Responsiveness and Reliability of a Sipping Device to Measure Motivation in Normal-Weight Individuals and Bariatric Surgery Patients
Abstract
1. Introduction
1.1. Background
1.1.1. Sweet Taste—Nutritive Versus Nonnutritive Sweeteners for Motivational Assessment
1.1.2. Motivation to Work for Sweets
1.1.3. Motivation to Work for and Consume Sweets in the Context of Bariatric Surgery in Animals and Humans
1.1.4. Novelty of the Sipometer
1.2. Aims, Hypothesis, and Expected Outcomes
1.2.1. Aims
1.2.2. Hypothesis
1.2.3. Expected Outcomes
Aim 1
Aim 2
2. Materials and Methods
2.1. Participants
2.2. Study Design
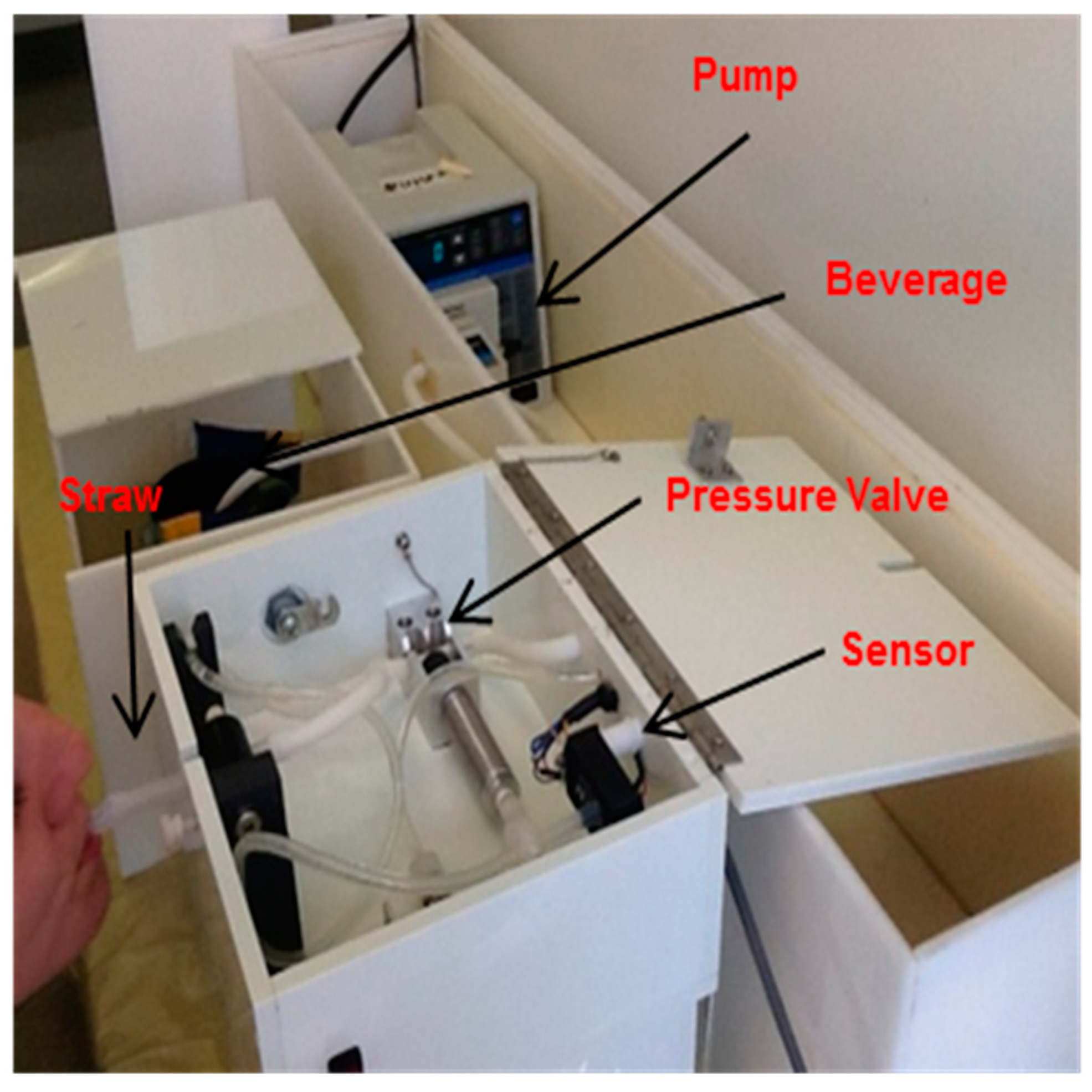
2.3. Instrumentation and Methods
2.4. Data Collection
2.5. Data Analyses
3. Results
3.1. Participant Characteristics
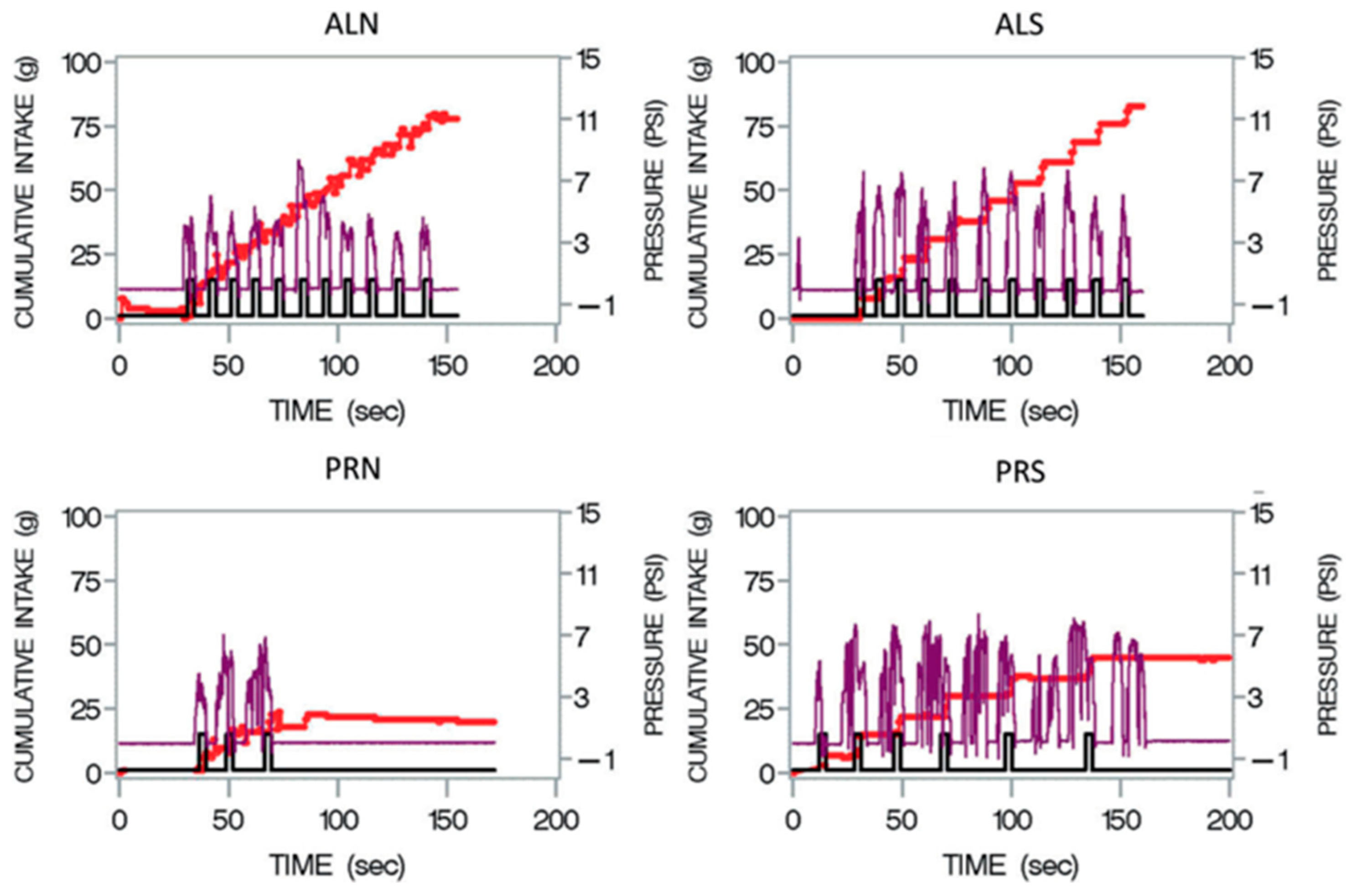
3.2. ANOVA Model 1—Differences across Groups, Visits, and Conditions (Schedule × Beverage)
3.2.1. Overall Model
3.2.2. Differences across Conditions at Baseline (E1 and E2)
3.2.3. Differences across Conditions at 3-Month Follow-Up (E1 and E2)
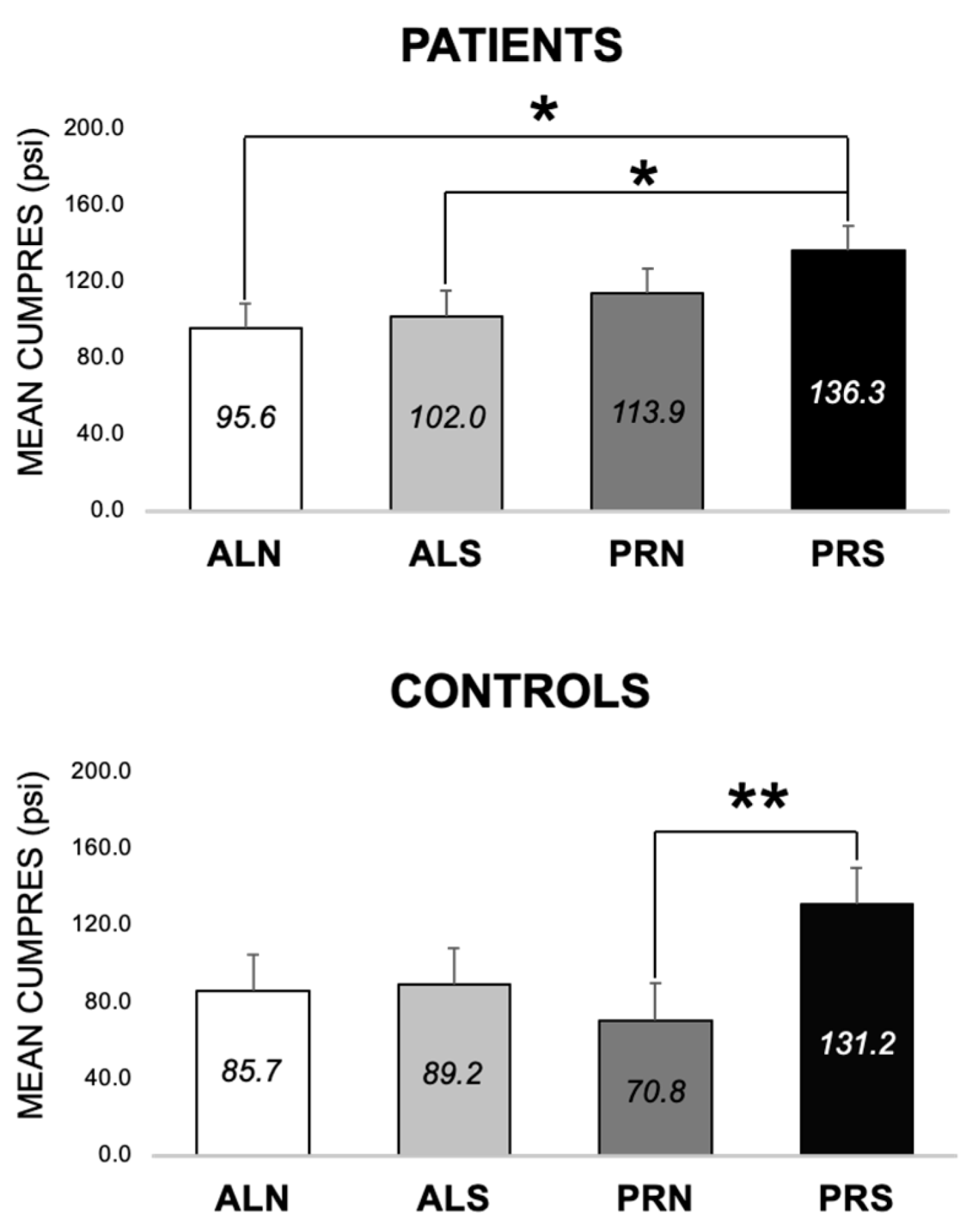
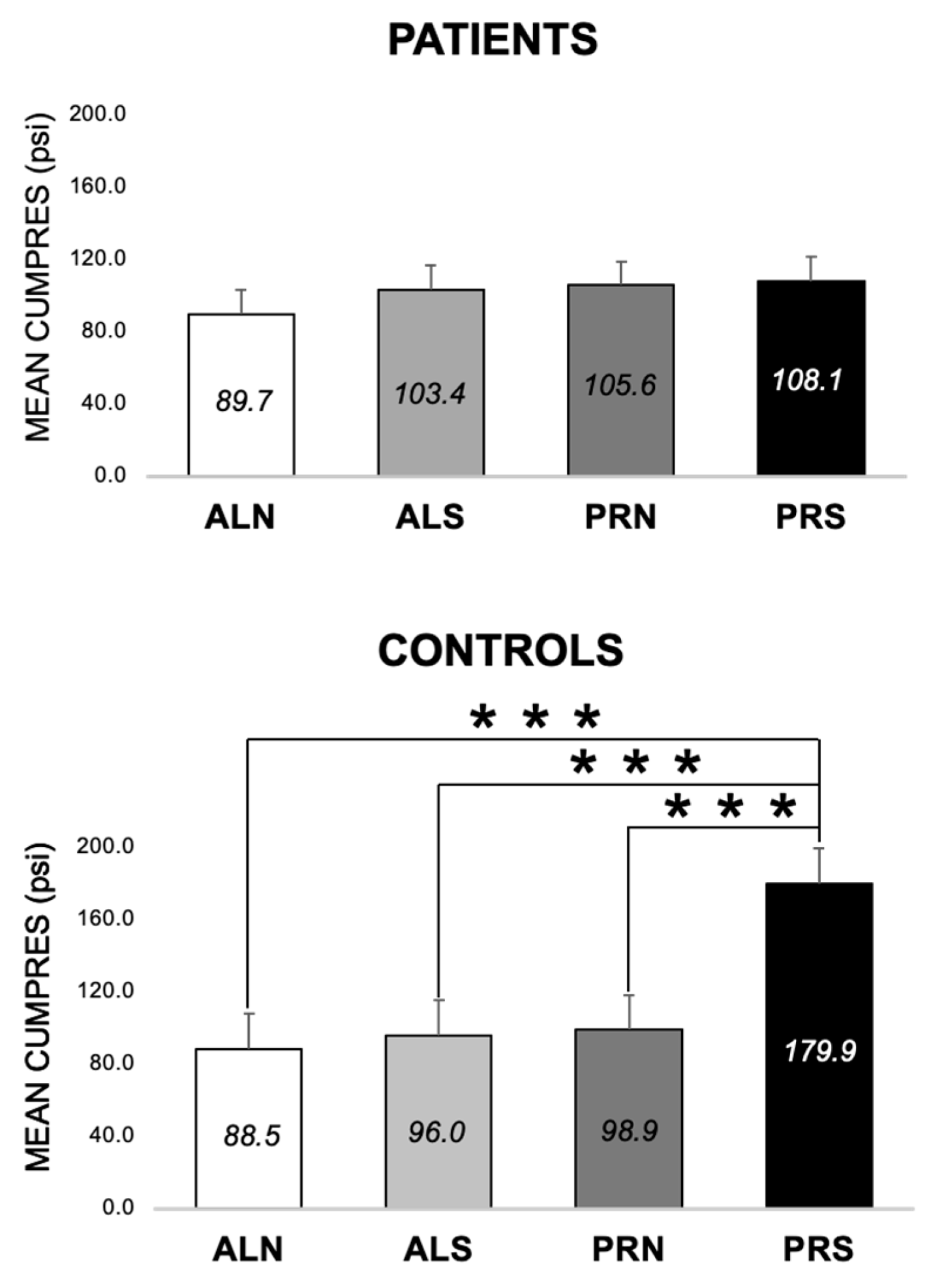
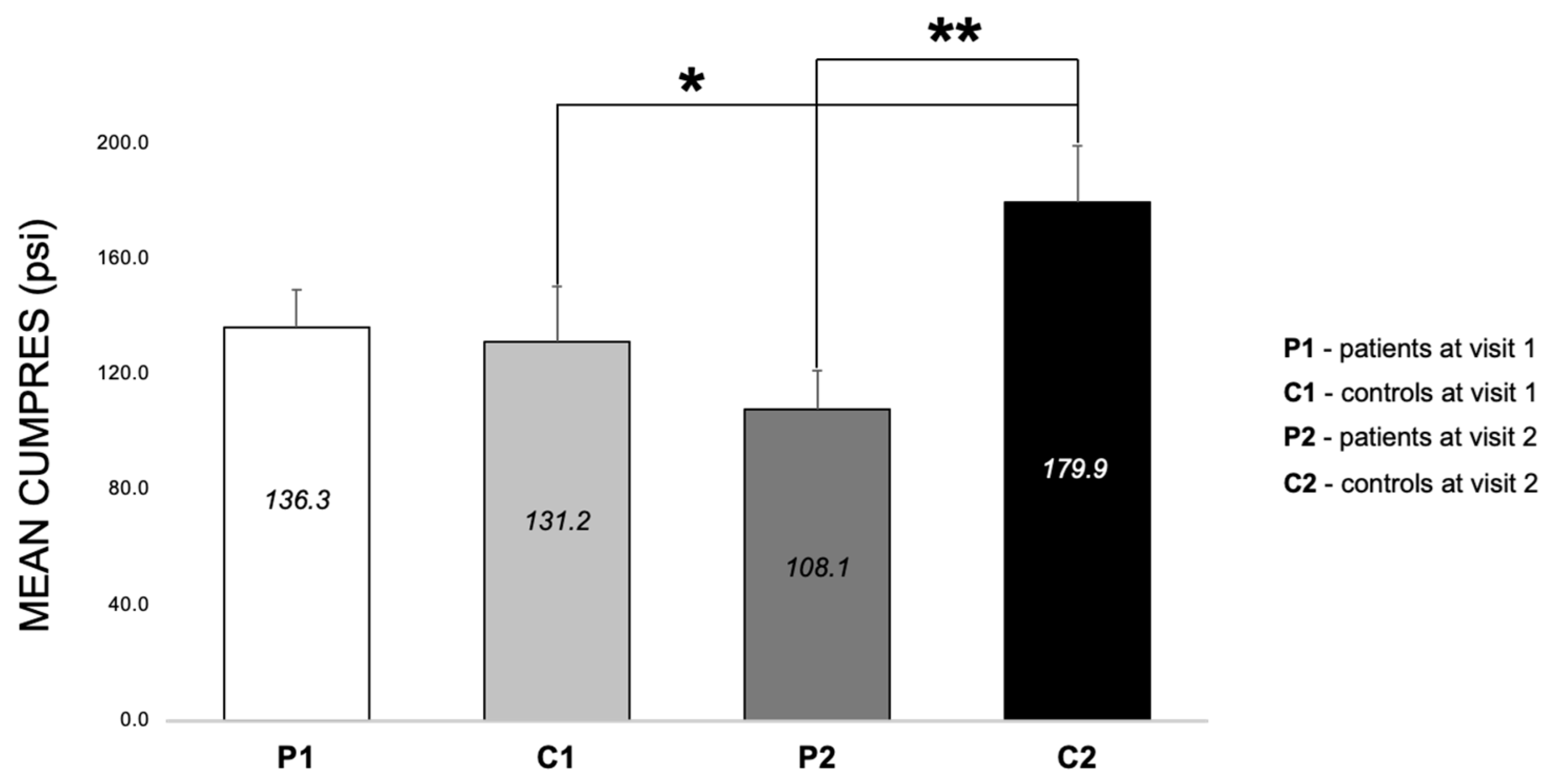
3.2.4. Differences between Patients and Controls for PRS at Both Visits (E3, E6–E8)
3.2.5. Specific Interactions
3.3. ANOVA Model 2—Differences across Visits and Conditions (Schedule × Beverage) in Patients
3.3.1. Overall Model
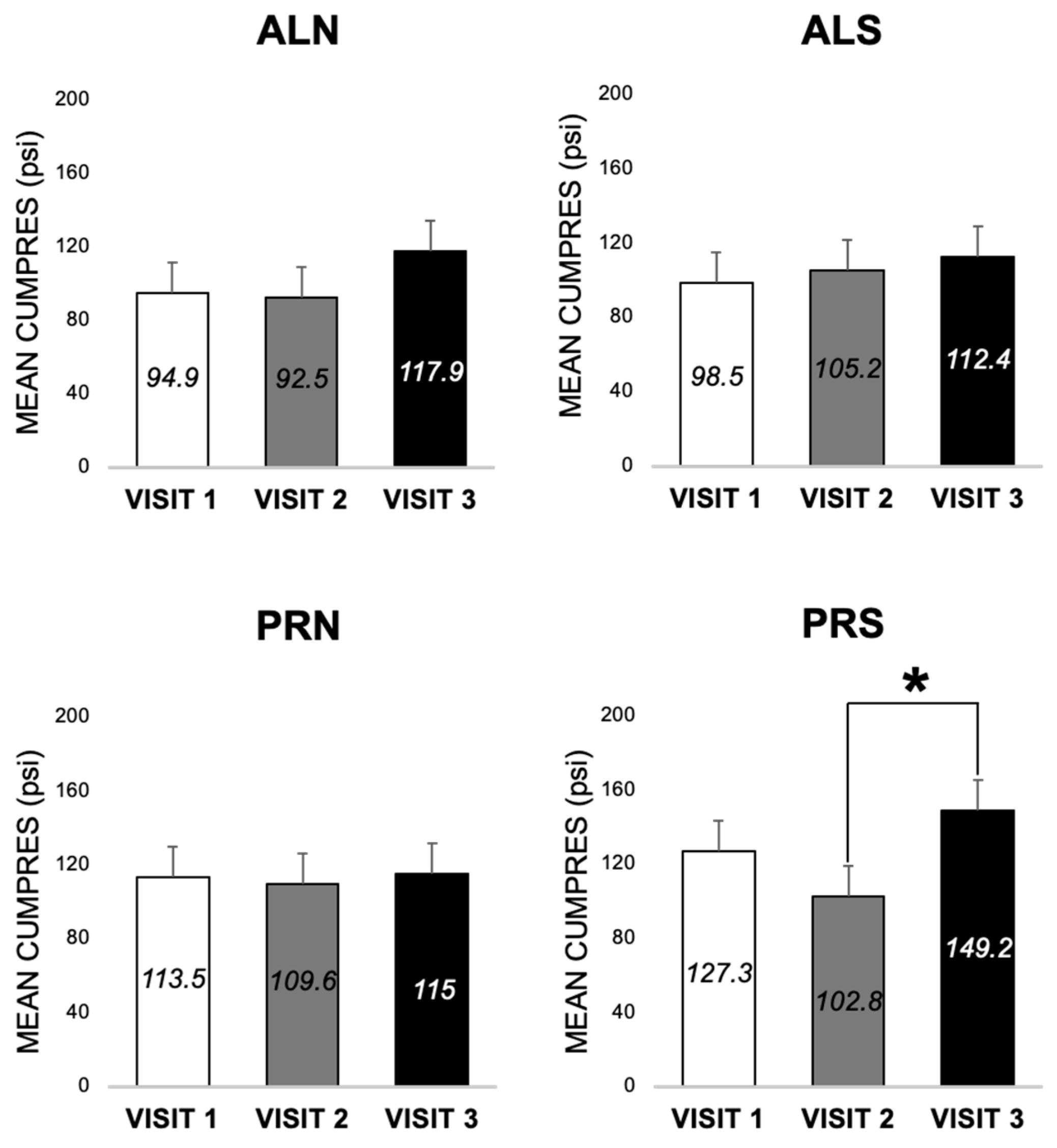
3.3.2. Differences across Visits for Each Condition in Patients (E3 and E4)
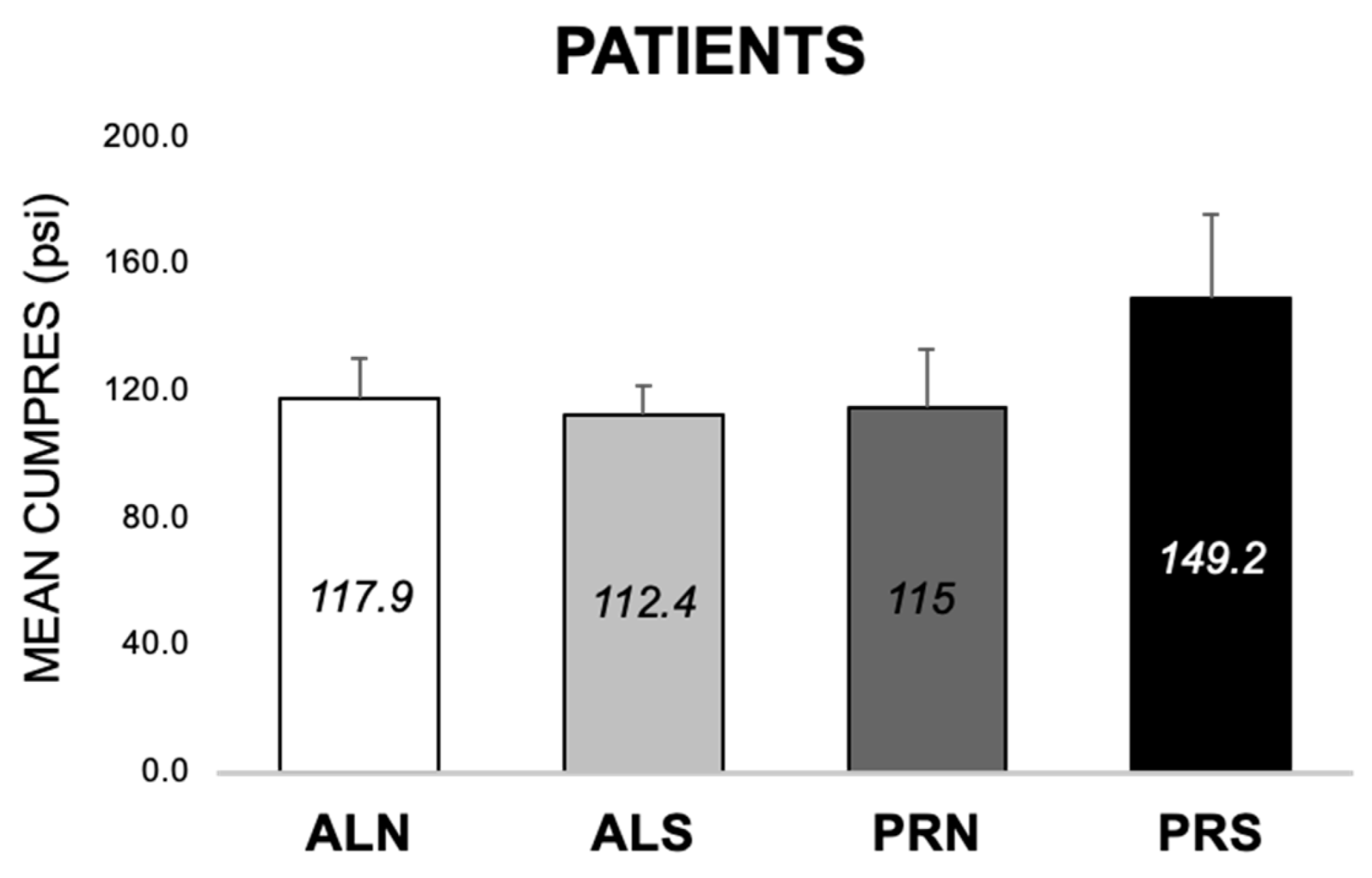
3.3.3. Differences across Conditions at 24-Month Follow-Up in Patients (E1, E2)
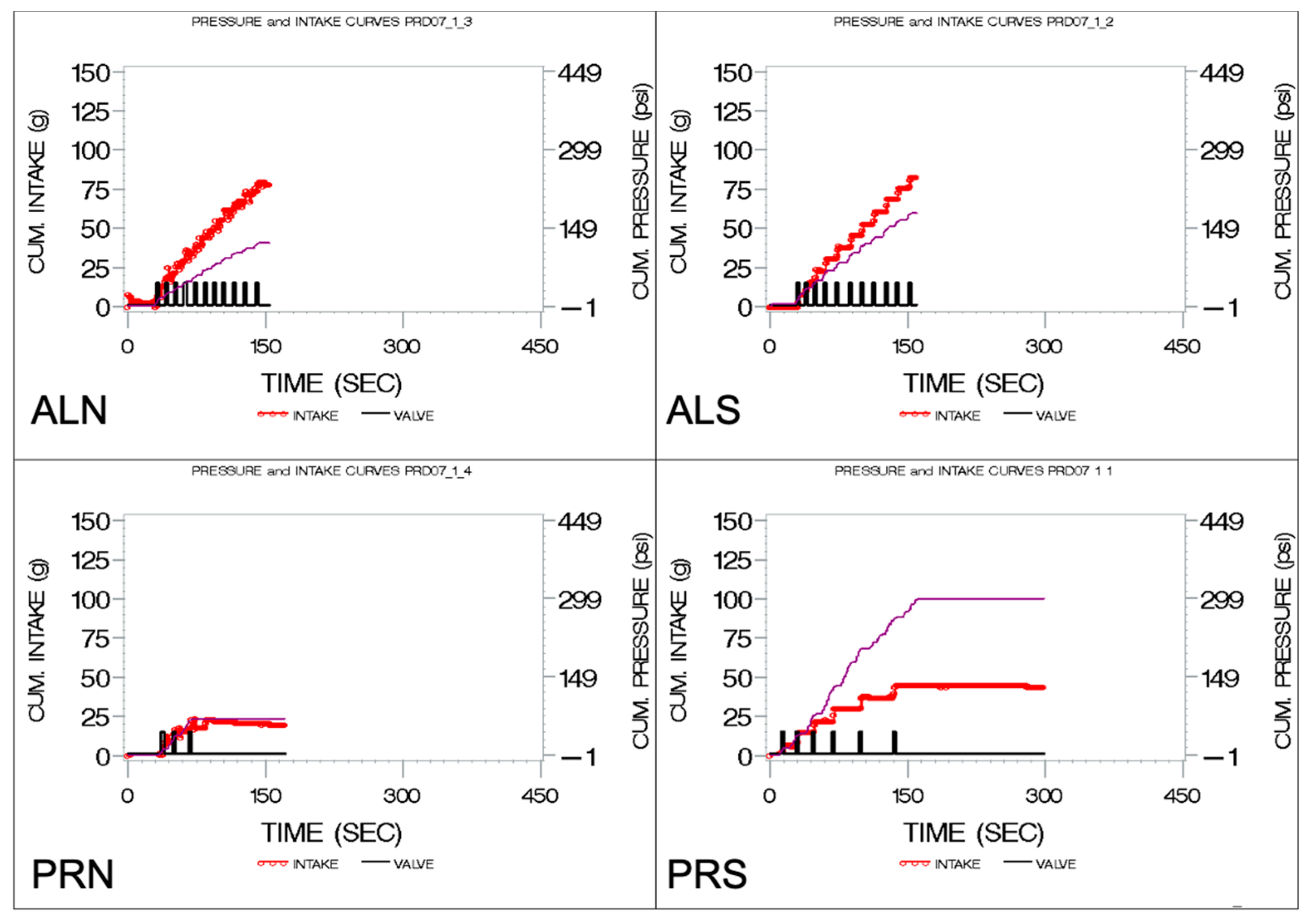
3.4. Linear Regressions of Cumulative Pressure (E5 and E9)
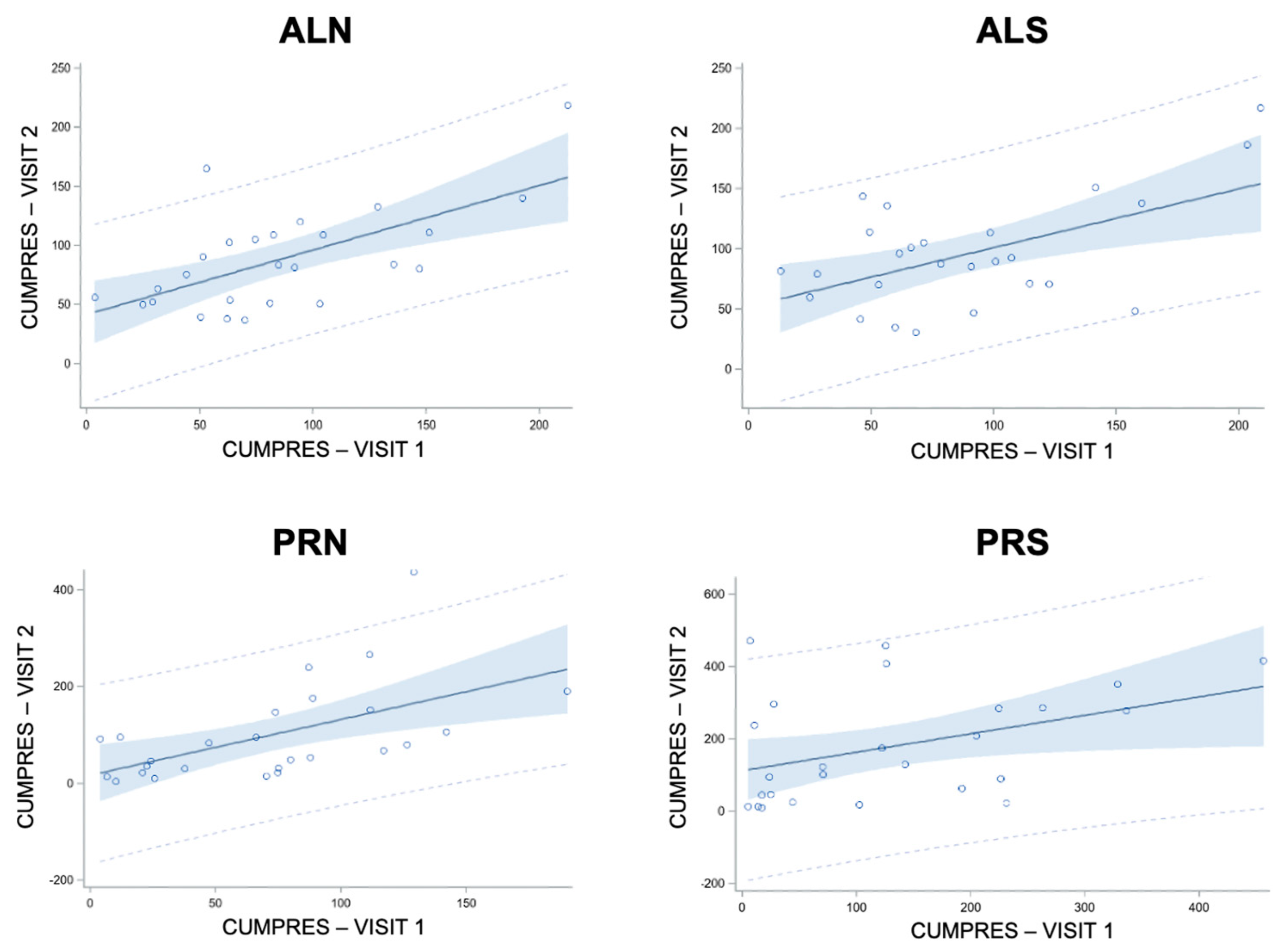
3.4.1. Linear Regressions of Cumulative Pressure at Follow-Up from Baseline in Controls (E9)
3.4.2. Linear Regressions of Cumulative Pressure at Follow-Up from Baseline in Patients (E5)
4. Discussion
4.1. Overall Findings
4.2. Specific Findings
4.2.1. Condition Differences (E1 and E2)
4.2.2. Visit and Surgery Differences (E3 and E4)
4.2.3. Group Differences (E6 and E7)
4.2.4. Control Differences (E8)
4.2.5. Regressions across Visits (E5 and E9)
4.3. Advantages
4.4. Limitations
