I. Evaluation of Muscle Mass and Malnutrition in Patients with Colorectal Cancer Using the Global Leadership Initiative on Malnutrition Criteria and Comparing Bioelectrical Impedance Analysis and Computed Tomography Measurements. Nutrients 2024, 16, 3035. https://doi.org/10.3390/nu16173035
de Luis Roman D, López Gómez JJ, Muñoz M, Primo D, Izaola O, Sánchez I. Evaluation of Muscle Mass and Malnutrition in Patients with Colorectal Cancer Using the Global Leadership Initiative on Malnutrition Criteria and Comparing Bioelectrical Impedance Analysis and Computed Tomography Measurements. Nutrients. 2024; 16(17):3035. https://doi.org/10.3390/nu16173035
Chicago/Turabian Stylede Luis Roman, Daniel, Juan José López Gómez, Marife Muñoz, David Primo, Olatz Izaola, and Israel Sánchez. 2024. "Evaluation of Muscle Mass and Malnutrition in Patients with Colorectal Cancer Using the Global Leadership Initiative on Malnutrition Criteria and Comparing Bioelectrical Impedance Analysis and Computed Tomography Measurements" Nutrients 16, no. 17: 3035. https://doi.org/10.3390/nu16173035
APA Stylede Luis Roman, D., López Gómez, J. J., Muñoz, M., Primo, D., Izaola, O., & Sánchez, I. (2024). Evaluation of Muscle Mass and Malnutrition in Patients with Colorectal Cancer Using the Global Leadership Initiative on Malnutrition Criteria and Comparing Bioelectrical Impedance Analysis and Computed Tomography Measurements. Nutrients, 16(17), 3035. https://doi.org/10.3390/nu16173035







