Abstract
Multiple sclerosis (MS) is a chronic autoimmune disorder characterized by inflammation, demyelination, and neurodegeneration, resulting in significant disability and reduced quality of life. Current therapeutic strategies primarily target immune dysregulation, but limitations in efficacy and tolerability highlight the need for alternative treatments. Plant-derived compounds, including alkaloids, phenylpropanoids, and terpenoids, have demonstrated anti-inflammatory effects in both preclinical and clinical studies. By modulating immune responses and promoting neuroregeneration, these compounds offer potential as novel adjunctive therapies for MS. This review provides insights into the molecular and cellular basis of MS pathogenesis, emphasizing the role of inflammation in disease progression. It critically evaluates emerging evidence supporting the use of plant-derived compounds to attenuate inflammation and MS symptomology. In addition, we provide a comprehensive source of information detailing the known mechanisms of action and assessing the clinical potential of plant-derived compounds in the context of MS pathogenesis, with a focus on their anti-inflammatory and neuroprotective properties.
1. Introduction
Multiple sclerosis (MS) is a chronic autoimmune disorder of the central nervous system (CNS) characterized by inflammation, demyelination, and neurodegeneration [1]. Its increasing prevalence positions it as the most common progressive neurologic pathology among young adults globally [2]. Multiple sclerosis typically manifests through a variety of neurological symptoms, including motor dysfunction, sensory deficits, fatigue, and cognitive impairment, leading to significant disability and reduced quality of life [3].
The etiology of MS involves complex interactions between genetic predisposition, environmental factors, and dysregulated immune responses [4]. Although the exact cause remains elusive, it is widely accepted that aberrant immune activation, particularly by autoreactive T cells, plays a pivotal role in initiating and perpetuating the inflammatory cascade within the CNS [5]. This immune dysregulation leads to the infiltration of immune cells across the blood-brain barrier (BBB), resulting in the release of pro-inflammatory cytokines, oxidative stress, and subsequent damage to myelin sheaths and neurons [6].
Current therapeutic strategies for MS primarily focus on modulating immune responses to reduce inflammation and halt disease progression [7]. Current treatments, including disease-modifying therapies (DMTs), symptomatic treatments, acute relapse treatments, rehabilitation therapies, and lifestyle modifications, have shown efficacy in mitigating relapses and delaying the onset of disability [8]. However, their long-term safety profiles and limited effectiveness in progressive forms of MS underscore the need for alternative treatment modalities [8].
Recently, especially in the past decade, there has been growing interest in exploring the therapeutic potential of plant-derived compounds for managing MS-associated inflammation [9,10]. Phytochemicals, including alkaloids, phenylpropanoids, and terpenoids, have demonstrated anti-inflammatory, antioxidant, and potential neuroprotective properties in both pre-clinical and clinical studies [11]. These compounds exert their effects through various mechanisms, including inhibiting pro-inflammatory mediators, modulating immune cell function, and promoting neuroregeneration.
A recently published review focused on the molecular mechanism of some polyphenols’ protective benefits against MS with a focus on the role of gut microbiota in MS etiopathogenesis [12]. This review aims to provide a comprehensive overview of the pathophysiology of MS, with a particular emphasis on the role of inflammation in disease progression. Furthermore, we will critically evaluate the emerging evidence supporting the use of select plant-derived compounds as adjunctive therapies to attenuate inflammation-induced MS symptomology. By elucidating the underlying mechanisms of action and assessing the clinical efficacy of these natural agents, it will encourage the development of novel therapeutic strategies that offer greater efficacy and tolerability for individuals living with MS.
2. Molecular and Cellular Basis of MS Pathogenesis
2.1. Immune Cell Dynamics in the Brain and Central Nervous System (CNS)
Antigen presentation is central to immune surveillance in the brain. In this process, antigen presenting cells (APCs) sample and present CNS-derived antigens to helper T cells, modulating immune responses [13]. Regulatory mechanisms within the CNS, including regulatory T cells, soluble immunomodulatory factors, and the BBB, tightly regulate immune activation and tolerance, ensuring immune homeostasis within the CNS microenvironment [14]. In this section, we will provide an overview of these normal immune responses, as they are crucial for understanding the pathogenesis of MS.
2.2. Antigen Presenting Cells and the Antigen
Upon encountering foreign or self-derived antigens, APCs, such as dendritic cells, macrophages, or B cells, utilize pattern recognition receptors (PRRs) to identify and internalize the antigen through a process known as phagocytosis [15]. Following antigen internalization, the APC processes the antigens into smaller peptide fragments within a specialized intracellular compartment called a phagolysosome, which is the product of the fusion of a phagosome and a lysosome [16]. These peptide fragments are then loaded onto major histocompatibility complexes (MHC), forming MHC–peptide complexes [17]. Through antigen presentation, APCs effectively display these MHC–peptide complexes on their cell surface, where they can be recognized and engaged by T cell receptors (TCRs) expressed on the surface of helper T cells [17]. The interaction between MHC–peptide complexes and TCRs initiates a cascade of immune responses, including T cell activation, proliferation, and differentiation, which collectively tailor the adaptive immune response to combat the specific antigen that was initially encountered by the APC [18].
2.3. Neuroinflammation and Demyelination: Molecular Mimicry
In-depth investigations, tracing back to seminal studies from 1935, have sought to explore the similarities and differences between MS and its experimental autoimmune encephalomyelitis (EAE) model [19]. Initially, certain types of immune cells were perceived as the main instigators of CNS demyelination, with specific subsets of CD4+ T cells having been believed to multiply in the brain and release cytokines that harm myelin [20]. However, recent findings have challenged this viewpoint, suggesting that additional immune cell populations, including macrophages, CD8+ T cells, and B cells, also play significant roles in the inflammatory response seen in both EAE and MS lesions [5,21].
Contrary to previous assumptions, doubts have been raised about the harmful effects of certain cytokines on myelin in MS lesions, with emerging evidence pointing towards the potential importance of cytotoxic CD8+ T cells in demyelination [22]. These cells can identify oligodendrocytes and/or myelin antigens due to their expression of specific molecules under inflammatory circumstances, implying a primary involvement in an antigen-focused inflammatory reaction [23]. Moreover, B lymphocytes have emerged as notable contributors to MS pathology, as evidenced by therapeutic trials targeting molecules on these cells, resulting in decreased lesion formation and relapses [24].
Overall, this developing comprehension of MS pathology emphasizes the roles of CD8+ T cells and B lymphocytes in addition to that of CD4+ T-helper cells [25]. Additionally, interactions between T cells and certain glial cells, particularly oligodendrocytes, are crucial for CNS balance and autoimmune diseases like MS [26]. The concept of molecular mimicry adds complexity to this interaction, as foreign substances that resemble CNS antigens can incite self-directed T cell responses, perpetuating neuroinflammatory processes [27].
2.4. Inflammatory Cytokine Pathways Involved in MS Pathogenesis
It is thought that the dysregulation of pro-inflammatory cytokine production, including IL-1, IL-6, IFN-γ, and TNF-α, contributes to the pathogenesis and progression of MS [28,29]. These cytokines are often produced in excess and contribute to chronic inflammation, demyelination, and neurodegeneration within the CNS [30,31]. These cytokines collectively promote the recruitment and activation of both helper T cells and macrophages in the CNS [32]. This leads to the formation of inflammatory lesions, breakdown of the BBB, and subsequent damage to myelin and neurons [33]. IFN-γ, primarily produced by activated T cells, further exacerbates inflammation and tissue damage by activating microglia, leading to the release of cytotoxic factors and oxidative stress [34]. Additionally, dysregulated cytokine signaling disrupts the balance between pro-inflammatory and anti-inflammatory mediators, contributing to the chronic and relapsing-remitting nature of MS [35,36,37]. Targeting these dysregulated cytokines through immunomodulatory therapies represents a promising approach for the treatment of MS as this would attenuate inflammation, protect neuronal integrity, and ultimately improve clinical outcomes for affected individuals [38].
2.4.1. TNF-α Signaling
TNF-α is produced by macrophages, monocytes, and T lymphocytes in response to various stimuli such as infection, inflammation, or tissue injury [39]. The production of TNF-α is tightly regulated at the transcriptional and post-transcriptional levels and is only upregulated when the aforementioned conditions are met [40]. The effect of TNF-α as a binding substrate depends largely on the tissue type and TNF-α receptor to which the cytokine binds [39]. To date, there are two known types of TNF-α receptors, TNFR1 (also known as p55 or CD120) and TNFR2 (also known as p75 or CD120b) [41]. TNFR1 is widely expressed and primarily involved in mediating pro-inflammatory and apoptotic signals, while TNFR2 is expressed on immune cells and contributes to immune regulation and tissue repair processes [42]. Under the same convention, the binding of TNF-α to either of these receptors will elicit unique signaling mechanisms, coined as the canonical and non-canonical pathways [43].
The canonical pathway, primarily associated with TNFR1 signaling, begins with the receptor trimerization and assembly of a multiprotein signaling complex along the intracellular domain of TNFR1 [44]. Within this complex, there is an array of adaptor proteins, the first of which is TNF-α receptor-associated death domain (TRADD), as both this adaptor protein and the receptor itself share complimentary death domains [45] (Figure 1). In a similar fashion, receptor-interacting protein-1 (RIP-1) possesses a death domain that allows its articulation with the upstream receptor complex [46]. Additionally, RIP-1 can undergo both polyubiquitination (K63-linked chains) and phosphorylation (S14, S15, S20, and S166) through autophosphorylation and feedback mechanisms from downstream/crosstalk interactions (IκB kinase β and associated TRAFs) [47,48]. Regardless of the catalyst, phosphorylation along these serine residues leads to the activation of RIP-1 as a serine/threonine kinase [48]. TNF-α receptor-associated factor 2 (TRAF2) is then recruited to the growing signaling complex and activated via RIP-1-mediated serine phosphorylation (residue unknown) [49]. Following the formation of the TNFR1 receptor/membrane complex, additional cellular inhibitors of apoptosis 1 and 2 (cIAP1/2) are recruited and bound to TRAF2 [50]. Once bound, cIAP1/2, known formally as E3 ubiquitin ligases, facilitates the ubiquitination of RIP-1 proteins within the same complex [51]. The polyubiquitinated K63-linked chains serve as molecular scaffolding for the IkB [Inhibitor of kB] kinase complex (IKK) [52]. This IKK complex is primarily composed of three domains: IKKα (catalytic), IKKβ (catalytic), and NF-κβ essential modulator (NEMO; regulatory) subunits [53]. At rest, NF-κβ dimers, typically composed of p50 and p65 (RelA), are sequestered in the cytoplasm by inhibitory proteins (IkB) [54]. The activated IKK complex, specifically IKKα, phosphorylates IkBα, the p50/RelA sequestration agent of IkB, along serine residues (S32 and S36) located on the destruction box [55,56]. This culminates in the generation of a phosphodegron motif, which serves as a recognition particle for Beta-Transducing Repeat-containing Protein (βTrCP) [55,56]. βTrCP bound to phosphorylated IkBα orchestrates the polyubiquitination and proteasomal degradation of the IkB subunit [57]. Successively, the liberated p50/RelA dimer freely enters the nucleus and regulates the transcription of antiapoptotic genes [58].
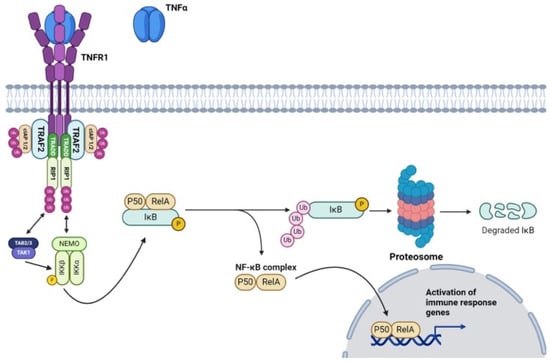
Figure 1.
The canonical TNF-α signaling pathway activates NF-κB through TNF receptor binding, involving TRADD, RIP-1, and the IKK complex, leading to the transcription of various genes that are involved in the immune response (Created with https://www.biorender.com/ accessed on 3 July 2024).
The non-canonical pathway, initiated primarily by TNFR2 receptor activation, does not involve the recruitment of TRADD [59]. Instead, TNFR2 activation leads to the formation of a complex involving TRAF2, TNF receptor-associated factor 3 (TRAF3), and the E3 ubiquitin ligases cIAPs [60]. Once this complex is formed, cIAPs facilitate the ubiquitination of TRAF3 and its subsequent degradation [61]. In its activated form, this complex activates the NF-κB-inducing kinase (NIK), which subsequently phosphorylates and activates IKKα [53]. If there is no receptor activation, TRAF3 remains bound to NIK and promotes its ubiquitination [62]. Activated IKKα then phosphorylates p100, leading to its proteasomal processing into the active form of NF-κβ subunit RelB [63]. The RelB is then dimerized with p52 and subsequently translocated to the nucleus to regulate the transcription of genes, including bcl-2 and bcl-xl, involved in immune cell survival, proliferation, and differentiation [64].
Nuclear translocation of both canonical- and noncanonical-activated dimers enhances the transcription of additional pro-inflammatory mediators, including IL-1β, IL-6, IL-8, and TNF-α [65]. Additionally, NF-κβ activation induces histone acetylation, facilitating the transcription of various genes, including those encoding pro-inflammatory cytokines [66]. When coupled, these pathways elicit an additive effect that continuously exacerbates pro-inflammatory mechanisms in a positive feedback fashion [67].
2.4.2. IL-1 Signaling
The IL-1 family consists of 11 known members, of which, IL-1α, IL-1β, and IL-18 are most notable in MS pathogenesis [68]. The synthesis of IL-1 family cytokines shares several similarities with the synthesis of other cytokines [69]. It is primarily regulated by the binding of antigens to PRRs on innate immune system cells [70]. Upon activation, immune cells initiate signaling cascades that lead to the activation of transcription factors such as NF-κB and activator protein 1 (AP-1) [71]. NF-κβ and AP-1 bind to specific regulatory regions in the IL-1 gene promoter, driving the transcription of IL-1 mRNA [72]. To date, there are two known isoforms of IL-1 (IL-1α and IL-1β) that contribute to inflammatory regulation in MS [73]. Once translated, the inactive, precursory Pro-IL-1 protein undergoes maturation through caspase activation, producing active IL-1 [74].
IL-1 signaling orchestrates a spectrum of pro- and anti-inflammatory tissue responses [75]. The macroscopic outcome of the immune cell response largely hinges on the plasma receptor to which the cytokine binds [76]. IL-1β, much like other cytokine ligands, has a dedicated heterodimeric receptor complex comprised of integral proteins IL-1 Receptor Type 1 (IL-1R1) and IL-1 Receptor Accessory Protein [77] (Figure 2). When IL-1β binds to this complex, the myeloid differentiation primary response 88 (MYD88) adaptor protein is recruited to the cytoplasmic domain of the receptor complex through its Toll/IL-1 receptor (TIR) domain [78]. MYD88 then undergoes homodimerization, which facilitates the formation of a signaling complex [79]. This event is followed by the recruitment of IL-1 Receptor-Associated Kinases 1, 2, and 4 (IRAK1/2/4) [80]. IRAK4, believed to undergo autophosphorylation upon complex binding, then phosphorylates IRAK1 (S376/T387) and IRAK2 (S386/T399) [81,82,83]. TNF-α Receptor-Associated Factor 6 (TRAF6) then binds to the phosphorylated IRAK1 and subsequently undergoes a conformational change and undergoes auto-ubiquitination, forming K63-linked polyubiquitin chains [84]. Activated TRAF6 serves as a crucial signaling node, mediating downstream signaling events through the activation of various intracellular signaling pathways, including the NF-κB and mitogen-activated protein kinase (MAPK) pathways [85]. Additionally, TRAF6 polyubiquitination can lead to proteasomal degradation in cell-mediated inflammatory responses, though what facilitates the fate of the IL-1β cascade remains unclear [86]. TRAF6 ubiquitination events lead to the recruitment and activation of transforming growth factor-beta-activated kinase 1 (TAK1) and its binding partners, TAK1-Binding Protein 1 (TAB1), TAB2, and TAB3. TAB2 and TAB3 possess specific K63-linked ubiquitin chain binding domains, known as ubiquitin-binding in ABIN and NEMO, which facilitate their articulation with the scaffold of polyubiquinated-TRAF6 [87,88]. TAB2 and TAB3 then recruit and activate TAK1 [89]. TAK1, a serine/threonine kinase, subsequently phosphorylates the IKK (S177 and S181) complex and MAPKs (MKK3; S189/T193, MKK4; S257/T261, MKK6; S207/T211, and MKK7; S271/T275), leading to the activation of NF-κB and MAPK signaling cascades, respectively [52,90,91]. Activation of these pathways results in the transcriptional regulation of genes involved in inflammatory responses, immune cell activation, and other cellular processes [92]. One such response is the further transcription of successive cytokines, including IL-6 and IL-17 [93,94].
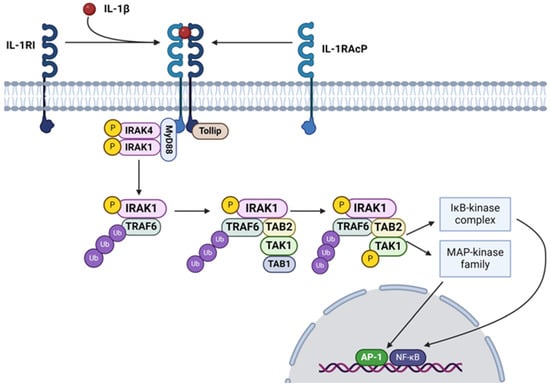
Figure 2.
The IL-1β signaling pathway activates NF-κB and MAPKs through the IL-1 receptor, leading to the transcription of genes involved in inflammation and immune responses (Created with https://www.biorender.com/ accessed on 3 July 2024).