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolpe, J. Need-reduction, drive-reduction, and reinforcement: A neurophysiological view. Psychol. Rev. 1950, 57, 19. [Google Scholar] [CrossRef]
- Seward, J.P. Drive, incentive, and reinforcement. Psychol. Rev. 1956, 63, 195. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, F.D.; Roby, T.B.; Campbell, B.A. Drive reduction versus consummatory behavior as determinants of reinforcement. J. Comp. Physiol. Psychol. 1954, 47, 349. [Google Scholar] [CrossRef] [PubMed]
- Pfaffmann, C. The pleasures of sensation. Psychol. Rev. 1960, 67, 253. [Google Scholar] [CrossRef]
- Weinstock, R.; White, R.T.; Bolles, R.C. Incentive value of saccharin as a function of concentration and deprivation conditions. Psychon. Sci. 1965, 3, 103–104. [Google Scholar] [CrossRef]
- De Francisco, J.C.; Dess, N.K. Aspartame consumption in rats selectively bred for high versus low saccharin intake. Physiol. Behav. 1998, 65, 393–396. [Google Scholar] [CrossRef]
- Cardello, H.M.A.B.; Da Silva, M.A.P.A.; Damasio, M.H. Measurement of the relative sweetness of stevia extract, aspartame and cyclamate/saccharin blend as compared to sucrose at different concentrations. Plant Foods Hum. Nutr. 1999, 54, 119–129. [Google Scholar] [CrossRef]
- Klein, D.A.; Schebendach, J.E.; Brown, A.J.; Smith, G.P.; Walsh, B.T. Modified sham feeding of sweet solutions in women with and without bulimia nervosa. Physiol. Behav. 2009, 96, 44–50. [Google Scholar] [CrossRef][Green Version]
- Perez-Leighton, C.E.; Hamm, J.D.; Shechter, A.; Tamura, S.; Laferrère, B.; Pi-Sunyer, X.; Albu, J.; Greenberg, D.; Kissileff, H.R. Preoperative liking and wanting for sweet beverages as predictors of body weight loss after Roux-en-Y gastric bypass and sleeve gastrectomy. Int. J. Obes. 2020, 44, 1350–1359. [Google Scholar] [CrossRef]
- Hogenkamp, P.S.; Shechter, A.; St-Onge, M.P.; Sclafani, A.; Kissileff, H. A sipometer for measuring motivation to consume and reward value of foods and beverages in humans: Description and proof of principle. Physiol. Behav. 2017, 171, 216–227. [Google Scholar] [CrossRef]
- Drewnowski, A.; Massien, C.; Louis-Sylvestre, J.; Fricker, J.; Chapelot, D.; Apfelbaum, M. Comparing the effects of aspartame and sucrose on motivational ratings, taste preferences, and energy intakes in humans. Am. J. Clin. Nutr. 1994, 59, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R. Effects of aspartame and sucrose on hunger and energy intake in humans. Physiol. Behav. 1990, 47, 1037–1044. [Google Scholar] [CrossRef]
- Booth, D.A.; Higgs, S.; Schneider, J.; Klinkenberg, I. Learned liking versus inborn delight: Can sweetness give sensual pleasure or is it just motivating? Psychol. Sci. 2010, 21, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Pfaffmann, C. Taste, its sensory and motivating properties. Am. Sci. 1964, 52, 187–206. [Google Scholar]
- Kissileff, H.R.; Herzog, M. Progressive ratio (PR) schedules and the sipometer: Do they measure wanting, liking, and/or reward? A tribute to Anthony Sclafani and Karen Ackroff. Appetite 2018, 122, 44–50. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol. Behav. 2003, 79, 663–670. [Google Scholar] [CrossRef]
- Al-Alsheikh, A.S.; Alabdulkader, S.; Johnson, B.; Goldstone, A.P.; Miras, A.D. Effect of obesity surgery on taste. Nutrients 2022, 14, 866. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, A.; Kovacs, P.; Ahmed, T.; Meirelles, K.; Lynch, C.J.; Cooney, R.N. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G967–G979. [Google Scholar] [CrossRef]
- Blonde, G.D.; Mathes, C.M.; Inui, T.; Hamel, E.A.; Price, R.K.; Livingstone, M.B.E.; le Roux, C.W.; Spector, A.C. Oromotor and somatic taste reactivity during sucrose meals reveals internal state and stimulus palatability after gastric bypass in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R204–R218. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Bradley, D.; Eagon, J.C.; Sullivan, S.; Abumrad, N.A.; Klein, S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity 2014, 22, E13–E20. [Google Scholar]
- Nance, K.; Eagon, J.C.; Klein, S.; Pepino, M.Y. Effects of sleeve gastrectomy vs. Roux-en-Y gastric bypass on eating behavior and sweet taste perception in subjects with obesity. Nutrients 2017, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Burge, J.C.; Schaumburg, J.Z.; Choban, P.S.; DiSilvestro, R.A.; Flancbaum, L. Changes in Patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J. Am. Diet. Assoc. 1995, 95, 666–670. [Google Scholar] [CrossRef]
- Bueter, M.; Miras, A.D.; Chichger, H.; Fenske, W.; Ghatei, M.A.; Bloom, S.R.; Unwun, R.J.; Lutz, T.A.; Spector, A.C.; le Roux, C.W. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol. Behav. 2011, 104, 709–721. [Google Scholar] [CrossRef]