2.4.3. IL-6 Signaling and the JAK/STAT Pathway
IL-6, like IL-1 and TNF-α, is characterized as a pro-inflammatory cytokine [95]. In its classical signaling pathway, IL-6 binds to its heterodimeric receptor complex comprising the IL-6 receptor (IL-6R) and glycoprotein 130 (Gp130) [96]. IL-6R exists in two forms: membrane-bound IL-6R (mIL-6R) and soluble IL-6R (sIL-6R) [97]. Membrane-bound IL-6R is expressed on the surfaces of several cell types, including hepatocytes, leukocytes, and some epithelial cells [98]. Upon binding to IL-6, mIL-6R undergoes conformational changes and forms a complex with the signal-transducing receptor subunit, Gp130 [99]. Gp130 lacks intrinsic kinase activity but is associated with Janus kinases (JAKs), particularly JAK1 and JAK2, which are activated in response to cytokine binding to the receptor complex [100]. Upon activation, JAKs phosphorylate tyrosine residues on the cytoplasmic tail of Gp130, providing docking sites for signal transducer and activator of transcription (STAT) proteins [101]. Once recruited to the receptor complex, STAT proteins become phosphorylated by JAKs along Src homology (SH2) domains and form homo- or hetero-dimers [94]. These STAT dimers translocate to the nucleus, where they regulate the transcription of target genes involved in immune responses, inflammation, cell proliferation, and differentiation [102].
2.4.4. IFN-γ Signaling
IFN-γ signaling is primarily associated with orchestrating inflammation and cell-mediated immune responses [103]. However, recent studies show that IFN-γ may be involved in promoting tumor progression [104]. The production of IFN-γ is tightly regulated by upstream cytokines, notably IL-12 and IL-18, which are primarily secreted by APCs [105]. There are three types of receptors for IFNs that are expressed in nucleated cells [106] (Figure 3). Type I IFN receptors exhibit the broadest specificity, responding to various IFNs, including IFN-α, IFN-β, IFN-τ, IFN-δ, IFN-ɛ, and IFN-ω [107]. Type II IFN receptors, on the other hand, are specifically activated by IFN-γ binding [108]. These receptors exist as tetramers composed of two IFNγR1 and two IFNγR2 subunits. In contrast, type III receptors are highly selective for IFN-λ signaling [109]. Mechanistically, IFN-γ transduces its signals through the JAK/STAT pathway, wherein STAT proteins serve as the primary transcription factors for IFN-γ-induced gene expression [110]. This intricate signaling cascade regulates a wide array of cellular responses, ranging from immune modulation to inflammatory processes, highlighting the diverse roles of IFN-γ in health and disease [111].
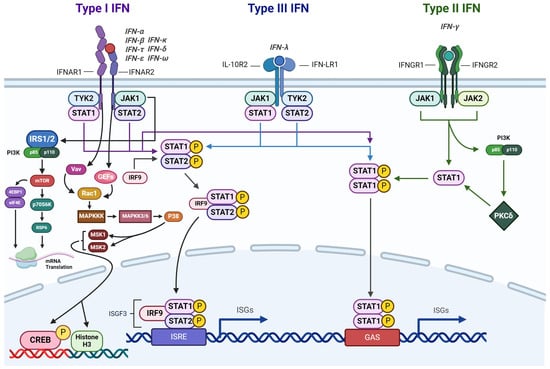
Figure 3.
IFN signaling activates the JAK-STAT pathway, leading to the transcription of genes involved in the immune response (Created with https://www.biorender.com/ accessed on 3 July 2024).
2.4.5. MAPK Pathway
In addition to the JAK/STAT pathway, the MAPK pathway is a parallel signaling cascade that plays a crucial role in regulating the expression of inflammatory cytokines [100]. The MAPK pathway is a complex network of signaling proteins, primarily consisting of three main kinases: extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase, and p38 MAP kinase [112]. The pathway begins with guanine nucleotide exchange factors (GEFs) catalyzing the exchange of GDP for GTP on RAS, a GTPase signaling protein [113]. Upon activation, RAS-GTP recruits and activates rapidly accelerated fibrosarcoma (Raf), which is a kinase [114]. Raf then phosphorylates and activates mitogen-activated protein kinase kinase (MEK; S218 and S222), which in turn phosphorylates and activates ERK1 (T202 and Y204) and ERK2 (T185 and Y187) [115,116,117]. Once activated, ERK1/2 translocates to the nucleus, where it phosphorylates various transcription factors such as Elk-1, c-Fos, and c-Jun [118]. The phosphorylated transcription factors then bind to the responsive elements in the promoter regions of target genes [119], subsequently leading to the transcription of genes encoding proliferative proteins, including cytokines, growth factors, and other regulatory molecules [120].
Among the MAPK pathway kinases, p38 MAPK is particularly relevant in the context of inflammation [121]. It is activated in response to various extracellular stimuli, including pro-inflammatory cytokines and cellular stress [92]. Once activated, p38 MAPK phosphorylates a variety of downstream targets, including ATF-2 and NF-κB, as well as other protein kinases and regulatory proteins involved in inflammatory signaling [122]. This phosphorylation cascade ultimately leads to the expression of inflammatory cytokines including, IL-1β, TNF-α, and IL-6, contributing to the inflammatory response [65].
The MAPK pathway is tightly regulated by various mechanisms, including phosphorylation, ubiquitination, and protein-protein interactions [123]. For instance, negative regulators such as MAPK phosphatases can dephosphorylate and inactivate MAPKs, while scaffold proteins and adaptor molecules facilitate the assembly of signaling complexes and enhance pathway efficiency [124]. Additionally, feedback loops and crosstalk between different signaling pathways further modulate MAPK activity, ensuring precise control of cellular responses to diverse stimuli [125]. Thus, the MAPK pathway serves as a critical mediator of inflammation and represents a promising target for therapeutic intervention in inflammatory diseases, including MS [126].
2.4.6. Sirtuins
Sirtuins represent a family of NAD+-dependent protein deacetylases and ADP-ribosyltransferases, prominently implicated in cellular stress, metabolism, and aging [127]. Among them, SIRT1 and SIRT3 have been extensively studied in the context of MS. In MS pathology, dysregulation of sirtuins is evident, notably in their roles in modulating the immune response, oxidative stress, and mitochondrial function [127,128]. SIRT1 exerts anti-inflammatory effects by deacetylating transcription factors, including NF-κB, and inhibiting microglial activation [129]. Conversely, SIRT3 regulates mitochondrial integrity and reactive oxygen species (ROS) detoxification, thereby mitigating neuroinflammation-induced oxidative damage [130]. Dysfunctional sirtuin signaling in MS leads to aberrant immune activation, compromised mitochondrial function, and heightened oxidative stress, which exacerbate neuronal injury, demyelination, and disease progression [130]. Targeting sirtuin pathways holds promise for developing therapeutic strategies aimed at ameliorating neuroinflammation and preserving neuronal integrity in MS [131,132].
2.5. Cytokine Dysfunction of MS and Its Effects
In individuals with MS, the levels of TNF-α, IL-1, IL-6, and IFN-γ in the cerebrospinal fluid (CSF) and lesions within the CNS are elevated [133]. Increased production of these cytokines contributes to the activation of macrophages and T cells within the CNS lesions [134]. These immune cells release additional cytokines as part of the inflammatory response, contributing to tissue damage and neuroinflammation [135]. TNF-α-mediated activation of TNFR1 can lead to pro-inflammatory responses, apoptosis, and neurotoxicity, while TNFR2 activation may have neuroprotective and immunoregulatory effects [136]. In MS, there appears to be an imbalance in TNFR1 and TNFR2 signaling, with increased expression of TNFR1 and altered downstream signaling pathways, promoting inflammation and tissue damage [137]. TNF-α has been implicated in the demyelination and neurodegeneration observed in MS [138]. It can directly induce oligodendrocyte apoptosis, leading to demyelination. In addition, TNF-α contributes to neuronal damage through other mechanisms, including excitotoxicity and oxidative stress [139]. Moreover, TNF-α can disrupt BBB integrity by increasing the expression of adhesion molecules on endothelial cells and promoting leukocyte infiltration into the CNS [140]. The disruption of BBB allows immune cells and inflammatory mediators to penetrate the CNS more easily, exacerbating neuroinflammation and tissue damage [141].
The macroscopic outcomes of IL-1 signaling, similar to TNF-α, hinge on the isoform of the IL-1 receptor to which IL-1 family cytokines bind [68]. Given the context of secretory increases in IL-1α and IL-1β expression during MS pathogenesis, understanding imbalanced ligand binding can aid in the development of receptor-antagonistic treatments [142]. While both IL-1α and IL-1β can bind to IL-1R1 on expressive tissues, eliciting a pro-inflammatory response, IL-1β can additionally bind to IL-1R2 [143]. This receptor has been classified as a decoy receptor, which serves to modulate the overproduction of IL-1β in inflamed states [144]. In the context of MS, there exists a dysregulation of the expression of these two receptors. Specifically, IL-1R1, which perpetuates pro-inflammation signaling, has been shown to be overexpressed relative to IL-1R2 [145,146]. Conversely, alterations in IL-1R2 expression or function may affect the regulation of IL-1 activity, further exacerbating inflammatory responses [147].
Regarding IFN-γ receptor dysregulation, the specific imbalance with type II IFNRs in patients with MS remains unclear. However, type II receptor dysregulation has been seen in other autoimmune conditions, such as systemic lupus and rheumatoid arthritis [148,149]. The respective imbalances between TNFR1 and 2 and IL-1R1 and 2 also warrant further investigation as possible therapeutic targets for MS [150].
3. Current Treatments for MS
The complexity of the myriad of pathways involved in its pathogenesis demands a multifaceted approach to the management of MS. Current treatment options for MS encompass a diverse array of interventions, including DMTs, symptomatic treatments, acute relapse treatments, rehabilitation therapies, and lifestyle modifications [151]. These treatments are tailored to the specific needs and characteristics of each patient, with the goal of slowing disease progression, minimizing relapses, managing symptoms, and preserving neurological function [152].
3.1. Disease-Modifying Therapies (DMT)
DMTs are a cornerstone of treatment for MS, particularly for individuals with relapsing forms of the disease [153]. These medications are designed to modify the immune response, thereby reducing the frequency and severity of relapses and slowing disability progression [154]. DMTs work through various mechanisms, including immunomodulation, anti-inflammatory effects, and modulation of lymphocyte trafficking [155]. Commonly used DMTs include glatiramer acetate (GA), dimethyl fumarate (DMF), teriflunomide, and fingolimod [156]. Treatment selection is based on factors such as disease activity, severity, as well as individual patient characteristics and preferences [157]. Overall, DMTs play a critical role in managing MS by reducing disease activity, delaying progression, and improving long-term outcomes for individuals living with MS [158].
3.1.1. Glatiramer Acetate
Glatiramer acetate (GA) is a DMT that exerts its therapeutic effects through a complex interplay of cellular mechanisms that modulate the immune response and convey neuroprotection [159,160]. GA is a synthetic polypeptide that resembles myelin basic protein, a component of the myelin sheath that is targeted by the autoimmune response [161]. Although the mechanism of action of GA in MS is not fully understood, several ideas have been proposed. One possibility is that it works by inducing immunomodulatory shifts in T cell responses, resulting in the generation of regulatory T cells (Tregs), thus shifting the balance from pro-inflammatory Th1 and Th17 cells to anti-inflammatory Th2 cells [162]. Additionally, GA may act as a decoy antigen, diverting autoreactive T cells away from myelin proteins and towards recognizing GA peptides instead [163]. Furthermore, GA has been shown to activate APCs, such as dendritic cells and macrophages, leading to the release of anti-inflammatory cytokines, such as IL-10, and the suppression of pro-inflammatory cytokines, such as IL-12 and TNF-α [159]. This shift in the cytokine profile contributes to the downregulation of the inflammatory response within the CNS [164]. Finally, GA may exert neuroprotective effects by promoting remyelination, enhancing neuronal survival, and reducing oxidative stress and excitotoxicity [165].
3.1.2. Dimethyl Fumarate
Upon administration, dimethyl fumarate (DMF) is rapidly metabolized to its active form, monomethyl fumarate (MMF), which exerts immunomodulatory and anti-inflammatory effects [166]. One of the primary mechanisms of DMF involves the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway [167]. MMF activates Nrf2 by covalently modifying cysteine residues on Kelch-like ECH-associated protein 1 (Keap1), a negative regulator of Nrf2 [168]. This modification leads to the dissociation of Nrf2 from Keap1, allowing Nrf2 to translocate to the nuclei, where it binds to antioxidant response elements (AREs) in the promoter regions of genes encoding various antioxidant and cytoprotective proteins [169,170]. Consequently, DMF/MMF upregulates the expression of the genes that code for the following proteins: heme oxygenase-1, NAD(P)H quinone dehydrogenase 1, and glutathione S-transferase [171]. These collectively help to mitigate oxidative stress and maintain cellular redox homeostasis [171]. Additionally, DMF/MMF inhibits the production of IL-17 and IL-23 by modulating the activation of dendritic cells and T cells [172]. DMF/MMF also suppresses the activation and proliferation of various immune cells, including T cells, B cells, and monocytes, through mechanisms that are not fully understood but may involve inhibition of the NF-κB signaling pathway and modulation of mitochondrial function [173,174].
3.1.3. Teriflunomide
As an active metabolite of leflunomide, teriflunomide inhibits dihydroorotate dehydrogenase, a key enzyme involved in pyrimidine synthesis, thereby disrupting the proliferation of activated lymphocytes [175]. By inhibiting pyrimidine synthesis, teriflunomide reduces the proliferation and expansion of autoreactive T and B lymphocytes, which play a central role in the pathogenesis of MS [176]. Furthermore, teriflunomide modulates immune cell function by interfering with the differentiation and activation of various subsets of immune cells [177]. Specifically, teriflunomide inhibits the production of pro-inflammatory cytokines, including IL-17 and IFN-γ, while promoting the secretion of anti-inflammatory cytokines, including IL-10 [175,178]. This shift in the cytokine profile helps to dampen the inflammatory response and restore immune balance within the CNS [179]. Additionally, teriflunomide may exert neuroprotective effects by reducing oxidative stress and mitochondrial dysfunction, which are implicated in the pathogenesis of MS-related neurodegeneration [180].
3.1.4. Fingolimod
Fingolimod acts as a sphingosine-1-phosphate (S1P) receptor modulator [181]. Upon ingestion, it is phosphorylated to fingolimod phosphate (FTY720-P), which resembles S1P [182]. FTY720-P binds to and downregulates S1P1 receptors on lymphocytes, which prevents their egress from lymphoid organs into circulation [183]. This reduces the peripheral pool of circulating lymphocytes available to infiltrate the CNS, thereby mitigating neuroinflammation in MS lesions [183]. Additionally, fingolimod may directly modulate CNS-resident cells, such as astrocytes and microglia, attenuating their activation and pro-inflammatory responses [184]. Beyond its immunomodulatory effects, fingolimod may promote neuroprotective and repair mechanisms, including enhanced neurotrophic factor expression, neuronal survival, and remyelination [184,185].
3.2. Symptomatic Treatments and Therapy for MS
Symptomatic treatments and therapies for MS aim to alleviate specific symptoms experienced by individuals living with the condition, thereby improving their quality of life [186]. These interventions target various manifestations of MS, including spasticity, fatigue, pain, bladder dysfunction, and cognitive impairment [187]. Medications like baclofen and tizanidine are commonly prescribed to manage spasticity, while amantadine may be used to address MS-related fatigue [186,188]. Additionally, anticonvulsants or antidepressants may help alleviate neuropathic pain, while medications like oxybutynin or mirabegron can aid in managing bladder dysfunction [189]. Cognitive rehabilitation regimens, including cognitive training and compensatory strategies, are also utilized to address cognitive impairment [190].
3.3. Acute Relapse Treatment
The purpose of acute relapse treatment for MS is to alleviate the severity and duration of symptoms associated with flare-ups, known as relapses or exacerbations [191]. These treatments aim to shorten the duration of neurological deficits and facilitate recovery following a relapse [192]. Acute relapse treatments often involve short courses of high-dose corticosteroids, such as intravenous injection of methylprednisolone, which exerts potent anti-inflammatory effects and suppresses immune responses [193]. Corticosteroids reduce inflammation around demyelinated lesions in the CNS, leading to faster resolution of symptoms [194]. In some cases, plasma exchange (also known as plasmapheresis) may be considered for severe relapses that are refractory to corticosteroid therapy [195,196]. Plasma exchange involves removing plasma from the blood and replacing it with a substitute solution, which can effectively remove circulating antibodies and inflammatory mediators implicated in the relapse [197].
3.4. Lifestyle Modifications
Lifestyle modifications play a crucial role in managing MS by enhancing overall well-being, minimizing symptoms, and improving quality of life [198]. Regular exercise can help maintain mobility, strength, and flexibility while reducing fatigue and depression, which are commonly associated with MS [199]. A balanced diet rich in fruits, vegetables, lean proteins, and omega-3 fatty acids can support immune function and promote brain health [200]. Adequate hydration is also essential to prevent urinary tract infections and manage the bladder symptoms often experienced by MS patients [201]. Additionally, the implementation of stress management techniques such as mindfulness meditation, yoga, and deep breathing exercises can help alleviate psychological stressors and potentially reduce the risk of MS exacerbations [202]. By incorporating these lifestyle modifications into daily routines, individuals with MS can better manage their symptoms and optimize their overall health and well-being [203].
4. Plant-Derived Compounds as Medications for MS
Despite significant advancements in MS, there remains a notable gap in addressing the multifaceted nature of the disease [204]. Current therapeutic strategies primarily target immune dysregulation to mitigate inflammation and slow disease progression [205]. While treatment modalities such as DMTs have demonstrated efficacy in reducing relapse rates and delaying disability accumulation, they often come with limitations such as incomplete efficacy, adverse side effects, and inadequate management of progressive forms of MS [206]. Furthermore, there is a growing recognition of the need for alternative treatment modalities that can offer greater efficacy, tolerability, and neuroprotective effects [207]. In this context, plant-derived compounds present a promising avenue for addressing these unmet needs in MS management [208]. With their diverse array of bioactivities, plant-derived medications have the potential to complement existing therapies by targeting inflammation, oxidative stress, and neurodegeneration in MS [209]. By harnessing the therapeutic properties of phytochemicals, such as phenylpropanoids, alkaloids, and terpenoids, there is an opportunity to fill some of the gaps in currently available treatment options for MS and to improve outcomes for individuals living with this complex autoimmune disorder [11].