- Bray, G.A.; Barry, R.E.; Benfield, J.R.; Castelnuovo-Tedesco, P.; Rodin, J. Intestinal bypass surgery for obesity decreases food intake and taste preferences. Am. J. Clin. Nutr. 1976, 29, 779–783. [Google Scholar] [CrossRef]
- Miras, A.D.; Jackson, R.N.; Jackson, S.N.; Goldstone, A.P.; Olbers, T.; Hackenberg, T.; Spector, A.C.; le Roux, C.W. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 2012, 96, 467–473. [Google Scholar] [CrossRef]
- Gero, D.; Bueter, M. Post-bariatric changes in ingestive behavior: Shift in macronutrient preferences in rats and dynamic adaptation of the within-meal microstructure in humans. Physiol. Behav. 2023, 263, 114113. [Google Scholar] [CrossRef]
- Geary, N.; Bächler, T.; Whiting, L.; Lutz, T.A.; Asarian, L. RYGB progressively increases avidity for a low-energy, artificially sweetened diet in female rats. Appetite 2016, 98, 133–141. [Google Scholar] [CrossRef][Green Version]
- Hodos, W. Progressive ratio as a measure of reward strength. Science 1961, 134, 943–944. [Google Scholar] [CrossRef]
- Bodell, L.P.; Keel, P.K. Weight suppression in bulimia nervosa: Associations with biology and behavior. J. Abnorm. Psychol. 2015, 124, 994. [Google Scholar] [CrossRef] [PubMed]
- Schebendach, J.; Broft, A.; Foltin, R.W.; Walsh, B.T. Can the reinforcing value of food be measured in bulimia nervosa? Appetite 2013, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Keel, P.K.; Kennedy, G.A.; Rogers, M.L.; Joyner, K.J.; Bodell, L.P.; Forney, K.J.; Duffy, M.E. Reliability and validity of a transdiagnostic measure of reward valuation effort. Psychol. Assess. 2022, 34, 419. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Carr, K.A. Food reinforcement and habituation to food are processes related to initiation and cessation of eating. Physiol. Behav. 2021, 239, 113512. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Monteleone, E. Food preferences and obesity. Endocrinol. Metab. 2021, 36, 209–219. [Google Scholar] [CrossRef]
- Iatridi, V.; Armitage, R.M.; Yeomans, M.R.; Hayes, J.E. Effects of sweet-liking on body composition depend on age and lifestyle: A challenge to the simple sweet-liking—Obesity hypothesis. Nutrients 2020, 12, 2702. [Google Scholar] [CrossRef]
- Sclafani, A.; Nissenbaum, J.W. Robust conditioned flavor preference produced by intragastric starch infusions in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1988, 255, R672–R675. [Google Scholar] [CrossRef]
- Bertino, M.; Beauchamp, G.K.; Riskey, D.R.; Engelman, K. Taste perception in three individuals on a low sodium diet. Appetite 1981, 2, 67–73. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Moran, M. Dietary experience and sweet taste preference in human infants. Appetite 1982, 3, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Watamura, S.E.; Donzella, B.; Kertes, D.A.; Gunnar, M.R. Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2004, 45, 125–133. [Google Scholar] [CrossRef]
- Capaldi, E.D.; Privitera, G.J. Decreasing dislike for sour and bitter in children and adults. Appetite 2008, 50, 139–145. [Google Scholar] [CrossRef]
- Klein, D.A.; Schebendach, J.E.; Gershkovich, M.; Smith, G.P.; Walsh, B.T. Modified sham feeding of sweet solutions in women with anorexia nervosa. Physiol. Behav. 2010, 101, 132–140. [Google Scholar] [CrossRef][Green Version]
- Hayes, J.E.; DePasquale, D.A.; Moser, S.E. Asymmetric dominance as a potential source of bias in hedonic testing. Food Qual. Prefer. 2011, 22, 559–566. [Google Scholar] [CrossRef]
- Privitera, G.J.; Mulcahey, C.P.; Orlowski, C.M. Human sensory preconditioning in a flavor preference paradigm. Appetite 2012, 59, 414–418. [Google Scholar] [CrossRef]
- Weafer, J.; Burkhardt, A.; de Wit, H. Sweet taste liking is associated with impulsive behaviors in humans. Front. Behav. Neurosci. 2014, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Schebendach, J.; Klein, D.A.; Mayer, L.E.; Attia, E.; Devlin, M.J.; Foltin, R.W.; Walsh, B.T. Assessment of the motivation to use artificial sweetener among individuals with an eating disorder. Appetite 2017, 109, 131–136. [Google Scholar] [CrossRef]
- Kissileff, H.R. The Universal Eating Monitor (UEM): Objective assessment of food intake behavior in the laboratory setting. Int. J. Obes. 2022, 46, 1114–1121. [Google Scholar] [CrossRef]
- Smith, K.R.; Papantoni, A.; Veldhuizen, M.G.; Kamath, V.; Harris, C.; Moran, T.H.; Carnell, S.; Steele, K.E. Taste-related reward is associated with weight loss following bariatric surgery. J. Clin. Investig. 2020, 130, 4370–4381. [Google Scholar] [CrossRef] [PubMed]