4.1. Polyphenols
4.1.1. Epigallocatechin-3-Gallate
Epigallocatechin-3-gallate (EGCG) (Figure 4A), a potent polyphenol abundant in green tea, has garnered considerable interest for its wide-ranging health benefits [210,211]. Derived primarily from the leaves of the Camellia sinensis plant, EGCG belongs to the catechin group of compounds [212]. While green tea serves as the primary dietary source of EGCG, smaller quantities can also be found in white tea and oolong tea [213]. EGCG has a bioavailability of 0.1% (Table 1) [214], and is available in concentrated supplement form for those seeking targeted consumption [215].
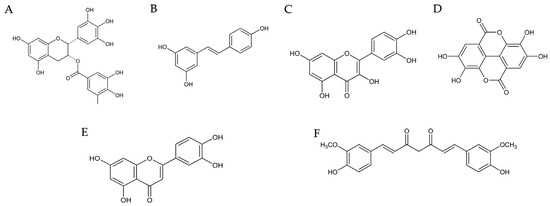
Figure 4.
Structures of the polyphenols described in this article. EGCG (A), resveratrol (B), quercetin (C), ellagic acid (D), luteolin (E), and curcumin (F) [216,217,218,219,220,221].

Table 1.
Summary of bioavailability of each polyphenol discussed with the potential to treat MS-associated symptoms. (#) denotes an in vivo study and (^) a clinical study.
Recent research highlights the ability of EGCG to inhibit the effects of several pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, by targeting key signaling pathways [222,223]. By blocking the IKK complex and preventing the phosphorylation of IkB, EGCG inhibits the release of NF-κB, a critical mediator of inflammation [224]. Moreover, EGCG disrupts the MAPK signaling pathway, further reducing IL-1β production [225]. This dual mechanism leads to a decrease in pro-inflammatory cytokine levels while promoting the production of the anti-inflammatory cytokines, IL-10 and TGF-β [179,226].
Beyond its immunomodulatory effects, EGCG demonstrates neuroprotective properties, shielding neurons from oxidative stress and apoptosis and potentially promoting remyelination [227]. The accumulation of EGCG within the mitochondria may protect against neuronal damage by reducing induced apoptosis [228]. Additionally, EGCG has shown promising results in enhancing cell viability, reducing markers of stress and apoptosis, and protecting against various forms of toxicity [229]. These protective effects extend to mitigating glutamate excitotoxicity and preserving mitochondrial function, ultimately enhancing cognitive function and prolonging lifespan [230]. Outside the realm of neurology, EGCG has shown promise in cancer treatment, cardiovascular health, weight management, diabetes management, and skin health, underscoring its versatility and potential as a multifaceted therapeutic agent [229,231].
In an 18-month clinical trial, although a dose of 800 mg EGCG was shown to be safe and bioavailable in patients with relapsing-remitting MS, it had no additional effect on the GA treatment on the MRI or immune parameters [232]. However, the same dose of EGCG combined with 60 mL of coconut oil showed a significant improvement in some gait parameters and balance in MS patients over a 4-month nutritional intervention study, which may suggest a neuroprotective effect [233]. From the same study, the combined effect of EDCG, coconut oil, and a Mediterranean isocaloric diet, a promising protective effect against cardiac risk by improving levels of albumin, beta-hydroxybutarate, and paraoxonase 1 and anthropometric parameters such as waist-to-hip ratio and muscle mass [234]. In an experimental study, different doses of EGCG had an anti-fatigue effect by improving associated blood parameters and increasing glycogen content in the liver and the muscles of the mice [235]. These findings suggest that, when combined with the appropriate nutritional intervention, EGCG is a promising polyphenol for the management of MS symptoms. However, more clinical research is needed to confirm this.
4.1.2. Resveratrol
Resveratrol (Figure 4B), a natural polyphenol abundant in plants such as grapes and berries, has emerged as a promising compound in the realm of MS research due to its notable anti-inflammatory properties [236]. Studies have revealed, similarly to EGCG, its capacity to target key inflammatory cytokines implicated in MS pathogenesis, including TNF-α, IL-1β, IL-6, IL-17, and IFN-γ, effectively dampening the inflammatory cascade [226,237]. Moreover, the neuroprotective effects of resveratrol and its ability to promote remyelination further underscore its potential in attenuating MS progression [238]. These beneficial effects are attributed to its modulation of various signaling pathways, such as suppressing NF-κB and MAPK while activating sirtuins, which collectively reduce inflammation and neurodegeneration [239,240]. The oral bioavailability of resveratrol is low, <1% (Table 1) [241]. However, in a mouse model of MS, resveratrol-loaded macrophage exosomes administered intranasally reduced inflammatory parameters in the CNS and relived disease progression via microglia targeting [242]. As such, resveratrol stands as a promising candidate for the development of novel therapeutic interventions against MS, although further research is necessary to delineate its precise mechanisms of action and therapeutic efficacy [243].
4.1.3. Quercetin
Quercetin (Figure 4C), a natural flavanol found widely in fruits, vegetables, and grains, exhibits numerous pharmacological effects that make it a promising candidate for neuroprotection and multiple sclerosis (MS) treatment. It reportedly has a bioavailability of 16% (Table 1) [244], which is surprisingly higher relative to most polyphenols. Its therapeutic potential is attributed to its ability to modulate key signaling pathways involved in oxidative stress and inflammation, particularly the nuclear factor erythroid 2–related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) pathways [245]. Quercetin activates Sirtuin 1 (SIRT1), which has been linked to neuroprotection and anti-aging effects [246]. Quercetin also stimulates autophagy in Schwann cells, enhancing their ability to cope with neurodegenerative stress [247]. The antioxidative and anti-apoptotic activities of quercetin contribute to reducing hypoxia-induced memory dysfunction and increasing neuronal survival [248,249].
With respect to MS, quercetin impacts both demyelination and remyelination [250,251]. Quercetin also modulates inflammatory responses by inhibiting key cytokines such as TNF-α, IL-1β, and IL-6, inhibiting dendritic cells and Th17 cells, and shifting microglial activation to a neuroprotective M2 phenotype [252,253,254]. Inhibition of Th17 cell differentiation is achieved by targeting STAT4 [255,256]. These effects underscore the potential of quercetin as a complementary MS treatment, addressing both inflammation and neurodegeneration.
In an ethidium bromide-induced demyelination rat model, quercetin treatment (50 mg/kg/day) prevented additional demyelination, improved remyelination, enhanced locomotor activity, inhibited lipid peroxidation, and preserved acetylcholinesterase (AChE) activity [257]. A similar model confirmed that quercetin protected Na+/K+-ATPase function in both demyelination and remyelination phases, decreased oxidative stress, and maintained AChE activity [258]. Additionally, in a lysolecithin-induced demyelination model in the optic chiasm, quercetin treatment led to reduced visual evoked potential latency, diminished demyelination, and enhanced remyelination [259]. In experimental allergic EAE models, quercetin reduced disease progression by controlling myeloperoxidase activity, nitric oxide levels, and lipid peroxidation [255,256]. It also inhibited IL-12-induced T cell proliferation and Th1 differentiation. In vitro studies further highlight the ability of quercetin to decrease cytokine levels, such as IL-1β and TNF-α, and modulate matrix metalloproteinases (MMPs) in peripheral blood mononuclear cells from MS patients [253]. Quercetin-loaded nanoparticles have also shown the potential to reduce demyelination and inflammation in preclinical models [260].
4.1.4. Ellagic Acid
Ellagic acid (Figure 4D), a polyphenol abundant in the Mediterranean diet, despite having a low bioavailability of <0.2% (Table 1) [261], shows significant promise for MS treatment [262] as it attenuates demyelination and neuroinflammation in MS models [263,264]. The anti-inflammatory effects of ellagic acid are partly mediated through its ability to inhibit NF-κB signaling [265,266]. By suppressing NF-κB activation, ellagic acid reduces the production of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, which are known to exacerbate neuroinflammation in MS [263]. In the MOG35−55-immunized EAE model, high-dose ellagic acid (50 mg/kg) alleviates clinical symptoms, improves motor function, and reduces neurological deficits [263]. It counteracts astrogliosis, astrocyte activation, demyelination, and axonal loss, attenuating neuroinflammation and axonal damage by modulating the NLRP3 inflammasome and pyroptotic pathways, reducing pro-inflammatory cytokines, and increasing IL-4 levels and GATA3 expression [264,265]. Ellagic acid has been shown to inhibit apoptosis in neural cells by modulating various signaling pathways, including the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathway. By enhancing PI3K/Akt signaling, ellagic acid promotes cell survival and inhibits the apoptotic pathways that are often triggered during neurodegeneration [267,268].
Human studies with ellagic acid supplementation (90 mg twice daily for 12 weeks) show improvements in health markers, including reduced BDI-II scores (depression index score), IFN-γ, NO, cortisol, and IDO gene expression, and increased brain-derived neurotrophic factor and serotonin levels [269,270]. In a cuprizone-induced demyelination model, ellagic acid ameliorates behavioral impairments and counters oxidative stress by enhancing antioxidant enzyme activities [271]. The compound also positively impacts gut microbiota, promoting beneficial bacteria and increasing propionate levels, which correlate with reduced EAE symptoms [262]. These findings highlight the role of ellagic acid in modulating immune responses and improving neurological health, thus offering a promising therapeutic avenue for MS.
4.1.5. Luteolin
Luteolin (Figure 4E) has demonstrated significant potential as a therapeutic agent for MS by modulating key pathways involved in remyelination and inflammation [272,273]. It has been shown to have a bioavailability of 4.1% (Table 1) [274]. In rodent models of MS, luteolin effectively inhibits the Nrf2 pathway in astrocytes, which is crucial for cholesterol biosynthesis and transfer to oligodendrocytes. This process is essential for myelin repair, as sustained Nrf2 activation impairs oligodendrocyte survival and remyelination. Luteolin’s inhibition of Nrf2 restores cholesterol biosynthesis and supports oligodendrocyte function, thereby promoting remyelination [275]. This mechanism highlights a novel therapeutic target in MS, where luteolin can modulate astrocyte-oligodendrocyte interactions to enhance central nervous system regeneration.
Furthermore, luteolin exhibits robust anti-inflammatory effects, which are critical in the context of MS pathology. It significantly reduces the production of pro-inflammatory cytokines, including IL-1β and TNF-α, and inhibits STAT3 phosphorylation, a key mediator in T cell activation [273,276,277]. This dual-action mechanism not only mitigates neuroinflammation but also supports oligodendrocyte survival and remyelination, positioning luteolin as a promising candidate for the treatment of MS.
4.1.6. Curcumin
Curcumin (Figure 4F), derived from turmeric, has a bioavailability of 60–66%, as reported in one study (Table 1) [278]. However, the circulating concentrations of curcumin after oral administration are low, with its various metabolites being primarily detected. Regardless, curcumin has demonstrated potential in enhancing IFN β-1a therapy for MS by improving radiological inflammation markers [279,280]. In EAE models, polymerized nano-curcumin (PNC) significantly reduced disease scores and symptoms like paralysis and motor deficits [281,282]. PNC modulates immune responses by lowering pro-inflammatory factors and raising anti-inflammatory cytokines such as IL-4, IL-10, and TGF-β [283,284]. It also boosts FOXP3 expression, a key transcription factor for regulatory T cells, and enhances HO-1 expression via the Nrf2 pathway, thereby reducing neuroinflammation [285,286].
The effects of curcumin extend to promoting myelin repair and reducing glial activation [282,287]. Curcumin-loaded nanoparticles show greater efficacy than free curcumin in decreasing immune cell infiltration and demyelination in the corpus callosum [287]. Clinical studies reveal benefits in reducing Expanded Disability Status Scale (EDSS) scores and increasing anti-inflammatory markers like TGF-β and IL-10 [285,286]. Although curcumin combined with IFN β-1a did not significantly alter EDSS scores, it improved the anti-inflammatory effects of IFN β-1a without increasing adverse reactions. Curcumin also influences gut microbiota, which affects neuroinflammation and disease progression, highlighting its potential as an adjunct therapy for MS [288].
4.2. Alkaloids
4.2.1. Caffeine
Caffeine (Figure 5A) is a purine alkaloid, which is subcategorized as a trimethylxanthine, a member of the methylxanthine class of pharmacologic agents [289,290]. Caffeine has a bioavailability of 99% (Table 2) [291], and prominent sources include coffee, tea, soda, and energy drinks [290]. Extensive research has demonstrated the ability of caffeine to protect against Alzheimer’s and Parkinson’s disease, to oppose oxidative stress and inflammation, and to act as a bronchodilator and vasodilator [292,293,294]. Relevant to MS, caffeine suppresses inflammation via inhibiting NF-κB [295]. Specifically, it disrupts the NF-κB signaling progression by blocking the translocation of p50 and p65 subunits into the nuclei [296]. Inhibition of NF-κB by caffeine also reduces NLRP3 inflammasome, which contributes to NF-κB-stimulated transcription of IL-1β and IL-18 [295,297]. Caffeine may also prevent inflammation by down-regulating the NLRC4 inflammasome [298], which is the main protease responsible for converting pro-IL-1β into active mature IL-1β. Thus, caffeine can reduce IL-1β production by inhibiting NF-κB, NLRP3, and NLRC4.
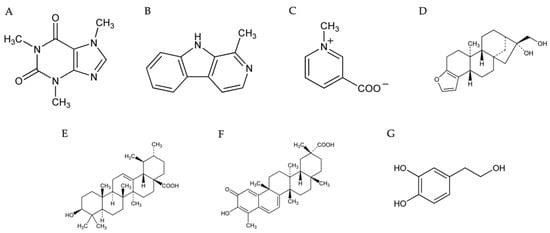
Figure 5.
Chemical structures of the alkaloids, terpenes, and catechol described in this article. Caffeine (A), harmane (B), trigonelline (C), cafestol (D), ursolic acid (E), celastrol (F), and hydroxytyrosol (G) [299,300,301,302,303,304,305].

Table 2.
Summary of bioavailability of each alkaloid, terpene, and catechol discussed with the potential to treat MS-associated symptoms. (#) denotes an in vivo study and (^) a clinical study.
While it is not fully elucidated as to how caffeine suppresses inflammation, it may modulate immune responses by acting as a non-specific antagonist of adenosine receptors (ARs), which are the main targets of ingested caffeine. There are four known subtypes of ARs, A1, A2A, A2B, and A3, all of which are coupled to G-proteins. However, each subtype of ARs has a distinct pharmacological profile, tissue distribution, and G-protein coupling [306]. Therefore, adenosine, the endogenous ligand for ARs, can elicit many physiological or pathological effects by acting on these receptors in a dose-dependent manner. Accordingly, caffeine exerts various effects depending on the concentration. Low doses of caffeine can stimulate cAMP production by blocking the A1 receptor, which, along with A3, is coupled to Gi/Go protein to inhibit adenylate cyclase (AC) activity [307]. Subsequently, increased cAMP concentrations suppress proinflammatory cytokine production partially via activating the repressor transcription factor CCAAT displacement protein (CDP) [308]. Cyclic AMP also directly binds to NLRP3, thus directly inhibiting the assembly of the inflammasome [309]. Further, caffeine has been shown to inhibit phosphodiesterases, which hydrolyze cAMP to AMP [310]. The A2 receptor is coupled to Gαs, and its activation stimulates AC activity and cAMP production. At high concentrations, caffeine antagonizes the A2 receptor, thereby inhibiting cAMP formation, leading to the increased production of proinflammatory cytokines [307]. However, the circulating concentrations of caffeine required for inhibiting the A2 receptor in vivo may not be achievable through dietary intake of coffee or caffeine. It was found that sufficient antagonism of A2 requires caffeine concentrations ≥ 100 µM [311]. Orally ingested caffeine typically reaches a peak plasma concentration (Cmax) between 15 and 120 min in healthy adults [291]. A recent study shows that oral administration of 200 mg of caffeine (roughly the amount of caffeine found in two 240 mL servings of coffee) in healthy men resulted in a Cmax of 3.4 mg/L, which equates to a plasma concentration of 17.51 µM [312]. To achieve the plasma concentrations of caffeine sufficient to inhibit A2 receptor and cAMP production would require ~1100 mg of orally ingested caffeine, which is about 2.75 times the USDA-published safe limit of 400 mg/day [313]. Thus, moderate caffeine consumption is unlikely to cause the A2 antagonism-mediated inhibition of cAMP production. Although further investigation is needed to more thoroughly elucidate the cellular mechanisms behind its anti-inflammatory effects, caffeine presents a promising avenue for future MS therapies.
4.2.2. Harmane
Harmane (Figure 5B), a β-carboline alkaloid, exhibits potential as an anti-inflammatory agent in MS treatment [314]. The compound is derived from the Peganum harmala plant. It has a bioavailability of 19% (Table 2) [315], and can be naturally found in several foods and beverages, including soy sauce, toasted bread, barley, coffee, and fermented alcohol-containing beverages [314,315,316,317]. Additionally, harmane can be found in many cooked meats such as beef, mutton, and chicken, with greater concentrations of harmane being found in meats that have been cooked for longer periods of time and at higher temperatures [314]. This is because harmane can be formed through the Maillard reaction, a process often used in food processing to imbue food products with appealing flavors and colors that involves interactions between the free amine groups of proteins and the carbonyl groups of carbohydrates [318].
Harmane is one of the alkaloids present in Peganum harmala, which was often used as a medicinal herb in ancient times in certain regions of the world to treat multiple diseases, including various cancers [319]. More recently, research demonstrated that harmane can counteract inflammation, primarily by inhibiting myeloperoxidase (MPO) [320]. MPO is expressed most abundantly in neutrophils and aids in defense against pathogens by catalyzing the formation of many ROS, including hypochlorous acid (HOCl), which is a potent antimicrobial agent and one of the strongest oxidant molecules produced in the human body [321]. In contrast to their beneficial contributions to the efficacy of the immune system, the ROS produced by MPO have also been implicated in a wide variety of cardiovascular, neurodegenerative, and autoimmune diseases [322]. In the context of MS, MPO causes the formation of ROS such as hypochlorous acid, tyrosyl radicals, and aldehydes, which can increase the production of proinflammatory cytokines (IL-1α and TNF-α, for example) [323]. ROS can activate the NF-κB pathway and subsequently increase IL-1 and TNF-α expression [324]. Interestingly, MPO inhibitors have been shown to decrease the expression of the proinflammatory cytokines IL-1α and TNF-α [325]. Thus, harmane, as an MPO inhibitor, might be useful in attenuating MS pathogenesis.
Although the current literature is limited with respect to the effects of harmane supplementation on the pathogenesis of MS in vivo, one study sought to determine the neuroactive effects of β-carbolines supplied as pure compounds versus a natural source (coffee substitute) in a murine model [326]. The results of the study indicated that the animal diets enriched with coffee substitutes resulted in a higher concentration of harmane in the blood and had a positive effect on animal activity [326]. Further, the lack of significant differences between the health parameters of the control and experimental groups suggested that there was no negative effect on the general health of the animals associated with the addition of harmane to the diet [326].
4.2.3. Trigonelline
Trigonelline (Figure 5C) is a pyridine alkaloid derived from nicotinic acid and is classified as a methylnicotinic acid [327]. Dietary sources of trigonelline include fenugreek seeds, garden peas, hemp seeds, oats, coffee, and coffee byproducts [328,329]. Trigonelline has a bioavailability of 64.42% (Table 2) [329] and has demonstrated promise as an anti-oxidative, anti-hyperglycemic, anti-hyperlipidemic, anti-hypercholesterolemic, anti-cariogenic, and anti-microbial agent [329]. In addition, trigonelline had neuroprotective effects in animal models of diabetes, Alzheimer’s disease, Parkinson’s disease, and modulated process that involves nervous system development and inflammation [330]. Specifically, regarding inflammation, trigonelline prevents the transcriptional upregulation of the p50 and p65 subunits of NF-κB [331,332], thereby reducing the expression of TNF-α, IL-1β, IL-6 [65], suggesting that it may potentially be used to prevent the pathogenesis of MS.
While trigonelline has yet to be thoroughly investigated for its effects on MS, studies evaluating its efficacy in the treatment of other neurodegenerative diseases evidence the value of trigonelline as a prospective compound for future investigation in the context of MS. One such study demonstrated that trigonelline significantly mitigated oxidative stress in LPS-treated mice by increasing the levels of antioxidant defense enzymes and decreasing the lipid peroxidation [333]. The study further showed that TNF-α and IL-6 levels, which had been significantly elevated in the mice after LPS administration, were significantly reduced following trigonelline administration at doses of 50 and 100 mg/kg [333].
Another study sought to evaluate the neuroprotective effects of trigonelline by examining the ability of the compound to restore amyloid β (Aβ)-induced axonal degeneration and improve memory function in Alzheimer’s disease 5XFAD model mice [334]. The results demonstrated that oral administration of trigonelline to 5XFAD mice for 14 days resulted in significantly improved object recognition memory and object location memory and normalized neurofilament light levels in the cerebral cortex, which is a biomarker of axonal damage [334].
4.3. Terpenoids
4.3.1. Cafestol
Cafestol (Figure 5D) is a fat-soluble ent-kaurene diterpenoid that is derived from the beans of the Coffea arabica plant and has been the subject of numerous pharmacological studies due to its various beneficial biological activities, including anti-inflammatory, anti-carcinogenic, anti-angiogenic, anti-diabetic, anti-oxidant [335,336,337] and neuroprotective [338] effects. The concentration of cafestol found in a cup of coffee can vary greatly depending on the quality, blend, and method of preparation. Unfiltered coffee has been shown to contain significantly larger concentrations of cafestol than filtered coffee [339]. The majority (64–70%) of cafestol is absorbed by the duodenum of healthy individuals (Table 2) [340].
Emerging evidence has shown that cafestol targets several biological pathways to exert its anti-inflammatory effects. This is primarily accomplished by regulating chemokines intercellular adhesion molecule-1 (ICAM1), monocyte chemoattractant protein-1 (MCP1), and IL8 [336,341]. It was shown that cafestol can inhibit the secretion of inflammatory mediators induced by cyclic strain in human umbilical vein endothelial cells (HUVECs) [341]. It is believed that cafestol attenuates ROS production, which in turn prevents MAPK phosphorylation, leading to the reduction in the production of these inflammatory mediators [341]. In addition, cafestol has been demonstrated to significantly reduce TNF-α and IL-1β levels and inhibit cardiac apoptosis by modulating tissue levels of Bax and Caspase-3 [342]. It has further been reported that cafestol can effectively block the AP-1 pathway by directly inhibiting the activity of ERK2 and consequently reducing the production of PEG2 and its associated pro-inflammatory activities [343].
Although the existing literature contains an abundance of in vitro studies investigating the biological activities of cafestol, the available in vivo studies pertaining to MS are limited due to greater emphasis being placed on its remarkable anti-diabetic effects. Although further research is needed, the affordability and abundance of cafestol, as well as its numerous benefits and minimal side effects, make it a promising option for future investigation in the context of MS.
4.3.2. Ursolic Acid
Ursolic acid (UA) (Figure 5E) is a pentacyclic triterpenoid and a secondary metabolite present in many commonly used plants, including fruits, vegetables, and herbs such as thyme, rosemary, lavender, oregano, and mint [344]. Despite its very low bioavailability (0.03%) (Table 2) [345], the anti-inflammatory and neuroprotective effects of UA make it a promising candidate with respect to the development of novel therapies for MS. Recent research has demonstrated that the administration of UA can significantly increase the expression of the anti-inflammatory cytokine IL-10 [346]. Additionally, UA has been shown to attenuate amyloid β (Aβ)-induced memory impairments through amelioration of oxidative stress and downregulation of IL-1β, IL-6, and TNF-α levels in the hippocampus of mice [347]. Finally, it was found that UA markedly inhibited LPS-induced IκBα phosphorylation and degradation, NF-κB p65 nuclear translocation, and p38 activation in the mouse brain but did not affect the activation of TLR4, MyD88, ERK, JNK, and Akt [348]. These data suggest that UA may hold the potential to mitigate inflammation-associated brain disorders by blocking the p38 and NF-κB signaling pathways and inhibiting the production of pro-inflammatory factors.
In addition to its anti-inflammatory properties, UA has also been shown to exert neuroprotective effects and actively combat demyelination in the central nervous system [349]. The results of a recent study indicated that treatment with UA increased the number of new oligodendrocyte lineage cells and myelination by reducing inflammation and preventing gliosis [349]. Although the study was primarily concerned with the investigation of UA in the context of Parkinson’s disease, microglial activation and proliferation are key features of MS pathology as well. Given its role as an anti-inflammatory mediator and its ability to preserve myelin and actively promote the remyelination of axons, UA holds immense therapeutic potential for treating MS.
4.3.3. Celestrol
Celastrol (Figure 5F), a pentacyclic triterpene derived from the root of the Tripterygium wilfordii plant, has shown significant therapeutic potential across various conditions, including diabetes, metabolic dysfunction, irritable bowel syndrome, and Alzheimer’s disease [350,351,352,353]. Its chemical structure is defined as 3-hydroxy-9β,13α-dimethyl-2-oxo-24,25,26-trinoroleana-1(10),3,5,7-tetraen-29-oic acid [350] and it has a bioavailability of 17% (Table 2) [354]. The efficacy of celestrol has been demonstrated in numerous human clinical trials, and it has been recognized for its ability to restore lipid metabolism and modulate protein homeostasis.
In MS research, celastrol exhibits potent anti-inflammatory and neuroprotective properties. Celastrol mechanistically inhibits pro-inflammatory cytokines, including IFN-γ and IL-17, while upregulating anti-inflammatory cytokines, such as IL-4 [355]. This modulation is achieved by blocking key transcription factors such as STAT3 and retinoid-related orphan receptor gamma t, which are involved in Th17 cell differentiation and inflammation [356]. The action of celestrol extends to the inhibition of NF-κB and AP-1, crucial transcription factors that drive inflammation [355,357]. It also inhibits LPS-induced production of IL-1β, TNF-α, and IL-6, potentially through cyclooxygenase-2 inhibition [358,359]. Additionally, celastrol promotes mitochondrial autophagy by acting as a Nur77 ligand, which contributes to its neuroprotective effects [360].
In MS models, the effects of celastrol are particularly pronounced in the spinal cord and optic nerve [355]. It significantly reduces neuroinflammation and apoptosis, as evidenced by lower levels of nitrites, reduced immunohistochemical expression of TLR2 and CD3+ T-lymphocytes, and improvements in histopathological scores [355]. The treatment also decreases levels of chemokines (including RANTES, MCP-1, MIP-1α, and GRO/KC) and cytokines (including TNF-α and IL-1β) [361]. In the optic nerve, Celastrol mitigates severe inflammatory responses and microgliosis, and it restores apoptotic balance. In EAE models of MS, celastrol reduces neurobehavioral abnormalities, inflammatory infiltration, and demyelination. It downregulates the pro-inflammatory cytokines IFN-γ and IL-17 while upregulating anti-inflammatory cytokines, including IL-4 [355]. Celastrol also mitigates severe inflammatory responses and microgliosis, reduces chemokines, and inhibits IL-17 expression [356].
Despite its promising preclinical results, the use of celestrol is associated with potential risks, including microglia cytotoxicity at elevated concentrations (100–1000 nM), and chronic use is associated with heart and liver damage [362,363,364].
4.4. Catechol
Hydroxytyrosol
Hydroxytyrosol (HT) (Figure 5G) is a potent phenolic compound primarily found in olive oil and olive leaves and is known for its robust antioxidant properties [365]. The bioavailability of HT is 75% when administered in an aqueous solution (Table 2) [366] and its amphipathic nature facilitates effective absorption and distribution [367]. HT primarily scavenges reactive oxygen species (ROS) both intracellularly and extracellularly, addressing oxidative stress associated with neurodegenerative diseases [368,369]. The ability of HT to cross the blood-brain barrier (BBB) is particularly relevant for treating CNS disorders, including MS [370,371].
In MS, HT counteracts chronic inflammation and oxidative stress that contribute to myelin degradation and neuronal damage [372]. By reducing the expression and activity of MMP-9 and MMP-2, HT helps maintain BBB integrity and limits immune cell infiltration into the CNS [373,374,375]. HT also diminishes oxidative stress and lipid peroxidation in MS, enhances antioxidant enzyme activity such as glutathione peroxidase, and regulates iron metabolism [374]. These actions collectively support the potential of HT as a treatment for alleviating symptoms and slowing the progression of MS.
5. Conclusions
The emerging evidence presented in this review underscores the promising potential of plant-derived compounds as adjunctive therapies for MS [376]. With their diverse array of bioactive compounds and multifaceted mechanisms of action, including anti-inflammatory and neuroprotective properties, plant-derived compounds offer a complementary approach to existing treatments [377]. By targeting inflammation, oxidative stress, and neurodegeneration, these compounds address critical aspects of MS pathogenesis. Moreover, their ability to modulate immune responses and promote neuroregeneration suggests a broader therapeutic scope beyond symptom management. However, further research is needed to elucidate the specific mechanisms of action, optimize dosing regimens, and evaluate long-term efficacy and safety profiles. By harnessing the therapeutic potential of phytochemicals, such as alkaloids, phenylpropanoids, and terpenoids, there is an opportunity to enhance the clinical management of MS and improve outcomes for affected individuals. Ultimately, the integration of plant-derived pharmacologic agents into the MS treatment paradigm holds promise for addressing the complex and multifaceted nature of this autoimmune disorder, offering hope for improved quality of life and functional outcomes for patients.
Author Contributions
Conceptualization, S.W., S.H. and W.M.; methodology, S.W., S.H., A.R., B.C., D.F., W.M., H.A. and D.L.; software, S.W., S.H. and W.M.; validation, S.W., D.F., W.M, H.A. and D.L.; formal analysis, S.W., S.H., D.F., W.M., H.A. and D.L.; investigation, S.W., S.H., A.R., D.F., W.M. and D.L.; resources, S.W., D.F., W.M., H.A. and D.L.; writing—original draft preparation, S.W., S.H., A.R., B.C. and W.M.; writing—review and editing, S.H., D.F., W.M, H.A. and D.L.; supervision, W.M.; project administration, W.M.; funding acquisition, W.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Liberty University Office of Sponsored Programs and Research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tafti, D.; Ehsan, M.; Xixis, K.L. Multiple Sclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499849/ (accessed on 6 May 2024).
- Hittle, M.; Culpepper, W.J.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Topol, B.; LaRocca, N.G.; Nelson, L.M.; et al. Population-Based Estimates for the Prevalence of Multiple Sclerosis in the United States by Race, Ethnicity, Age, Sex, and Geographic Region. JAMA Neurol. 2023, 80, 693–701. [Google Scholar] [CrossRef]
- Silveira, C.; Guedes, R.; Maia, D.; Curral, R.; Coelho, R. Neuropsychiatric Symptoms of Multiple Sclerosis: State of the Art. Psychiatry Investig. 2019, 16, 877–888. [Google Scholar] [CrossRef]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and genetic risk factors for MS: An integrated review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef] [PubMed]
- Pukoli, D.; Vécsei, L. Smouldering Lesion in MS: Microglia, Lymphocytes and Pathobiochemical Mechanisms. Int. J. Mol. Sci. 2023, 24, 12631. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Talanki Manjunatha, R.; Habib, S.; Sangaraju, S.L.; Yepez, D.; Grandes, X.A. Multiple Sclerosis: Therapeutic Strategies on the Horizon. Cureus 2022, 14, e24895. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Amato, M.P.; Centonze, D.; Gallo, P.; Gasperini, C.; Inglese, M.; Patti, F.; Pozzilli, C.; Preziosa, P.; Torjano, M. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: An expert opinion. J. Neurol. 2022, 269, 5382–5394. [Google Scholar] [CrossRef]
- Furgiuele, A.; Cosentino, M.; Ferrari, M.; Marino, F. Immunomodulatory Potential of Cannabidiol in Multiple Sclerosis: A Systematic Review. J. Neuroimmune Pharmacol. 2021, 16, 251–269. [Google Scholar] [CrossRef]
- Costantini, E.; Masciarelli, E.; Casorri, L.; Di Luigi, M.; Reale, M. Medicinal herbs and multiple sclerosis: Overview on the hard balance between new therapeutic strategy and occupational health risk. Front. Cell. Neurosci. 2022, 16, 985943. [Google Scholar] [CrossRef]
- Zahedipour, F.; Hosseini, S.A.; Henney, N.C.; Barreto, G.E.; Sahebkar, A. Phytochemicals as inhibitors of tumor necrosis factor alpha and neuroinflammatory responses in neurodegenerative diseases. Neural Regen. Res. 2022, 17, 1675–1684. [Google Scholar]
- La Rosa, G.; Lonardo, M.S.; Cacciapuoti, N.; Muscariello, E.; Guida, B.; Faraonio, R.; Santillo, M.; Damiano, S. Dietary Polyphenols, Microbiome, and Multiple Sclerosis: From Molecular Anti-Inflammatory and Neuroprotective Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 7247. [Google Scholar] [CrossRef] [PubMed]
- Ousman, S.S.; Kubes, P. Immune surveillance in the central nervous system. Nat. Neurosci. 2012, 15, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, L.L.; Alvarez, J.I.; Begley, D.J.; Boado, R.J.; del Zoppo, G.J.; Doolittle, N.D.; Engelhardt, B.; Hallenbeck, J.M.; Lonser, R.R.; Ohlfest, J.R. Immunologic privilege in the central nervous system and the blood–brain barrier. J. Cereb. Blood Flow. Metab. 2013, 33, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. T Cells and MHC Proteins. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26926/ (accessed on 6 May 2024).