- Gero, D.; File, B.; Alceste, D.; Frick, L.D.; Serra, M.; Ismaeil, A.E.; Steinert, R.E.; Spector, A.C.; Bueter, M. Microstructural changes in human ingestive behavior after Roux-en-Y gastric bypass during liquid meals. JCI Insight 2021, 6, e136842. [Google Scholar] [CrossRef]
- Nicanor-Carreón, J.G.; Seyedsadjadi, N.; Rowitz, B.; Pepino, M.Y. Weight Regain and Ingestive Behavior in Women after Metabolic Surgery. Nutrients 2023, 15, 3670. [Google Scholar] [CrossRef]
- Freire, R.H.; Borges, M.C.; Alvarez-Leite, J.I.; Correia MI, T.D. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition 2012, 28, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Dagan, S.S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Sandbank, G.K.; Ben-Porat, T.; Sinai, T. Nutritional recommendations for adult bariatric surgery patients: Clinical practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Alceste, D.; Serra, M.; Raguz, I.; Gero, D.; Thalheimer, A.; Widmer, J.; File, B.; Ismaeil, A.; Steinert, R.E.; Spector, A.C.; et al. Association between microstructure of ingestive behavior and body weight loss in Patients one year after Roux-en-Y gastric bypass. Physiol. Behav. 2022, 248, 113728. [Google Scholar] [CrossRef]
- Klein, D.A.; Schebendach, J.S.; Devlin, M.J.; Smith, G.P.; Walsh, B.T. Intake, sweetness and liking during modified sham feeding of sucrose solutions. Physiol. Behav. 2006, 87, 602–606. [Google Scholar] [CrossRef]
- Jayasinghe, S.N.; Kruger, R.; Walsh, D.C.; Cao, G.; Rivers, S.; Richter, M.; Breier, B.H. Is sweet taste perception associated with sweet food liking and intake? Nutrients 2017, 9, 750. [Google Scholar] [CrossRef]
- McInnis, K.; Brown, J.L.; Finlayson, G.; Dent, R.; Doucet, É. Appetite changes in weight regain and weight maintenance after Roux-en-Y gastric bypass. Obes. Surg. 2022, 32, 1–12. [Google Scholar] [CrossRef]
- Lynch, A. “When the honeymoon is over, the real work begins”: Gastric bypass Patients’ weight loss trajectories and dietary change experiences. Soc. Sci. Med. 2016, 151, 241–249. [Google Scholar] [CrossRef]
- Nasirzadeh, Y.; Kantarovich, K.; Wnuk, S.; Okrainec, A.; Cassin, S.E.; Hawa, R.; Sockalingam, S. Binge eating, loss of control over eating, emotional eating, and night eating after bariatric surgery: Results from the Toronto Bari-PSYCH Cohort Study. Obes. Surg. 2018, 28, 2032–2039. [Google Scholar] [CrossRef]