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T cell receptor (TCR) signaling in health and disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Rostami, A.; Ciric, B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013, 333, 76–87. [Google Scholar] [CrossRef]
- Radandish, M.; Khalilian, P.; Esmaeil, N. The Role of Distinct Subsets of Macrophages in the Pathogenesis of MS and the Impact of Different Therapeutic Agents on These Populations. Front. Immunol. 2021, 20, 667705. Available online: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2021.667705/full (accessed on 6 May 2024). [CrossRef]
- Coutinho Costa, V.G.; Araújo, S.E.S.; Alves-Leon, S.V.; Gomes, F.C.A. Central nervous system demyelinating diseases: Glial cells at the hub of pathology. Front. Immunol. 2023, 14, 1135540. [Google Scholar] [CrossRef]
- Lubetzki, C.; Stankoff, B. Demyelination in multiple sclerosis. Handb. Clin. Neurol. 2014, 122, 89–99. [Google Scholar] [PubMed]
- Kumar, G.; Axtell, R.C. Dual Role of B Cells in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 2336. [Google Scholar] [CrossRef]
- Comi, G.; Bar-Or, A.; Lassmann, H.; Uccelli, A.; Hartung, H.P.; Montalban, X.; Sorensen, P.S.; Hohlfeld, R.; Hauser, S.L. Expert Panel of the 27th Annual Meeting of the European Charcot Foundation. The role of B cells in Multiple Sclerosis and related disorders. Ann. Neurol. 2021, 89, 13–23. [Google Scholar] [CrossRef]
- Huseby, E.S.; Kamimura, D.; Arima, Y.; Parello, C.S.; Sasaki, K.; Murakami, M. Role of T cell—Glial cell interactions in creating and amplifying central nervous system inflammation and multiple sclerosis disease symptoms. Front. Cell. Neurosci. 2015, 9, 295. [Google Scholar] [CrossRef]
- Suliman, B.A. Potential clinical implications of molecular mimicry-induced autoimmunity. Immun. Inflamm. Dis. 2024, 12, e1178. [Google Scholar] [CrossRef]
- Meyer-Arndt, L.; Kerkering, J.; Kuehl, T.; Infante, A.G.; Paul, F.; Rosiewicz, K.S.; Siffrin, V.; Alisch, M. Inflammatory Cytokines Associated with Multiple Sclerosis Directly Induce Alterations of Neuronal Cytoarchitecture in Human Neurons. J. Neuroimmune Pharmacol. 2023, 18, 145–159. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Farooq, M.; Hwang, M.J.; Haseeb, M.; Choi, S. Autoimmune Neuroinflammatory Diseases: Role of Interleukins. Int. J. Mol. Sci. 2023, 24, 7960. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, Inflammation and Pain. Int. Anesth. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Papiri, G.; D’Andreamatteo, G.; Cacchiò, G.; Alia, S.; Silvestrini, M.; Paci, C.; Luzzi, S.; Vignini, A. Multiple Sclerosis: Inflammatory and Neuroglial Aspects. Curr. Issues Mol. Biol. 2023, 45, 1443–1470. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Grunwald, C.; Krętowska-Grunwald, A.; Adamska-Patruno, E.; Kochanowicz, J.; Kułakowska, A.; Chorąży, M. The Role of Selected Interleukins in the Development and Progression of Multiple Sclerosis—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 2589. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Eva, L.; Pleș, H.; Covache-Busuioc, R.A.; Glavan, L.A.; Bratu, B.G.; Bordeianu, A.; Dumitrascu, D.l.; Corlatescu, A.D.; Ciurea, A.V. A Comprehensive Review on Neuroimmunology: Insights from Multiple Sclerosis to Future Therapeutic Developments. Biomedicines 2023, 11, 2489. [Google Scholar] [CrossRef] [PubMed]
- Jang D in Lee, A.H.; Shin, H.Y.; Song, H.R.; Park, J.H.; Kang, T.B.; Lee, S.R.; Yang, S.H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Stathopoulou, C.; Kapsetaki, M.; Stratigi, K.; Spilianakis, C. Long non-coding RNA SeT and miR-155 regulate the Tnfα gene allelic expression profile. PLoS ONE 2017, 12, e0184788. [Google Scholar] [CrossRef]
- Ruiz, A.; Palacios, Y.; Garcia, I.; Chavez-Galan, L. Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections. Int. J. Mol. Sci. 2021, 22, 5461. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Brand, D.D.; Zheng, S.G. Role of TNF–TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front. Immunol. 2018, 9, 784. [Google Scholar] [CrossRef]
- Park, K.M.; Bowers, W.J. Tumor Necrosis Factor-alpha Mediated Signaling in Neuronal Homeostasis and Dysfunction. Cell Signal. 2010, 22, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Goeddel, D.V. TNF-R1 signaling: A beautiful pathway. Science 2002, 296, 1634–1635. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, W.; Lin, Z. Functional roles in cell signaling of adaptor protein TRADD from a structural perspective. Comput. Struct. Biotechnol. J. 2020, 18, 2867–2876. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, J.; Han, J. Receptor-interacting protein (RIP) kinase family. Cell. Mol. Immunol. 2010, 7, 243–249. [Google Scholar] [CrossRef]
- Tang, Y.; Tu, H.; Zhang, J.; Zhao, X.; Wang, Y.; Qin, J.; Lin, X. K63-linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat. Commun. 2019, 10, 4157. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, S.S.; Zhao, S.; Yang, Z.; Zhong, C.Q.; Chen, X.; Cai, Q.; Yang, Z.H.; Huang, D.; Wu, R.; et al. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017, 8, 14329. [Google Scholar] [CrossRef]
- Siegmund, D.; Wagner, J.; Wajant, H. TNF Receptor Associated Factor 2 (TRAF2) Signaling in Cancer. Cancers 2022, 14, 4055. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.E.; Pantaki, D.; Feltham, R.; Mace, P.D.; Cordier, S.M.; Schmukle, A.C.; Davidson, A.J.; Callus, B.A.; Wong, W.W.L. TRAF2 Must Bind to Cellular Inhibitors of Apoptosis for Tumor Necrosis Factor (TNF) to Efficiently Activate NF-κB and to Prevent TNF-induced Apoptosis. J. Biol. Chem. 2009, 284, 35906–35915. [Google Scholar] [CrossRef]
- Bertrand, M.J.M.; Lippens, S.; Staes, A.; Gilbert, B.; Roelandt, R.; De Medts, J.; Gebaert, K.; Declercq, W.; Vandenabeele, P. cIAP1/2 Are Direct E3 Ligases Conjugating Diverse Types of Ubiquitin Chains to Receptor Interacting Proteins Kinases 1 to 4 (RIP1–4). PLoS ONE 2011, 6, e22356. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Israël, A. The IKK Complex, a Central Regulator of NF-κB Activation. Cold Spring Harb. Perspect. Biol. 2010, 2, a000158. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Traenckner, E.B.; Pahl, H.L.; Henkel, T.; Schmidt, K.N.; Wilk, S.; Baeuerle, P.A. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 1995, 14, 2876–2883. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Hung, M.C. Beyond NF-κB activation: Nuclear functions of IκB kinase α. J. Biomed. Sci. 2013, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; London, N.; Schueler-Furman, O.; Ben-Neriah, Y. Ubiquitination and Degradation of the Inhibitors of NF-κB. Cold Spring Harb. Perspect. Biol. 2010, 2, a000166. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2828279/ (accessed on 7 May 2024). [CrossRef]
- Ghosh, G.; Ya-Fan Wang, V.; Huang, D.B.; Fusco, A. NF-κB Regulation: Lessons from Structures. Immunol. Rev. 2012, 246, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Moatti, A.; Cohen, J.L. The TNF-α/TNFR2 Pathway: Targeting a Brake to Release the Anti-tumor Immune Response. Front. Cell Dev. Biol. 2021, 9, 725473. [Google Scholar] [CrossRef]
- Shi, J.H.; Sun, S.C. Tumor Necrosis Factor Receptor-Associated Factor Regulation of Nuclear Factor κB and Mitogen-Activated Protein Kinase Pathways. Front. Immunol. 2018, 9, 1849. [Google Scholar] [CrossRef]
- Chen, Z.J. Ubiquitination in Signaling to and Activation of IKK. Immunol. Rev. 2012, 246, 95–106. [Google Scholar] [CrossRef]
- Guven-Maiorov, E.; Keskin, O.; Gursoy, A.; VanWaes, C.; Chen, Z.; Tsai, C.J.; Nassinov, R. TRAF3 signaling: Competitive binding and evolvability of adaptive viral molecular mimicry. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2646–2655. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Guldenpfennig, C.; Teixeiro, E.; Daniels, M. NF-kB’s contribution to B cell fate decisions. Front. Immunol. 2023, 14, 1214095. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6277637/ (accessed on 8 May 2024). [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Evavold, C.L.; Kagan, J.C. Diverse Control Mechanisms of the Interleukin-1 Cytokine Family. Front. Cell Dev. Biol. 2022, 10, 910983. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Sig. Transduct. Target. Ther. 2021, 6, 1–24. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Sig. Transduct. Target. Ther. 2020, 5, 1–23. [Google Scholar] [CrossRef]
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 Transcription Factors as Regulators of Immune Responses in Cancer. Cancers 2019, 11, 1037. [Google Scholar] [CrossRef]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2019, 217, e20190314. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. USA 2009, 106, 9021–9026. [Google Scholar] [CrossRef]
- Macleod, T.; Berekmeri, A.; Bridgewood, C.; Stacey, M.; McGonagle, D.; Wittmann, M. The Immunological Impact of IL-1 Family Cytokines on the Epidermal Barrier. Front. Immunol. 2021, 12, 808012. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8733307/ (accessed on 8 May 2024). [CrossRef] [PubMed]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- Behzadi, P.; Sameer, A.S.; Nissar, S.; Banday, M.Z.; Gajdács, M.; García-Perdomo, H.A.; Akhtar, K.; Pinheiro, M.; Magnusson, P.; Sarshar, M.; et al. The Interleukin-1 (IL-1) Superfamily Cytokines and Their Single Nucleotide Polymorphisms (SNPs). J. Immunol. Res. 2022, 2022, 2054431. [Google Scholar] [CrossRef]
- Davis, C.N.; Mann, E.; Behrens, M.M.; Gaidarova, S.; Rebek, M.; Rebek, J.; Bartfai, T. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc. Natl. Acad. Sci. USA 2006, 103, 2953–2958. [Google Scholar] [CrossRef]
- Bayer, A.L.; Alcaide, P. MyD88: At the Heart of Inflammatory Signaling and Cardiovascular Disease. J. Mol. Cell. Cardiol. 2021, 161, 75–85. [Google Scholar] [CrossRef]
- Pereira, M.; Durso, D.F.; Bryant, C.E.; Kurt-Jones, E.A.; Silverman, N.; Golenbock, D.T.; Gazzinelli, R.T. The IRAK4 scaffold integrates TLR4-driven TRIF and MYD88 signaling pathways. Cell Rep. 2022, 40, 111225. [Google Scholar] [CrossRef]
- Vollmer, S.; Strickson, S.; Zhang, T.; Gray, N.; Lee, K.L.; Rao, V.R.; Cohen, P. The mechanism of activation of IRAK1 and IRAK4 by interleukin-1 and Toll-like receptor agonists. Biochem. J. 2017, 474, 2027. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, Q.; Ferrao, R.; Shen, C.; Hatcher, J.M.; Buhrlage, S.J.; Gray, N.S.; Wu, H. Crystal structure of human IRAK1. Proc. Natl. Acad. Sci. USA 2017, 114, 13507–13512. [Google Scholar] [CrossRef]
- Zhou, H.; Bulek, K.; Li, X.; Herjan, T.; Yu, M.; Qian, W.; Wang, H.; Zhou, G.; Chen, X.; Yang, H.; et al. IRAK2 directs stimulus-dependent nuclear export of inflammatory mRNAs. eLife 2017, 6, e29630. [Google Scholar] [CrossRef]
- Yamamoto, M.; Gohda, J.; Akiyama, T.; Inoue, J.I. TNF receptor-associated factor 6 (TRAF6) plays crucial roles in multiple biological systems through polyubiquitination-mediated NF-κB activation. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2021, 97, 145–160. [Google Scholar] [CrossRef]
- Walsh, M.C.; Lee, J.; Choi, Y. Tumor necrosis factor receptor associated factor 6 (TRAF6) regulation of development, function, and homeostasis of the immune system. Immunol. Rev. 2015, 266, 72–92. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Jiang, M.; Tai, G. Mechanism by which TRAF6 Participates in the Immune Regulation of Autoimmune Diseases and Cancer. Biomed. Res. Int. 2020, 2020, 4607197. [Google Scholar] [CrossRef] [PubMed]
- Kishida, S.; Sanjo, H.; Akira, S.; Matsumoto, K.; Ninomiya-Tsuji, J. TAK1-binding protein 2 facilitates ubiquitination of TRAF6 and assembly of TRAF6 with IKK in the IL-1 signaling pathway. Genes Cells 2005, 10, 447–454. [Google Scholar] [CrossRef]
- Walsh, M.C.; Kim, G.K.; Maurizio, P.L.; Molnar, E.E.; Choi, Y. TRAF6 Autoubiquitination-Independent Activation of the NFκB and MAPK Pathways in Response to IL-1 and RANKL. PLoS ONE 2008, 3, e4064. [Google Scholar] [CrossRef]
- Ali, T.; Nguyen, H.M.; Abbas, N.; Takeuchi, O.; Akira, S.; Suzuki, T.; Matsuzaki, G.; Takaesu, G. TAK1-binding protein 2 (TAB2) and TAB3 are redundantly required for TLR-induced cytokine production in macrophages. Int. Immunol. 2024, 36, 439–450. [Google Scholar] [CrossRef]
- Wang, P.N.; Huang, J.; Duan, Y.H.; Zhou, J.M.; Huang, P.Z.; Fan, X.J.; Huang, Y.; Wang, L.; Liu, H.L.; Wang, J.P.; et al. Downregulation of phosphorylated MKK4 is associated with a poor prognosis in colorectal cancer patients. Oncotarget 2017, 8, 34352–34361. [Google Scholar] [CrossRef][Green Version]
- Raingeaud, J.; Whitmarsh, A.J.; Barrett, T.; Dérijard, B.; Davis, R.J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 1996, 16, 1247–1255. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Hou, W.; Jin, Y.H.; Kang, H.S.; Kim, B.S. Interleukin-6 (IL-6) and IL-17 Synergistically Promote Viral Persistence by Inhibiting Cellular Apoptosis and Cytotoxic T Cell Function. J. Virol. 2014, 88, 8479–8489. [Google Scholar] [CrossRef]
- Escartín-Gutiérrez, J.R.; Ponce-Figueroa, M.; Torres-Vega, M.Á.; Aguilar-Faisal, L.; Figueroa-Arredondo, P. Transcriptional Activation of a Pro-Inflammatory Response (NF-κB, AP-1, IL-1β) by the Vibrio cholerae Cytotoxin (VCC) Monomer through the MAPK Signaling Pathway in the THP-1 Human Macrophage Cell Line. Int. J. Mol. Sci. 2023, 24, 7272. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Baran, P.; Hansen, S.; Waetzig, G.H.; Akbarzadeh, M.; Lamertz, L.; Huber, H.J.; Ahmadian, M.R.; Moll, J.M.; Scheller, J. The balance of interleukin (IL)-6, IL-6·soluble IL-6 receptor (sIL-6R), and IL-6·sIL-6R·sgp130 complexes allows simultaneous classic and trans-signaling. J. Biol. Chem. 2018, 293, 6762–6775. [Google Scholar] [CrossRef]
- Villar-Fincheira, P.; Sanhueza-Olivares, F.; Norambuena-Soto, I.; Cancino-Arenas, N.; Hernandez-Vargas, F.; Troncoso, R.; Gabrielli, L.; Chiong, M. Role of Interleukin-6 in Vascular Health and Disease. Front. Mol. Biosci. 2021, 8, 641734. [Google Scholar] [CrossRef] [PubMed]
- Adam, N.; Rabe, B.; Suthaus, J.; Grötzinger, J.; Rose-John, S.; Scheller, J. Unraveling Viral Interleukin-6 Binding to gp130 and Activation of STAT-Signaling Pathways Independently of the Interleukin-6 Receptor. J. Virol. 2009, 83, 5117. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef]
- Kuchipudi, S.V. The Complex Role of STAT3 in Viral Infections. J. Immunol. Res. 2015, 2015, 272359. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Solanki, H.S.; Advani, J.; Khan, A.A.; Keshava Prasad, T.S.; Gowda, H.; Thiyagarajan, S.; Chatterjee, A. Comprehensive network map of interferon gamma signaling. J. Cell Commun. Signal. 2018, 12, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef]
- de Weerd, N.A.; Nguyen, T. The interferons and their receptors—Distribution and regulation. Immunol. Cell Biol. 2012, 90, 483–491. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of Signaling and Immune Responses by IFN-γ and STAT1. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Jun, J.E.; Rubio, I.; Roose, J.P. Regulation of Ras Exchange Factors and Cellular Localization of Ras Activation by Lipid Messengers in T Cells. Front. Immunol. 2013, 4, 239. [Google Scholar] [CrossRef]
- Matallanas, D.; Birtwistle, M.; Romano, D.; Zebisch, A.; Rauch, J.; von Kriegsheim, A.; Kolch, W. Raf Family Kinases. Genes Cancer 2011, 2, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, G.D.; Shi, Z.; Qi, L.L.; Zhou, L.Y.; Fu, Z.X. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol. Lett. 2016, 12, 3045–3050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.F.; Guan, K.L. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 1994, 13, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Targeting ERK1/2 protein-serine/threonine kinases in human cancers. Pharmacol. Res. 2019, 142, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Maik-Rachline, G.; Hacohen-Lev-Ran, A.; Seger, R. Nuclear ERK: Mechanism of Translocation, Substrates, and Role in Cancer. Int. J. Mol. Sci. 2019, 20, 1194. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Wortzel, I.; Seger, R. The ERK Cascade. Genes Cancer 2011, 2, 195–209. [Google Scholar] [CrossRef]
- Kim, C.; Sano, Y.; Todorova, K.; Carlson, B.A.; Arpa, L.; Celada, A.; Lawrence, T.; Otsu, K.; Brissette, J.L.; Arthur, J.S.C.; et al. p38α MAP kinase serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat. Immunol. 2008, 9, 1019–1027. [Google Scholar] [CrossRef]
- Koul, H.K.; Pal, M.; Koul, S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer 2013, 4, 342–359. [Google Scholar] [CrossRef]
- Mathien, S.; Tesnière, C.; Meloche, S. Regulation of Mitogen-Activated Protein Kinase Signaling Pathways by the Ubiquitin-Proteasome System and Its Pharmacological Potential. Pharmacol. Rev. 2021, 73, 263–296. [Google Scholar] [CrossRef]
- Musi, C.A.; Agrò, G.; Santarella, F.; Iervasi, E.; Borsello, T. JNK3 as Therapeutic Target and Biomarker in Neurodegenerative and Neurodevelopmental Brain Diseases. Cells 2020, 9, 2190. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The role of sirtuins in cellular homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Keogh, J.B.; Clifton, P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018, 31, 35–51. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013, 48, 634–639. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Yang, G.; Chi, X.; Liang, X.; Zhang, Y. Sirtuins: Promising Therapeutic Targets to Treat Ischemic Stroke. Biomolecules 2023, 13, 1210. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Sun, Y.; Luo, Y.; Shen, Y.; Shao, A. Will Sirtuins Be Promising Therapeutic Targets for TBI and Associated Neurodegenerative Diseases? Front. Neurosci. 2020, 14, 791. [Google Scholar] [CrossRef]
- Magliozzi, R.; Pezzini, F.; Pucci, M.; Rossi, S.; Facchiano, F.; Marastoni, D.; Montagnana, M.; Lippi, G.; Reynolds, R.; Calabrese, M. Changes in Cerebrospinal Fluid Balance of TNF and TNF Receptors in Naïve Multiple Sclerosis Patients: Early Involvement in Compartmentalised Intrathecal Inflammation. Cells 2021, 10, 1712. [Google Scholar] [CrossRef]
- Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 2009, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Ortí-Casañ, N.; Boerema, A.S.; Köpke, K.; Ebskamp, A.; Keijser, J.; Zhang, Y.; Chen, T.; Dolga, A.M.; Broersen, K.; Rischer, R.; et al. The TNFR1 antagonist Atrosimab reduces neuronal loss, glial activation and memory deficits in an acute mouse model of neurodegeneration. Sci. Rep. 2023, 13, 10622. [Google Scholar] [CrossRef] [PubMed]
- Fresegna, D.; Bullitta, S.; Musella, A.; Rizzo, F.R.; De Vito, F.; Guadalupi, L.; Caioli, S.; Balletta, S.; Sanna, K.; Dolcetti, E.; et al. Re-Examining the Role of TNF in MS Pathogenesis and Therapy. Cells 2020, 9, 2290. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Busmail, A.; Penumetcha, S.S.; Ahluwalia, S.; Irfan, R.; Khan, S.A.; Reddy, S.R.; Lopez, M.E.V.; Mohammed, L. Tumor Necrosis Factor Alpha Blockade and Multiple Sclerosis: Exploring New Avenues. Cureus 2021, 13, e18847. [Google Scholar] [CrossRef]
- Lei, Z.; Lin, W. Mechanisms Governing Oligodendrocyte Viability in Multiple Sclerosis and Its Animal Models. Cells 2024, 13, 116. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Lin, C.C.; Edelson, B.T. New Insights into the Role of IL-1β in EAE and MS. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1α and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Rivera, K.; Andia, M.E.; Martínez Rodriguez, G. The IL-1 Family and Its Role in Atherosclerosis. Int. J. Mol. Sci. 2022, 24, 17. [Google Scholar] [CrossRef]
- Duarte-Silva, E.; Ulrich, H.; Oliveira-Giacomelli, Á.; Hartung, H.P.; Meuth, S.G.; Peixoto, C.A. The adenosinergic signaling in the pathogenesis and treatment of multiple sclerosis. Front. Immunol. 2022, 13, 946698. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9368763/ (accessed on 9 May 2024). [CrossRef] [PubMed]
- Peters, V.A.; Joesting, J.J.; Freund, G.G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 2013, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Supino, D.; Minute, L.; Mariancini, A.; Riva, F.; Magrini, E.; Garlanda, C. Negative Regulation of the IL-1 System by IL-1R2 and IL-1R8: Relevance in Pathophysiology and Disease. Front. Immunol. 2022, 13, 804641. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bao, R.; Li, L.; Deisenhammer, F.; Arnason, B.G.W.; Reder, A.T. Interferon-β corrects massive gene dysregulation in multiple sclerosis: Short-term and long-term effects on immune regulation and neuroprotection. EBioMedicine 2019, 49, 269–283. [Google Scholar] [CrossRef]
- Choi, M.Y.; Costenbader, K.H. Understanding the Concept of Pre-Clinical Autoimmunity: Prediction and Prevention of Systemic Lupus Erythematosus: Identifying Risk Factors and Developing Strategies Against Disease Development. Front. Immunol. 2022, 13, 890522. [Google Scholar] [CrossRef]
- Fiedler, T.; Fairless, R.; Pichi, K.; Fischer, R.; Richter, F.; Kontermann, R.E.; Pfizenmaier, K.; Diem, R.; Williams, S.K. Co-modulation of TNFR1 and TNFR2 in an animal model of multiple sclerosis. J. Neuroinflamm. 2023, 20, 100. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- Inojosa, H.; Proschmann, U.; Akgün, K.; Ziemssen, T. The need for a strategic therapeutic approach: Multiple sclerosis in check. Ther. Adv. Chronic Dis. 2022, 13, 20406223211063032. [Google Scholar] [CrossRef]
- Higuera, L.; Carlin, C.S.; Anderson, S. Adherence to Disease-Modifying Therapies for Multiple Sclerosis. J. Manag. Care Spec. Pharm. 2016, 22, 1394–1401. [Google Scholar] [CrossRef]
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.E.; de Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple Sclerosis: Immunopathology and Treatment Update. Brain Sci. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.J.; Buckle, G.J.; Singer, B.; Singh, V.; Boster, A. Lymphopenia and DMTs for relapsing forms of MS. Neurol. Clin. Pract. 2019, 9, 53–63. [Google Scholar] [CrossRef]
- Finkelsztejn, A. Multiple Sclerosis: Overview of Disease-Modifying Agents. Perspect. Med. Chem. 2014, 6, 65–72. [Google Scholar] [CrossRef]
- Laurenti, L.; Gaidano, G.; Mauro, F.R.; Molica, S.; Pasqualetti, P.; Scarfò, L.; Ghia, P. What Are the Attributes Prioritized in the Choice of Therapy in Chronic Lymphocytic Leukemia? A Patient-physician Cross-matching Analysis of a Discrete Choice Experiment. HemaSphere 2022, 6, e771. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9436277/ (accessed on 10 May 2024). [CrossRef]
- Amin, M.; Hersh, C.M. Updates and advances in multiple sclerosis neurotherapeutics. Neurodegener. Dis. Manag. 2022, 13, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Lalive, P.H.; Neuhaus, O.; Benkhoucha, M.; Burger, D.; Hohlfeld, R.; Zamvil, S.S.; Weber, M.S. Glatiramer Acetate in the Treatment of Multiple Sclerosis. CNS Drugs 2011, 25, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Kasindi, A.; Fuchs, D.T.; Koronyo, Y.; Rentsendorj, A.; Black, K.L.; Koronyo-Hamaoui, M. Glatiramer Acetate Immunomodulation: Evidence of Neuroprotection and Cognitive Preservation. Cells 2022, 11, 1578. [Google Scholar] [CrossRef] [PubMed]
- Schrempf, W.; Ziemssen, T. Glatiramer acetate: Mechanisms of action in multiple sclerosis. Autoimmun. Rev. 2007, 6, 469–475. [Google Scholar] [CrossRef]
- Adalid-Peralta, L.; Fragoso, G.; Fleury, A.; Sciutto, E. Mechanisms Underlying the Induction of Regulatory T cells and Its Relevance in the Adaptive Immune Response in Parasitic Infections. Int. J. Biol. Sci. 2011, 7, 1412–1426. [Google Scholar] [CrossRef]
- Duda, P.W.; Schmied, M.C.; Cook, S.L.; Krieger, J.I.; Hafler, D.A. Glatiramer acetate (Copaxone®) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J. Clin. Investig. 2000, 105, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Herges, K.; Millward, J.M.; Hentschel, N.; Infante-Duarte, C.; Aktas, O.; Zipp, F. Neuroprotective Effect of Combination Therapy of Glatiramer Acetate and Epigallocatechin-3-Gallate in Neuroinflammation. PLoS ONE 2011, 6, e25456. [Google Scholar] [CrossRef]
- Michell-Robinson, M.A.; Moore, C.S.; Healy, L.M.; Osso, L.A.; Zorko, N.; Grouza, V.; Touil, H.; Poliquin-Lasnier, L.; Trudelle, A.M.; Giacomini, P.S.; et al. Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann. Clin. Transl. Neurol. 2015, 3, 27–41. [Google Scholar] [CrossRef]
- Marques, E.S.; Severance, E.G.; Min, B.; Arsenault, P.; Conlin, S.M.; Timme-Laragy, A.R. Developmental impacts of Nrf2 activation by Dimethyl fumarate (DMF) in the developing Zebrafish (Danio rerio) embryo. Free Radic. Biol. Med. 2023, 194, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Crisman, E.; Duarte, P.; Dauden, E.; Cuadrado, A.; Rodríguez-Franco, M.I.; López, M.G.; Leon, R. KEAP1-NRF2 protein–protein interaction inhibitors: Design, pharmacological properties and therapeutic potential. Med. Res. Rev. 2023, 43, 237–287. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Song, Y.; Qu, Y.; Mao, C.; Zhang, R.; Jiang, D.; Sun, X. Post-translational modifications of Keap1: The state of the art. Front. Cell Dev. Biol. 2023, 11, 1332049. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10801156/ (accessed on 10 May 2024). [CrossRef]
- Wang, Y.; Gao, L.; Chen, J.; Li, Q.; Huo, L.; Wang, Y.; Wang, H.; Du, J. Pharmacological Modulation of Nrf2/HO-1 Signaling Pathway as a Therapeutic Target of Parkinson’s Disease. Front. Pharmacol. 2021, 12, 757161. [Google Scholar] [CrossRef]
- McGuire, V.A.; Ruiz-Zorrilla Diez, T.; Emmerich, C.H.; Strickson, S.; Ritorto, M.S.; Sutavani, R.V.; Weiβ, A.; Houslay, K.F.; Axel, K.; Meakin, P.J.; et al. Dimethyl fumarate blocks pro-inflammatory cytokine production via inhibition of TLR induced M1 and K63 ubiquitin chain formation. Sci. Rep. 2016, 6, 31159. [Google Scholar] [CrossRef]
- Gill, A.J.; Kolson, D.L. Dimethyl fumarate modulation of immune and antioxidant responses: Application to HIV therapy. Crit. Rev. Immunol. 2013, 33, 307–359. [Google Scholar] [CrossRef]
- Cross, S.A.; Cook, D.R.; Chi, A.W.S.; Vance, P.J.; Kolson, L.L.; Wong, B.J.; Jordan-Sciutto, K.L.; Kolson, D.L. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity; a novel candidate for HIV-neuroprotection. J. Immunol. 2011, 187, 5015–5025. [Google Scholar] [CrossRef]
- Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Teriflunomide and Its Mechanism of Action in Multiple Sclerosis. Drugs 2014, 74, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Tilly, G.; Cadoux, M.; Garcia, A.; Morille, J.; Wiertlewski, S.; Pecqueur, C.; Brouard, S.; Laplaud, D.; Degauque, N. Teriflunomide Treatment of Multiple Sclerosis Selectively Modulates CD8 Memory T Cells. Front. Immunol. 2021, 12, 730342. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; O’Connor, P.W. An update of teriflunomide for treatment of multiple sclerosis. Ther. Clin. Risk Manag. 2013, 9, 177–190. [Google Scholar]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Malla, B.; Cotten, S.; Ulshoefer, R.; Paul, F.; Hauser, A.E.; Niesner, R.; Bros, H.; Infante-Duarte, C. Teriflunomide preserves peripheral nerve mitochondria from oxidative stress-mediated alterations. Ther. Adv. Chronic Dis. 2020, 11, 2040622320944773. [Google Scholar] [CrossRef]
- Huwiler, A.; Zangemeister-Wittke, U. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: Recent findings and new perspectives. Pharmacol. Ther. 2018, 185, 34–49. [Google Scholar] [CrossRef]
- Chun, J.; Hartung, H.P. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 2010, 33, 91–101. [Google Scholar] [CrossRef]
- Brinkmann, V. FTY720 (fingolimod) in Multiple Sclerosis: Therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 2009, 158, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.F.; Bowen, J.D.; Reder, A.T. The Direct Effects of Fingolimod in the Central Nervous System: Implications for Relapsing Multiple Sclerosis. CNS Drugs 2016, 30, 135–147. [Google Scholar] [CrossRef]
- Bascuñana, P.; Möhle, L.; Brackhan, M.; Pahnke, J. Fingolimod as a Treatment in Neurologic Disorders Beyond Multiple Sclerosis. Drugs R D 2020, 20, 197–207. [Google Scholar] [CrossRef] [PubMed]
- de Sa, J.C.C.; Airas, L.; Bartholome, E.; Grigoriadis, N.; Mattle, H.; Oreja-Guevara, C.; O’Riordan, J.; Sellebjerg, F.; Stankoff, B.; Vass, K.; et al. Symptomatic therapy in multiple sclerosis: A review for a multimodal approach in clinical practice. Ther. Adv. Neurol. Disord. 2011, 4, 139–168. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, O.; Costa-Frossard, L.; Martínez-Ginés, M.L.; Montero, P.; Prieto-González, J.M.; Ramió-Torrentà, L. Integrated Management of Multiple Sclerosis Spasticity and Associated Symptoms Using the Spasticity-Plus Syndrome Concept: Results of a Structured Specialists’ Discussion Using the Workmat® Methodology. Front. Neurol. 2021, 12, 722801. [Google Scholar] [CrossRef]
- Joy, J.; Johnston, R., Jr. Characterisitics and Management of Major Symptoms. In Multiple Sclerosis: Current Status and Strategies for the Future; National Academies Press (US): Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222388/ (accessed on 10 May 2024).
- Cameron, A.P. Medical management of neurogenic bladder with oral therapy. Transl. Androl. Urol. 2016, 5, 51–62. [Google Scholar]
- Barman, A.; Chatterjee, A.; Bhide, R. Cognitive Impairment and Rehabilitation Strategies after Traumatic Brain Injury. Indian, J. Psychol. Med. 2016, 38, 172–181. [Google Scholar] [CrossRef]
- Ramo-Tello, C.; Blanco, Y.; Brieva, L.; Casanova, B.; Martínez-Cáceres, E.; Ontaneda, D.; Ramio-Torrenta, L.; Rovira, A. Recommendations for the Diagnosis and Treatment of Multiple Sclerosis Relapses. J. Pers. Med. 2021, 12, 6. [Google Scholar] [CrossRef]
- Bindawas, S.M.; Vennu, V.S. Stroke rehabilitation. Neurosciences 2016, 21, 297–305. [Google Scholar] [CrossRef]
- Ross, A.P.; Ben-Zacharia, A.; Harris, C.; Smrtka, J. Multiple Sclerosis, Relapses, and the Mechanism of Action of Adrenocorticotropic Hormone. Front. Neurol. 2013, 4, 21. [Google Scholar] [CrossRef]
- Ontaneda, D.; Rae-Grant, A.D. Management of acute exacerbations in multiple sclerosis. Ann. Indian Acad. Neurol. 2009, 12, 264–272. [Google Scholar]
- Jacob, S.; Mazibrada, G.; Irani, S.R.; Jacob, A.; Yudina, A. The Role of Plasma Exchange in the Treatment of Refractory Autoimmune Neurological Diseases: A Narrative Review. J. Neuroimmune Pharmacol. 2021, 16, 806–817. [Google Scholar] [CrossRef]
- Ikeda, K.M.; Lee, D.H.; Fraser, J.A.; Mirsattari, S.; Morrow, S.A. Plasma Exchange in a Patient with Tumefactive, Corticosteroid-Resistant Multiple Sclerosis. Int. J. MS Care 2015, 17, 231–235. [Google Scholar] [CrossRef]
- Hussein, G.; Liu, B.; Yadav, S.K.; Warsame, M.; Jamil, R.; Surani, S.R.; Khan, S.A. Plasmapheresis in the ICU. Medicina 2023, 59, 2152. [Google Scholar] [CrossRef] [PubMed]
- Neate, S.L.; Donald, A.; Jelinek, G.A.; Nag, N. Experiences of and attitudes to lifestyle modification for the management of multiple sclerosis: A qualitative analysis of free-text survey data. Health Expect. 2022, 25, 214–222. [Google Scholar] [CrossRef]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef]
- Cincotta, M.C.; Engelhard, M.M.; Stankey, M.; Goldman, M.D. Fatigue and fluid hydration status in multiple sclerosis: A hypothesis. Mult. Scler. 2016, 22, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.B.; Hadgkiss, E.J.; Weiland, T.J.; Jelinek, G.A. Meditation as an Adjunct to the Management of Multiple Sclerosis. Neurol. Res. Int. 2014, 2014, 704691. [Google Scholar] [CrossRef]
- Coyle, P.K. Symptom Management and Lifestyle Modifications in Multiple Sclerosis. Continuum 2016, 22, 815–836. [Google Scholar] [CrossRef]
- Racke, M.K. Challenges in Developing New Multiple Sclerosis Therapies. Ther. Adv. Neurol. Disord. 2008, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Mowry, E.M.; Newsome, S.D. Early Aggressive Treatment Approaches for Multiple Sclerosis. Curr. Treat. Opt. Neurol. 2021, 23, 19. [Google Scholar] [CrossRef]
- Nguyen, S.A.; Lavretsky, H. Emerging Complementary and Integrative Therapies for Geriatric Mental Health. Curr. Treat. Opt. Psychiatry 2020, 7, 447–470. [Google Scholar] [CrossRef]
- Mehr, S.R.; Zimmerman, M.P. Reviewing the Unmet Needs of Patients with Multiple Sclerosis. Am. Health Drug Benefits 2015, 8, 426–431. [Google Scholar]
- Angeloni, S.; Navarini, L.; Khamitova, G.; Sagratini, G.; Vittori, S.; Caprioli, G. Quantification of lignans in 30 ground coffee samples and evaluation of theirs extraction yield in espresso coffee by HPLC-MS/MS triple quadrupole. Int. J. Food Sci. Nutr. 2020, 71, 193–200. [Google Scholar] [CrossRef]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxid. Med. Cell Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Warden, B.A.; Smith, L.S.; Beecher, G.R.; Balentine, D.A.; Clevidence, B.A. Catechins are bioavailable in men and women drinking black tea throughout the day. J. Nutr. 2001, 131, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Merck &, Co. EGCG. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m4843 (accessed on 28 August 2024).
- Merck &, Co. Quercetin. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m9420 (accessed on 28 August 2024).
- Merck &, Co. Resveratrol. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m9549 (accessed on 28 August 2024).
- Merck &, Co. Ellagic Acid. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m4872 (accessed on 28 August 2024).
- Merck &, Co. Luteolin. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m6945 (accessed on 28 August 2024).
- Merck &, Co. Curcumin. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m3933 (accessed on 28 August 2024).