- Ünal, Ş.; Sevçnçer, G.M.; Maner, A.F. Prediction of Weight Regain after Bariatric Surgery by Night Eating, Emotional Eating, Eating Concerns, Depression and Demographic Characteristics. Turk. J. Psychiatry 2019, 30, 31–41. [Google Scholar]
- Novelli, I.R.; Fonseca, L.G.; Gomes, D.L.; Dutra, E.S.; de Carvalho, K.M.B. Emotional eating behavior hinders body weight loss in women after Roux-en-Y gastric bypass surgery. Nutrition 2018, 49, 13–16. [Google Scholar] [CrossRef]
- Miller-Matero, L.R.; Bryce, K.; Saulino, C.K.; Dykhuis, K.E.; Genaw, J.; Carlin, A.M. Problematic eating behaviors predict outcomes after bariatric surgery. Obes. Surg. 2018, 28, 1910–1915. [Google Scholar] [CrossRef]
- Hamm, J.D.; Dotel, J.; Tamura, S.; Shechter, A.; Herzog, M.; Brunstrom, J.M.; Albu, J.; Pi-Sunyer, F.X.; Laferrère, B.; Kissileff, H.R. Reliability and responsiveness of virtual portion size creation tasks: Influences of context, foods, and a bariatric surgical procedure. Phys. Behav. 2020, 223, 113001. [Google Scholar] [CrossRef] [PubMed]

| Demographics | Patients n = 56 | Controls n = 28 |
|---|---|---|
| Age, y | 34.9 ± 1.3 | 34.0 ± 2.1 |
| Sex | 89% F/11% M | 79% F/21% M |
| Race | 55% B/45% NB | 61% B/39% NB |
| Ethnicity | 64% H/36% NH | 68% H/32% NH |
| Surgery type | 38% R/62% V | - |
| Weight, kg | 121.7 ± 2.6 2 | 59.8 ± 1.3 |
| BMI, kg/m2 | 44.8 ± 0.8 2 | 21.8 ± 0.32 |
| Weight loss, 3 months, kg | 20.7 ± 0.7 | −0.4 ± 0.4 |
| Weight loss, 3 months, % | 17.1 ± 0.5 | −0.7 ± 0.6 |
| Weight loss, 24 months, kg 3 | 34.4 ± 2.1 | - |
| Weight loss, 24 months, % 3 | 28.1 ± 1.6 | - |
| Effect | df | F-Value | p-Value |
|---|---|---|---|
| Group | 1 | 0.71 | 0.40 |
| Visit | 1 | 0.05 | 0.83 |
| Condition | 3 | 2.02 | 0.16 |
| Group × Visit | 1 | 10.90 | <0.0001 |
| Group × Condition | 3 | 4.17 | 0.006 |
| Visit × Condition | 3 | 0.28 | 0.84 |
| Group × Visit × Condition | 3 | 0.40 | 0.75 |
| Effect | df | F-Value | p-Value |
|---|---|---|---|
| Visit | 2 | 2.22 | 0.11 |
| Condition | 3 | 1.68 | 0.17 |
| Visit × Condition | 6 | 0.50 | 0.81 |
| Cond | F-Value | Rsq | Slope ± SE | Slope P | Intercept ± SE | Intercept P |
|---|---|---|---|---|---|---|
| ALN | 16.79 | 0.41 | 0.55 ± 0.13 | 0.0004 | 41.72 ± 13.21 | 0.004 |
| ALS | 10.59 | 0.31 | 0.49 ± 0.15 | 0.003 | 52.41 ± 15.39 | 0.002 |