- Talib, W.H.; Awajan, D.; Alqudah, A.; Alsawwaf, R.; Althunibat, R.; Abu AlRoos, M.; Safadi, A.A.; Asab, S.A.; Hadi, R.W.; Kury, L.T.A. Targeting Cancer Hallmarks with Epigallocatechin Gallate (EGCG): Mechanistic Basis and Therapeutic Targets. Molecules 2024, 29, 1373. [Google Scholar] [CrossRef]
- James, A.; Wang, K.; Wang, Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [PubMed]
- Afzal, O.; Dalhat, M.H.; Altamimi, A.S.A.; Rasool, R.; Alzarea, S.I.; Almalki, W.H.; Murtaza, B.N.; Iftikhar, S.; Nadeem, S.; Nadeem, M.S.; et al. Green Tea Catechins Attenuate Neurodegenerative Diseases and Cognitive Deficits. Molecules 2022, 27, 7604. [Google Scholar] [CrossRef]
- Schroeder, E.K.; Kelsey, N.A.; Doyle, J.; Breed, E.; Bouchard, R.J.; Loucks, F.A.; Harbison, R.A.; Linseman, D.A. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid. Redox Signal. 2009, 11, 469–480. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell. Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Ntamo, Y.; Jack, B.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Nkambule, B.B.; Nyambuya, T.M.; Mabhida, S.E.; Hanser, S.; Orlando, P.; Tiano, L.; et al. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: Novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit. Rev. Food Sci. Nutr. 2022, 64, 87–109. Available online: https://www.tandfonline.com/doi/abs/10.1080/10408398.2022.2104805 (accessed on 10 May 2024). [CrossRef] [PubMed]
- Bellmann-Strobl, J.; Paul, F.; Wuerfel, J.; Dörr, J.; Infante-Duarte, C.; Heidrich, E.; Kortgen, B.; Brandt, A.; Pfuller, C.; Radbruch, H.; et al. Epigallocatechin Gallate in Relapsing-Remitting Multiple Sclerosis: A Randomized, Placebo-Controlled Trial. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e981. [Google Scholar] [CrossRef]
- Cuerda-Ballester, M.; Proaño, B.; Alarcón-Jimenez, J.; de Bernardo, N.; Villaron-Casales, C.; Lajara Romance, J.M.; Orti, J.E.d.l.R. Improvements in gait and balance in patients with multiple sclerosis after treatment with coconut oil and epigallocatechin gallate. A pilot study. Food Funct. 2023, 14, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, M.; Cuerda Ballester, M.; Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; López-Rodríguez, M.M.; Ceron, J.J.; Tvarijonaviciute, A.; Navarro, M.A.; Moreno, M.L.; et al. Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients 2020, 12, 3792. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.S.; Wu, D. Anti-Fatigue Effect of Green Tea Polyphenols (-)-Epigallocatechin-3-Gallate (EGCG). Pharmacogn. Mag. 2017, 13, 326–331. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Ghaiad, H.R.; Nooh, M.M.; El-Sawalhi, M.M.; Shaheen, A.A. Resveratrol Promotes Remyelination in Cuprizone Model of Multiple Sclerosis: Biochemical and Histological Study. Mol. Neurobiol. 2017, 54, 3219–3229. [Google Scholar] [CrossRef]
- Jiao, F.; Gong, Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid. Med. Cell Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Margină, D. Sirtuins, resveratrol and the intertwining cellular pathways connecting them. Ageing Res. Rev. 2023, 88, 101936. [Google Scholar] [CrossRef] [PubMed]
- Sergides, C.; Chirila, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, K.; Liu, Y.; Yin, X.; Zhu, H.; Yu, F.; Zhao, W. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J. Control. Release 2023, 353, 675–684. [Google Scholar] [CrossRef]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef] [PubMed]
- Kasikci, M.B.; Bagdathoglu, N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- Bayazid, A.B.; Lim, B.O. Quercetin is an active agent in berries against neurodegenerative diseases progression through modulation of Nrf2/HO1. Nutrients 2022, 14, 5132. [Google Scholar] [CrossRef]
- De Boer, V.C.J.; de Goffau, M.C.; Arts, I.C.W.; Hollman, P.C.H.; Keijer, J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006, 127, 618–627. [Google Scholar] [CrossRef]
- Qu, L.; Liang, X.; Gu, B.; Liu, W. Quercetin alleviates high glucose-induced Schwann cell damage by autophagy. Neural Regen. Res. 2014, 15, 1195–1203. [Google Scholar] [CrossRef]
- Wu, X.; Qu, X.; Zhang, Q.; Dong, F.; Yu, H.; Yan, C.; Qi, D.; Wang, M. Quercetin promotes proliferation and differentiation of oligodendrocyte precursor cells after oxygen/glucose deprivation-induced injury. Cell Mol. Neurobiol. 2014, 34, 463–471. [Google Scholar] [CrossRef]
- Prasad, J.; Baitharu, I.; Sharma, A.K.; Dutta, R.; Prasd, D.; Singh, S.B. Quercetin reverses hypobaric hypoxia-induced hippocampal neurodegeneration and improves memory function in the rat. High Alt. Med. Biol. 2013, 14, 383–394. [Google Scholar] [CrossRef]
- Yu, J.; Sun, Y.; Zhao, H.; Li, K.; Wang, L. Study on the mechanism of quercetin promoting myelin regeneration in CPZ induced demyelinating mice model. Int. J. of Trad. Chin. Med. 2020, 6, 39–45. [Google Scholar]
- Hendriks, J.J.A.; de Vries, H.E.; van der Pol, S.M.A.; van den Berg, T.K.; van Tol, E.A.F.; Dijkstra, C.D. Flavonoids inhibit myelin phagocytosis by macrophages; a structure-activity relationship study. Biochem Pharmacol. 2003, 65, 877–885. [Google Scholar] [CrossRef]
- Ahmadi, L.; Eskandari, N.; Ghanadian, M.; Rahmati, M.; Kasiri, N.; Etamadifar, M.; Toghyani, M.; Alsahebfosoul, F. The immunomodulatory aspect of quercetin penta acetate on Th17 cells proliferation and gene expression in multiple sclerosis. Cell, J. 2023, 25, 110–117. [Google Scholar] [PubMed]
- Sternberg, Z.; Chadha, K.; Lieberman, A.; Hojnacki, D.; Drake, A.; Zamboni, P.; Rocco, P.; Grazioli, E.; Weinstock-Guttman, B.; Munschauer, F. Quercetin and interferon-beta modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J. Neuroimmunol. 2008, 205, 142–147. [Google Scholar] [CrossRef]
- Tan, Z.; Yang, G.; Qiu, J.; Yan, W.; Liu, Y.; Ma, Z.; Li, J.; Liu, J.; Shan, N. Quercetin alleviates demyelination Through Regulating Microglial Phenotype Transformation to Mitigate Neuropsychiatric Symptoms in Mice with Vascular Dementia. Mol. Neurobiol. 2022, 59, 3140–3158. [Google Scholar] [CrossRef]
- Muthian, G.; Bright, J.J. Experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J. Clin. Immunol. 2004, 24, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential implications of quercetin in autoimmune diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, D.V.; Carvalho, F.B.; Mazzanti, C.M.; Dos Santos, R.P.; Andrades, A.O.; Aiello, G.; Rippilinger, A.; Graca, D.L.; Abdalla, F.H.; Oliveira, L.S.; et al. Neuroprotective role of quercetin in locomotor activities and cholinergic neurotransmission in rats experimentally demyelinated with ethidium bromide. Life Sci. 2014, 103, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.B.; Gutierres, J.M.; Beckmann, D.; Santos, R.P.; Thomé, G.R.; Baldissarelli, J.; Stefanello, N.; Andrades, A.; Aiello, G.; Ripplinger, A.; et al. Quercetin treatment regulates the Na+, K+-ATPase activity, peripheral cholinergic enzymes, and oxidative stress in a rat model of demyelination. Natr. Res. 2018, 55, 45–56. [Google Scholar] [CrossRef]
- Naeimi, R.; Baradaran, S.; Ashrafpour, M.; Moghadamnia, A.A.; Ghasemi-Kasman, M. Querectin improves myelin repair of optic chiasm in lyolecithin-induced focal demyelination model. Biomed. Pharmacother. 2018, 101, 485–493. [Google Scholar] [CrossRef]
- Danshdoust, D.; Khalili-Fomeshi, M.; Ghasemi-Kasman, M.; Ghorbanian, D.; Hashemian, M.; Gholami, M.; Moghadamnia, A.; Shojaei, A. Pregabalin enhances myelin repair and attenuates glial activation in lysolecithin-induced demyelination model of rat optic chiasm. Neuroscience 2017, 6, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Vadhanam, M.V.; Aqil, F.; Ravoori, S.; Gupta, R.C. Bioavailability of ellagic acid/ellagitannins from black raspberry and pomegranate. Cancer Res. 2011, 71, 4603. [Google Scholar] [CrossRef]
- Han, B.; Shi, L.; Bao, M.Y.; Yu, F.L.; Zhang, Y.; Lu, X.Y.; Wang, Y.; Li, D.X.; Lin, J.C.; Jia, W.; et al. Dietary ellagic acid therapy for CNS autoimmunity: Targeting on Alloprevotella rava and propionate metabolism. Microbiome 2024, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Kiasalari, Z.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Azadi-Ahmadabadi, E.; Esmaeil-Jamaat, E.; Fahanick-Babaei, J.; Fakour, M.; Fereidouni, F.; Ghasemi-Tarie, R.; Jalalzade-Ogvar, S. Ellagic acid ameliorates neuroinflammation and demyelination in experimental autoimmune encephalomyelitis: Involvement of NLRP3 and pyroptosis. J. Chem. Neuroanat. 2021, 111, 101891. [Google Scholar] [CrossRef] [PubMed]
- Karegar, S.J.; Aryaeian, N.; Hajiluian, G.; Suzuki, K.; Shidfar, F.; Salehi, M.; Ashtiani, B.H.; Farhangnia, P.; Delbandi, A.A. Ellagic acid effects on disease severity, levels of cytokines and T-bet, RORγt, and GATA3 genes expression in multiple sclerosis patients: A multicentral-triple blind randomized clinical trial. Front. Nutr. 2023, 10, 128846. [Google Scholar]
- He, X.M.; Zhou, Y.Z.; Sheng, S.; Li, J.J.; Wang, G.Q.; Zhang, F. Ellagic Acid Protects Dopamine Neurons via Inhibition of NLRP3 Inflammasome Activation in Microglia. Oxidative Med. Cell. Longev. 2020, 2020, 2963540. [Google Scholar] [CrossRef]
- Nabil-Adam, A.; Ashour, M.L.; Shreadah, M.A. Modulation of MAPK/NF-κB Pathway and NLRP3 Inflammasome by Secondary Metabolites from Red Algae: A Mechanistic Study. ACS Omega 2023, 8, 37971–37990. [Google Scholar] [CrossRef]
- Zhao, J.; Li, G.; Wei, J.; Dang, S.; Yu, X.; Ding, L.; Shang, C.; Zhang, H.; Zhang, Z.; Chen, H.; et al. Ellagic acid induces cell cycle arrest and apoptosis via the TGF-β1/Smad3 signaling pathway in human colon cancer HCT-116 cells. Oncol. Rep. 2020, 44, 768–776. [Google Scholar] [CrossRef]
- Yousef, A.I.; El-Masry, O.S.; Abdel Mohsen, M.A. Impact of Cellular Genetic Make-up on Colorectal Cancer Cell Lines Response to Ellagic Acid: Implications of Small Interfering RNA. Asian Pac. J. Cancer Prev. 2016, 17, 743–748. [Google Scholar] [CrossRef]
- Hajiluian, G.; Karegar, S.J.; Shidfar, F.; Aryaeian, N.; Salehi, M.; Lotfi, T.; Farhangnia, P.; Heshmati, J.; Delbandi, A.A. The effects of Ellagic acid supplementation on neurotrophic, inflammation, and oxidative stress factors, and indoleamine 2, 3-dioxygenase gene expression in multiple sclerosis patients with mild to moderate depressive symptoms: A randomized, triple-blind, placebo-controlled trial. Phytomedicine 2023, 121, 155094. [Google Scholar]
- Sacco, R.; Santangelo, G.; Stamenova, S.; Bisecco, A.; Bonavita, S.; Lavorgna, L.; Trojano, L.; D’Ambrosio, A.; Tedeschi, G.; Gallo, A. Psychometric properties and validity of Beck Depression Inventory II in multiple sclerosis. Eur. J. Neurol. 2016, 23, 744–750. [Google Scholar] [CrossRef]
- Khodaei, F.; Khoshnoud, M.J.; Heidaryfar, S.; Heidari, R.; Karimpour Baseri, M.H.; Azarpira, N.; Rashedinia, M. The effect of ellagic acid on spinal cord and sciatica function in a mice model of multiple sclerosis. J. Biochem. Mol. Toxicol. 2020, 34, e22564. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, O.S.; Ghanem, H.B.; El-Esawy, R.O.; Sadek, M.T. The modulatory effects of luteolin on cyclic AMP/Ciliary neurotrophic factor signaling pathway in experimentally induced autoimmune encephalomyelitis. IUBMB Life 2019, 71, 1401–1408. [Google Scholar] [CrossRef]
- Contarini, G.; Franceschini, D.; Facci, L.; Barbierato, M.; Giusti, P.; Zusso, M. A co-ultramicronized palmitoylethanolamide/luteolin composite mitigates clinical score and disease-relevant molecular markers in a mouse model of experimental autoimmune encephalomyelitis. J. Neuroinflammation 2019, 16, 126. [Google Scholar] [CrossRef] [PubMed]
- Sarawek, S.; Deredorf, H.; Butterweck, V. Pharmacokinetics of luteolin and metabolites in rats. Nat. Prod. Commun. 2008, 3, 2029–2036. [Google Scholar] [CrossRef]
- Molina-Gonzalez, I.; Holloway, R.K.; Jiwaji, Z.; Dando, O.; Kent, S.A.; Emelianova, K.; Lloyd, A.F.; Forbes, L.H.; Mahmood, A.; Skripuletz, T.; et al. Astrocyte-oligodendrocyte interaction regulates central nervous system regeneration. Nat. Commun. 2023, 14, 3372. [Google Scholar] [CrossRef]
- Sternberg, Z.; Chadha, K.; Lieberman, A.; Drake, A.; Hojnacki, D.; Weinstock-Guttman, B.; Munschauer, F. Immunomodulatory responses of peripheral blood mononuclear cells from multiple sclerosis patients upon in vitro incubation with the flavonoid luteolin: Additive effects of IFN-beta. J. Neuroinflammation 2009, 6, 28. [Google Scholar] [CrossRef]
- Xia, N.; Chen, G.; Liu, M.; Ye, X.; Pan, Y.; Ge, J.; Mao, Y.; Wang, H.; Wang, J.; Xie, S. Anti-inflammatory effects of luteolin on experimental autoimmune thyroiditis in mice. Exp. Ther. Med. 2016, 12, 4049–4054. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Petracca, M.; Quarantelli, M.; Moccia, M.; Vacca, G.; Satelliti, B.; D’Ambrosio, G.; Carotenuto, A.; Ragucci, M.; Assogna, F.; Capacchione, A.; et al. ProspeCtive study to evaluate efficacy, safety and tOlerability of dietary supplemeNT of curcumin (BCM95) in subjects with active relapsing multIple sclerosis treated with subcutaNeous interferon beta 1a 44 mcg TIW (CONTAIN): A randomized, controlled trial. Mult. Scler. Relat. Disord. 2021, 56, 103274. [Google Scholar] [CrossRef]
- Xie, L.; Li, X.K.; Takahara, S. Curcumin has bright prospects for the treatment of multiple sclerosis. Int. Immunopharmacol. 2011, 11, 323–330. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 17, 4449–4460. [Google Scholar] [CrossRef]
- Mohajeri, M.; Sadeghizadeh, M.; Najafi, F.; Javan, M. Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 2015, 99, 156–167. [Google Scholar] [CrossRef]
- Khosropour, S.; Shahvarooghi, E.; Rezaeizadeh, H.; Esmaeelzadeh, M. Curcumin and Its Semisynthetic Derivative F-Curcumin Ameliorate the Expression of Cytokines in Autoimmune Encephalomyelitis Mouse Models of Multiple Sclerosis. Iran. J. Allergy Asthma Immunol. 2023, 22, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Ayromlou, H.; Ahmadi, M.; Rikhtegar, R.; Rostamzadeh, D.; Roshangar, L.; Nouri, M.; Mehdizadeh, A.; Younesi, V.; et al. Nanocurcumin improves regulatory T-cell frequency and function in patients with multiple sclerosis. J Neuroimmunol. 2019, 327, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Ahmadi, M.; Rikhtegar, R.; Babaloo, Z.; Ayromlou, H.; Aghebati-Maleki, L.; Nouri, M.; Yousefi, M. Changes in Th17 cells function after nanocurcumin use to treat multiple sclerosis. Int. Immunopharmacol. 2018, 61, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, R.; Safarpour, F.; Hashemian, M.; Tashakorian, H.; Ahmadian, S.R.; Ashrafpour, M.; Ghasemi-Kasman, M. Curcumin-loaded nanoparticles ameliorate glial activation and improve myelin repair in lyolecithin-induced focal demyelination model of rat corpus callosum. Neurosci. Lett. 2018, 674, 1–10. [Google Scholar] [CrossRef]
- Khadka, S.; Omura, S.; Sato, F.; Nishio, K.; Kakeya, H.; Tsunoda, I. Curcumin β-D-Glucuronide Modulates an Autoimmune Model of Multiple Sclerosis with Altered Gut Microbiota in the Ileum and Feces. Front. Cell. Infect. Microbiol. 2021, 11, 772962. [Google Scholar] [CrossRef]
- Nuhu, A.A. Bioactive Micronutrients in Coffee: Recent Analytical Approaches for Characterization and Quantification. ISRN Nutr. 2014, 2014, 384230. [Google Scholar] [CrossRef]
- Drewnowski, A.; Rehm, C.D. Sources of Caffeine in Diets of US Children and Adults: Trends by Beverage Type and Purchase Location. Nutrients 2016, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Military Nutrition Research. Caffeine for the Sustainment of Mental Task Performance: Formulations for Military Operations; National Academies Press (US): Washington, DC, USA, 2001. Available online: http://www.ncbi.nlm.nih.gov/books/NBK223802/ (accessed on 11 July 2024).
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and Neuroprotective Effects of Caffeine against Alzheimer’s and Parkinson’s Disease: Insight into the Role of Nrf-2 and A2AR Signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef]
- Welsh, E.J.; Bara, A.; Barley, E.; Cates, C.J. Caffeine for asthma. Cochrane Database Syst. Rev. 2010, 2010, CD001112. [Google Scholar] [CrossRef] [PubMed]
- Supplements PC for a W on PHHA with C of C in F and D, Board F and N, Policy B on HS, Medicine I of. Caffeine Effects on the Cardiovascular System. In Caffeine in Food and Dietary Supplements: Examining Safety: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2014. Available online: https://www.ncbi.nlm.nih.gov/books/NBK202224/ (accessed on 31 May 2024).
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-κB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Jayasooriya, R.G.P.T.; Dilshara, M.G.; Choi, Y.H.; Jeong, Y.K.; Kim, N.D.; Kim, G.Y. Caffeine suppresses lipopolysaccharide-stimulated BV2 microglial cells by suppressing Akt-mediated NF-κB activation and ERK phosphorylation. Food Chem. Toxicol. 2012, 50, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R. NF-κB Restricts inflammasome activation via elimination of damaged mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef]
- Merck &, Co. Caffeine. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m2909 (accessed on 28 August 2024).
- Merck &, Co. Harmine. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m5919 (accessed on 28 August 2024).
- Merck &, Co. Trigonelline. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m11133 (accessed on 28 August 2024).
- Merck &, Co. Cafestol. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m2907 (accessed on 28 August 2024).
- Merck &, Co. Ursolic Acid. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m11345 (accessed on 28 August 2024).
- Merck &, Co. Celestrol. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m3227 (accessed on 28 August 2024).
- Merck &, Co. Hydroxytyrosol. Merck Index Online. Available online: https://merckindex.rsc.org/monographs/m6157 (accessed on 28 August 2024).
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef]
- Tavares, L.P.; Negreiros-Lima, G.L.; Lima, K.M.; E Silva, P.M.R.; Pinho, V.; Teixeira, M.M.; Sousa, L.P. Blame the signaling: Role of cAMP for the resolution of inflammation. Pharmacol. Res. 2020, 159, 105030. [Google Scholar] [CrossRef]
- Lee, G.S.; Subramanian, N.; Kim, A.I.; Aksentijevich, I.; Goldbach-Mansky, R.; Sacks, D.B.; Germain, R.N.; Kastner, D.L.; Chae, J.J. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 2012, 492, 123–127. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sebastião, A.M. Caffeine and adenosine. J. Alzheimers Dis. 2010, 20 (Suppl. S1), S3–S15. [Google Scholar] [CrossRef]
- Kovács, E.G.; Alatshan, A.; Budai, M.M.; Czimmerer, Z.; Bíró, E.; Benkő, S. Caffeine Has Different Immunomodulatory Effect on the Cytokine Expression and NLRP3 Inflammasome Function in Various Human Macrophage Subpopulations. Nutrients 2021, 13, 2409. [Google Scholar] [CrossRef]
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Planning Committee for a Workshop on Potential Health Hazards Associated with Consumption of Caffeine in Food and Dietary Supplements, Food and Nutrition Board, Board on Health Sciences Policy, Institute of Medicine. In Caffeine in Food and Dietary Supplements: Examining Safety: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2014. Available online: http://www.ncbi.nlm.nih.gov/books/NBK202230/ (accessed on 11 July 2024).
- Piechowska, P.; Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S. Bioactive β-Carbolines in Food: A Review. Nutrients 2019, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke. Food Addit. Contam. 2004, 21, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. Identification and occurrence of the bioactive beta-carbolines norharman and harman in coffee brews. Food Addit. Contam. 2002, 19, 748–754. [Google Scholar] [CrossRef]
- Guan, Y.; Louis, E.D.; Zheng, W. Toxicokinetics of tremorogenic natural products, harmane and harmine, in male sprague-dawley rats. J. Toxicol. Environ. Environ. Health A 2001, 64, 645–660. [Google Scholar] [CrossRef]
- Chen, C.; Jiao, Y.; Zeng, M.; He, Z.; Shen, Q.; Chen, J.; Quan, W. The Simultaneous Formation of Acrylamide, β-carbolines, and Advanced Glycation End Products in a Chemical Model System: Effect of Multiple Precursor Amino Acids. Front. Nutr. 2022, 9, 852717. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.H.; Shayegh, J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199–212. [Google Scholar] [CrossRef]
- Bensalem, S.; Soubhye, J.; Aldib, I.; Bournine, L.; Nguyen, A.T.; Vanhaeverbeek, M.; Rousseau, A.; Boudjeltia, K.Z.; Sarakbi, A.; Kauffmann, J.M.; et al. Inhibition of myeloperoxidase activity by the alkaloids of Peganum harmala L. (Zygophyllaceae). J. Ethnopharmacol. 2014, 154, 361–369. [Google Scholar] [CrossRef]
- Lockhart, J.S.; Sumagin, R. Non-Canonical Functions of Myeloperoxidase in Immune Regulation, Tissue Inflammation and Cancer. Int. J. Mol. Sci. 2022, 23, 12250. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef]
- Kim, H.J.; Wei, Y.; Wojtkiewicz, G.R.; Lee, J.Y.; Moskowitz, M.A.; Chen, J.W. Reducing myeloperoxidase activity decreases inflammation and increases cellular protection in ischemic stroke. J. Cereb. Blood Flow. Metab. 2019, 39, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- MohanKumar, S.M.J.; Murugan, A.; Palaniyappan, A.; MohanKumar, P.S. Role of cytokines and reactive oxygen species in brain aging. Mech. Ageing Dev. 2023, 214, 111855. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Wang, M.; Restrepo, R.J.; Wang, D.; Kalogeris, T.J.; Neumann, W.L.; Ford, D.A.; Korthuis, R.J. Myeloperoxidase instigates proinflammatory responses in a cecal ligation and puncture rat model of sepsis. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H705–H721. [Google Scholar] [CrossRef]
- Zawirska-Wojtasiak, R.; Fedoruk-Wyszomirska, A.; Piechowska, P.; Mildner-Szkudlarz, S.; Bajerska, J.; Wojtowicz, E.; Przygoński, K.; Gurda, D.; Kubicka, W.; Wyszko, E. β-Carbolines in Experiments on Laboratory Animals. Int. J. Mol. Sci. 2020, 21, 5245. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H. Biosynthetic Pathways of Purine and Pyridine Alkaloids in Coffee Plants. Nat. Prod. Commun. 2016, 11, 1934578X1601100742. [Google Scholar] [CrossRef]
- Garg, R.C. Chapter 44—Fenugreek: Multiple Health Benefits. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 599–617. Available online: https://www.sciencedirect.com/science/article/pii/B9780128021477000449 (accessed on 31 May 2024).
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Trigonelline in Coffee and Coffee By-Products. Molecules 2023, 28, 3460. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Pharmacological Activities, Therapeutic Effects, and Mechanistic Actions of Trigonelline. Int. J. Mol. Sci. 2024, 25, 3385. [Google Scholar] [CrossRef]
- Liang, Y.; Dai, X.; Cao, Y.; Wang, X.; Lu, J.; Xie, L.; Liu, K.; Li, X. The neuroprotective and antidiabetic effects of trigonelline: A review of signaling pathways and molecular mechanisms. Biochimie 2023, 206, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Antonisamy, P.; Arasu, M.V.; Dhanasekaran, M.; Choi, K.C.; Aravinthan, A.; Kim, N.S.; Kang, C.W.; Kim, J.H. Protective effects of trigonelline against indomethacin-induced gastric ulcer in rats and potential underlying mechanisms. Food Funct. 2016, 7, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.A.; Gawali, N.B.; Munshi, R.; Juvekar, A.R. Trigonelline insulates against oxidative stress, proinflammatory cytokines and restores BDNF levels in lipopolysaccharide induced cognitive impairment in adult mice. Metab. Brain Dis. 2018, 33, 681–691. [Google Scholar] [CrossRef]
- Farid, M.M.; Yang, X.; Kuboyama, T.; Tohda, C. Trigonelline recovers memory function in Alzheimer’s disease model mice: Evidence of brain penetration and target molecule. Sci. Rep. 2020, 10, 16424. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and Kahweol: A Review on Their Bioactivities and Pharmacological Properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef]
- Eldesouki, S.; Qadri, R.; Abu Helwa, R.; Barqawi, H.; Bustanji, Y.; Abu-Gharbieh, E.; El-Hneidi, W. Recent Updates on the Functional Impact of Kahweol and Cafestol on Cancer. Molecules 2022, 27, 7332. [Google Scholar] [CrossRef]
- Mellbye, F.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Cafestol, a Bioactive Substance in Coffee, Stimulates Insulin Secretion and Increases Glucose Uptake in Muscle Cells: Studies In Vitro. J. Nat. Prod. 2015, 78, 2447–2451. [Google Scholar] [CrossRef]
- Trinh, K.; Andrews, L.; Krause, J.; Hanak, T.; Lee, D.; Gelb, M.; Pallanck, L. Decaffeinated Coffee and Nicotine-Free Tobacco Provide Neuroprotection in Drosophila Models of Parkinson’s Disease through an NRF2-Dependent Mechanism. J. Neurosci. 2010, 30, 5525–5532. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Izumi, K.; Hiratsuka, K.; Kano, H.; Shimada, T.; Nakano, T.; Kadomoto, S.; Naito, R.; Iwamoto, H.; Yaegashi, H.; et al. Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells. Sci. Rep. 2021, 11, 675. [Google Scholar] [CrossRef]
- De Roos, B.; Meyboom, S.; Kosmeijer-Schuil, T.G.; Katan, M.B. Absorption and urinary excretion of the coffee diterpenes cafestol and kahweol in healthy ileostomy volunteers. J. Intern. Med. 1998, 244, 451–460. [Google Scholar] [CrossRef]
- Hao, W.R.; Sung, L.C.; Chen, C.C.; Chen, P.Y.; Cheng, T.H.; Chao, H.H.; Liu, J.C.; Chen, J.J. Cafestol Inhibits Cyclic-Strain-Induced Interleukin-8, Intercellular Adhesion Molecule-1, and Monocyte Chemoattractant Protein-1 Production in Vascular Endothelial Cells. Oxid. Med. Cell Longev. 2018, 2018, 7861518. [Google Scholar] [CrossRef] [PubMed]
- Al-Kenany, S.A.; Al-Shawi, N.N. Protective effect of cafestol against doxorubicin-induced cardiotoxicity in rats by activating the Nrf2 pathway. Front. Pharmacol. 2023, 14, 1206782. [Google Scholar] [CrossRef]
- Shen, T.; Lee, J.; Lee, E.; Kim, S.H.; Kim, T.W.; Cho, J.Y. Cafestol, a coffee-specific diterpene, is a novel extracellular signal-regulated kinase inhibitor with AP-1-targeted inhibition of prostaglandin E2 production in lipopolysaccharide-activated macrophages. Biol. Pharm. Bull. 2010, 33, 128–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bielecka, E.; Zubrzycka, N.; Marzec, K.; Maksylewicz, A.; Sochalska, M.; Kulawik-Pióro, A.; Lason, E.; Sliwa, K.; Malinowska, M.; Sikora, E.; et al. Ursolic Acid Formulations Effectively Induce Apoptosis and Limit Inflammation in the Psoriasis Models In Vitro. Biomedicines 2024, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, H.; Guo, S.; Li, P.; Qin, S.; Shi, M.; Zeng, C. Effect and mechanism of edible oil co-digestion on the bioaccessibility and bioavailability of ursolic acid. Food Chem. 2023, 423, 136220. [Google Scholar] [CrossRef]
- Honarvar, F.; Hojati, V.; Zare, L.; Bakhtiari, N.; Javan, M. Ursolic Acid Enhances Myelin Repair in Adult Mice Brains and Stimulates Exhausted Oligodendrocyte Progenitors to Remyelinate. J. Mol. Neurosci. 2022, 72, 2081–2093. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, X.; Feng, J.; Song, F.; Pan, Y. Ursolic acid attenuates beta-amyloid-induced memory impairment in mice. Arq. Neuropsiquiatr. 2016, 74, 482–488. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.J.; Lu, J.; Wu, D.M.; Zheng, Z.H.; Zheng, Y.L.; Wang, X.H.; Ruan, J.; Sun, X.; Shan, Q.; Zhang, Z.f. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-κB mediated inflammatory pathways. Neurobiol. Learn. Mem. 2011, 96, 156–165. [Google Scholar] [CrossRef]
- Honarvar, F.; Hojati, V.; Bakhtiari, N.; Vaezi, G.; Javan, M. Myelin Protection by Ursolic Acid in Cuprizone-Induced Demyelination in Mice. Iran. J. Pharm. Res. 2019, 18, 1978–1988. [Google Scholar]
- Cascão, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.J.; Yu, J.; Li, T.; Han, Y. Protective effect of celastrol on type 2 diabetes mellitus with nonalcoholic fatty liver disease in mice. Food Sci. Nutr. 2020, 8, 6207–6216. [Google Scholar] [CrossRef] [PubMed]
- Pinna, G.F.; Fiorucci, M.; Reimund, J.M.; Taquet, N.; Arondel, Y.; Muller, C.D. Celastrol inhibits pro-inflammatory cytokine secretion in Crohn’s disease biopsies. Biochem. Biophys. Res. Commun. 2004, 322, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Su, C.; Iyaswamy, A.; Krishnamoorthi, S.K.; Zhu, Z.; Yang, S.; Tong, B.C.; Liu, J.; Sreenivasmurthy, S.G.; Guan, X.; et al. Celastrol enhances transcription factor EB (TFEB)-mediated autophagy and mitigates Tau pathology: Implications for Alzheimer’s disease therapy. Acta Pharm. Sin. B 2022, 12, 1707–1722. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.Y.; Xu, M.J.; Wu, T.; Chu, J.H.; Liu, S.J.; Ju, W.Z. Oral bioavailability and gender-related pharmacokinetics of celastrol following administration of pure celastrol and its related tablets in rats. J. Ethnopharmacol. 2012, 144, 195–200. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Jiang, J.; Wang, Y.; Zhang, X. Celastrol Attenuates Multiple Sclerosis and Optic Neuritis in an Experimental Autoimmune Encephalomyelitis Model. Front. Pharmacol. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.H.; Dudics, S.; Astry, B.; Moudgil, K.D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016, 74, ftw059. [Google Scholar] [CrossRef]
- Youn, G.S.; Kwon, D.J.; Ju, S.M.; Rhim, H.; Bae, Y.S.; Choi, S.Y.; Park, J. Celastrol ameliorates HIV-1 Tat-induced inflammatory responses via NF-kappaB and AP-1 inhibition and heme oxygenase-1 induction in astrocytes. Toxicol. Appl. Pharmacol. 2014, 280, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.M.; Zhang, L.X.; Cheng, Z.H.; Cai, W.Z.; Miao, H.H.; Pan, D.J. Inhibitory effect of tripterine on activities of IL-1, IL-2 and release of PGE2. Yao Xue Xue Bao 1991, 26, 641–645. [Google Scholar]
- Kim, D.H.; Shin, E.K.; Kim, Y.H.; Lee, B.W.; Jun, J.G.; Park, J.H.; Kim, J.K. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur. J. Clin. Investig. 2009, 39, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Luo, Q.; Alitongbieke, G.; Chong, S.; Xu, C.; Xie, L.; Chen, X.; Zhang, D.; Zhou, Y.; Wang, Z.; et al. Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol. Cell 2017, 66, 141–153.e6. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.H.; Astry, B.; Nanjundaiah, S.M.; Yu, H.; Moudgil, K.D. Suppression of autoimmune arthritis by Celastrus-derived Celastrol through modulation of pro-inflammatory chemokines. Bioorganic Med. Chem. 2012, 20, 5229–5234. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, K.; Kitani, H.; Takayama-Ito, M.; Morimoto, K.; Kurane, I.; Saijo, M. Celastrol suppresses morphological and transcriptional responses in microglial cells upon stimulation with double-stranded RNA. Int. J. Neurosci. 2010, 120, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, X.; Xiong, Q.; Shikano, S.; Li, M. Chronic inhibition of cardiac Kir2.1 and HERG potassium channels by celastrol with dual effects on both ion conductivity and protein trafficking. J. Biol.Chem. 2006, 281, 5877–5884. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; He, X.; Zhang, F.; He, L.; Chen, J.; Wang, L.; An, L.; Fan, Y. Inhibitory mechanisms of celastrol on human liver cytochrome P450 1A2, 2C19, 2D6, 2E1 and 3A4. Xenobiotica 2015, 45, 571–577. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; de la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Cerezo, A.B.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin, protocatechuic acid and hydroxytyrosol effects on vitagenes system against alpha-synuclein toxicity. Food Chem. Toxicol. 2019, 134, 110817. [Google Scholar] [CrossRef]
- Agrawal, S.M.; Lau, L.; Yong, V.W. MMPs in the central nervous system: Where the good guys go bad. Semin. Cell Dev. Biol. 2008, 19, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Miranda, B.; Gallardo, I.; Melliou, E.; Cabero, I.; Álvarez, Y.; Magiatis, P.; Hernández, M.; Nieto, M.L. Oleacein Attenuates the Pathogenesis of Experimental Autoimmune Encephalomyelitis through Both Antioxidant and Anti-Inflammatory Effects. Antioxidants 2020, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef]
- Chen, C.; Ai, Q.d.; Wei, Y.h. Potential role of hydroxytyrosol in neuroprotection. J. Funct. Foods 2021, 82, 104506. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Latronico, T.; Branà, M.T.; Gramegna, P.; Coniglio, M.G.; Rossano, R.; Larocca, M.; Riccio, P. Structure-dependent inhibition of gelatinases by dietary antioxidants in rat astrocytes and sera of multiple sclerosis patients. Neurochem. Res. 2011, 36, 518–527. [Google Scholar] [CrossRef]
- Alam, M.Z. A review on plant-based remedies for the treatment of multiple sclerosis. Ann. Pharm. Françaises 2023, 81, 775–789. [Google Scholar] [CrossRef]
- Gangwar, V.; Garg, A.; Lomore, K.; Korla, K.; Bhat, S.S.; Rao, R.P.; Rafiq, M.; Kumawath, R.; Uddagiri, B.V.; Kareenhalli, V.V. Immunomodulatory Effects of a Concoction of Natural Bioactive Compounds—Mechanistic Insights. Biomedicines 2021, 9, 1522. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).