| PRN | 11.03 | 0.31 | 1.15 ± 0.35 | 0.003 | 17.48 ± 29.53 | 0.56 |
| PRS | 4.82 | 0.17 | 0.51 ± 0.23 | 0.04 | 112.96 ± 41.35 | 0.01 |
| Dep | Indep | Cond | F-Value | Rsq | Slope ± SE | Slope P | Intercept ± SE | Intercept P |
|---|---|---|---|---|---|---|---|---|
| V2 | V1 | ALN | 0.00 | 0.00 | −0.005 ± 0.19 | 0.98 | 92.90 ± 20.44 | <0.0001 |
| ALS | 0.23 | 0.005 | 0.09 ± 0.19 | 0.64 | 96.37 ± 21.34 | <0.0001 | ||
| PRN | 0.00 | 0.00 | 0.004 ± 0.12 | 0.97 | 109.14 ± 25.63 | 0.0001 | ||
| PRS | 6.87 | 0.14 | 0.31 ± 0.12 | 0.01 | 63.40 ± 19.86 | 0.003 | ||
| V3 | V2 | ALN | 4.92 | 0.10 | 0.39 ± 0.18 | 0.03 | 81.59 ± 20.25 | 0.0002 |
| ALS | 21.61 | 0.34 | 0.53 ± 0.11 | <0.0001 | 56.21 ± 14.48 | 0.0004 | ||
| PRN | 2.99 | 0.07 | 0.22 ± 0.13 | 0.09 | 90.60 ± 22.79 | 0.0003 | ||
| PRS | 4.89 | 0.10 | 0.63 ± 0.29 | 0.03 | 83.39 ± 39.10 | 0.04 | ||
| V3 | V1 | ALN | 0.83 | 0.02 | 0.20 ± 0.22 | 0.37 | 98.63 ± 24.51 | 0.0002 |
| ALS | 7.29 | 0.15 | 0.43 ± 0.16 | 0.01 | 70.09 ± 18.09 | 0.0004 | ||
| PRN | 8.93 | 0.18 | 0.29 ± 0.10 | 0.005 | 82.16 ± 20.09 | 0.0002 | ||
| PRS | 6.99 | 0.14 | 0.61 ± 0.23 | 0.01 | 71.67 ± 38.73 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamm, J.D.; Laferrère, B.; Albu, J.B.; Kini, S.; Pi-Sunyer, X.; Kissileff, H.R. Responsiveness and Reliability of a Sipping Device to Measure Motivation in Normal-Weight Individuals and Bariatric Surgery Patients. Nutrients 2024, 16, 3001. https://doi.org/10.3390/nu16173001
Hamm JD, Laferrère B, Albu JB, Kini S, Pi-Sunyer X, Kissileff HR. Responsiveness and Reliability of a Sipping Device to Measure Motivation in Normal-Weight Individuals and Bariatric Surgery Patients. Nutrients. 2024; 16(17):3001. https://doi.org/10.3390/nu16173001
Chicago/Turabian StyleHamm, Jeon D., Blandine Laferrère, Jeanine B. Albu, Subhash Kini, Xavier Pi-Sunyer, and Harry R. Kissileff. 2024. "Responsiveness and Reliability of a Sipping Device to Measure Motivation in Normal-Weight Individuals and Bariatric Surgery Patients" Nutrients 16, no. 17: 3001. https://doi.org/10.3390/nu16173001
APA StyleHamm, J. D., Laferrère, B., Albu, J. B., Kini, S., Pi-Sunyer, X., & Kissileff, H. R. (2024). Responsiveness and Reliability of a Sipping Device to Measure Motivation in Normal-Weight Individuals and Bariatric Surgery Patients. Nutrients, 16(17), 3001. https://doi.org/10.3390/nu16173001





