Effect of Oral Nutritional Supplementation on Health-Related Outcomes and Nutritional Biomarkers in Children and Adolescents with Undernutrition: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Review Protocol and Registration
2.2. Study Selection Criteria
2.3. Search Strategy
2.4. Data Extraction and Synthesis
2.5. Meta-Analysis
2.6. Study Quality Assessment
3. Results
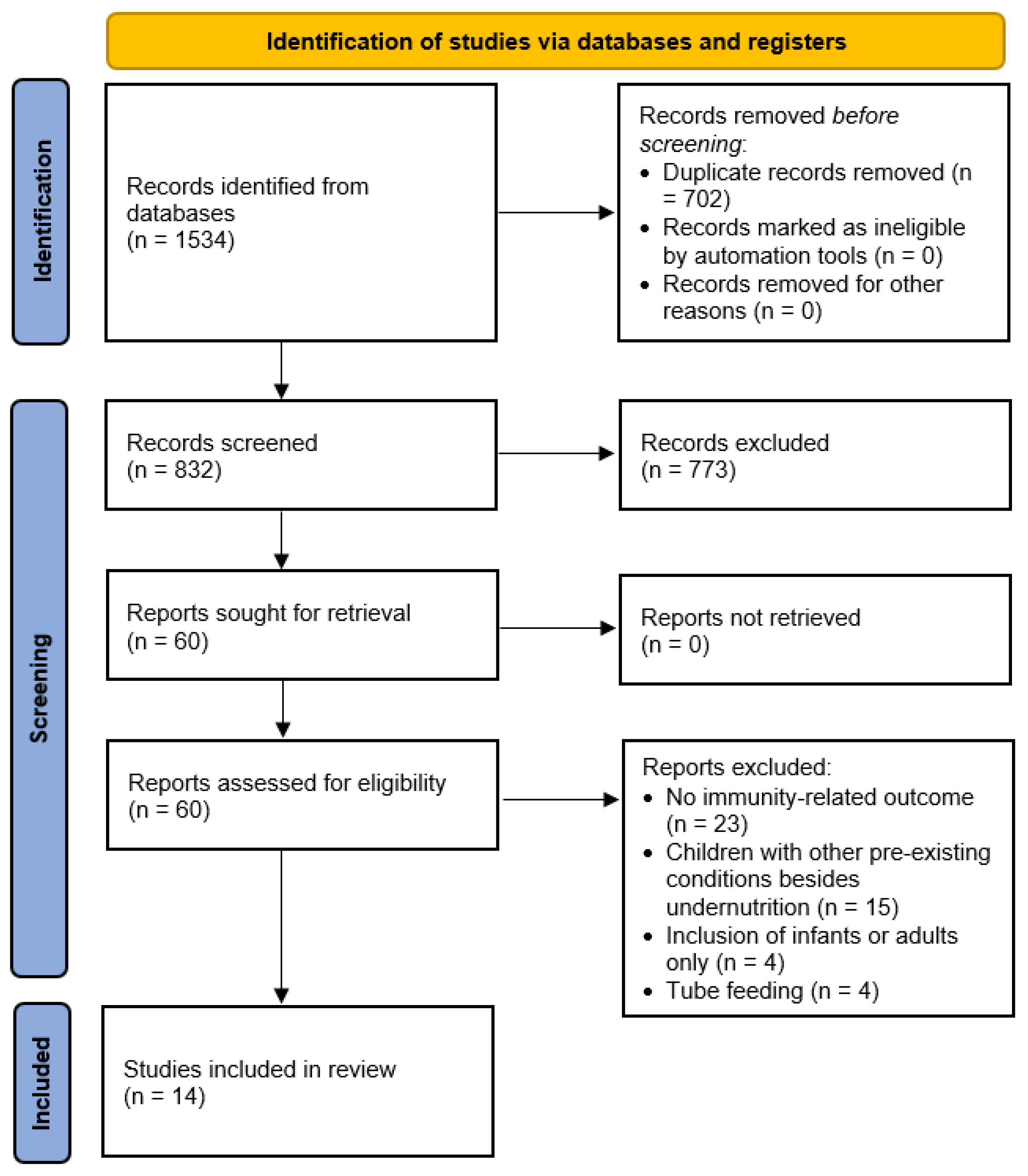
3.1. Identification of Studies
3.2. Characteristics of Study Participants
3.3. Characteristics of the Included Studies
3.4. Intervention Effectiveness
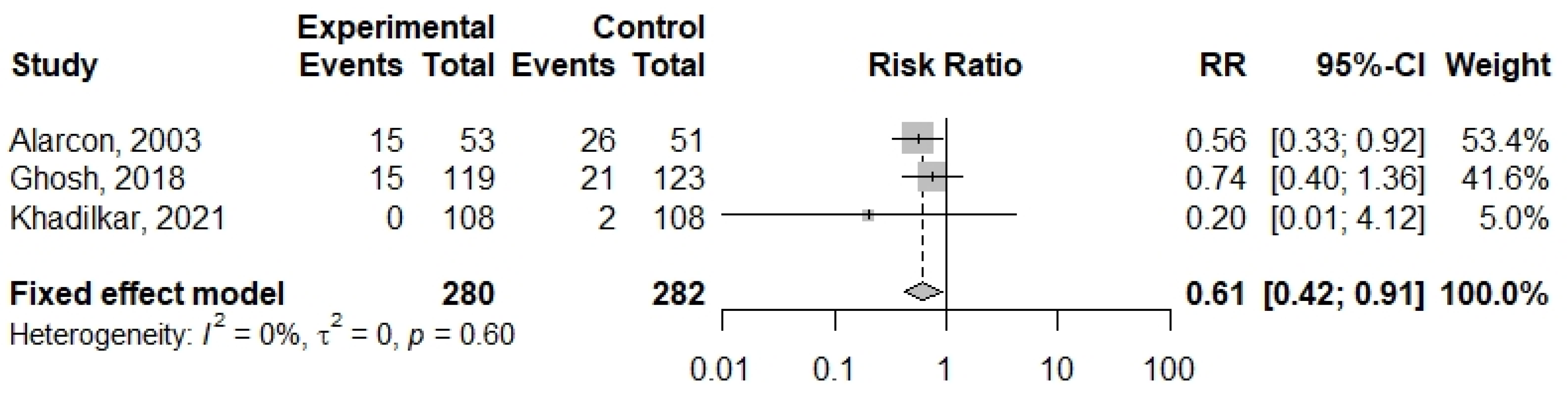
3.5. Meta-Analysis
3.6. Study Quality Assessment
4. Discussion
4.1. Health-Related Outcomes
4.2. Nutritional Biomarkers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Search Algorithm
| Search Platform | Databases | Search Algorithm |
|---|---|---|
| EBSCO |
| ( AB (“oral nutritional supplement*” or “oral nutrition supplement*” or “young child formula*” or PediaSure or Sustagen or NUTREN or “Boost Kid Essentials” or Kindercal or “Bright Beginnings” or “Peptamen Junior” or “S-26 PE Gold” or “Junior Horlicks” or Bournvita or Complan or Fortisip or Fortini or “Protinex Junior” or “Nutrilite Kids” or Dinoshake or “Nutraherbs Kids” or “Amul Pro” or Wyeth or “NG Solutions” or “Mead Johnson” or Nestle or Nutricia or ((nutrition or nutritional or nutrient or nutrients) N10 (“oral supplement*” or “dietary supplement*” or “diet supplement*”)) or “supplementary oral nutrition” or “supplemented oral nutrition” or “supplementary enteral nutrition” or “supplemented enteral nutrition” or “supplemental enteral nutrition” or ((oral or orally) and (“enteral formula*” or “enteric formula*” or “polymeric formula*” or “polymeric diet*”)) or “oral nutritional intervention*” or “sip feed*” or “nutrition drink” or “nutrition drinks” or “nutritional drink*” or “milk drink” or “milk drinks” or “fortified milk*” or “enriched milk*” or “fortified beverage*” or “liquid nutrition* supplement*” or “oral enteral nutrition*” or “oral enteric nutrition*” or “oral enteral feeding*” or “protein energy supplement*” or “protein-energy supplement*” or “protein and energy supplement*” or “protein calorie supplement*” or “protein-calorie supplement*” or “protein and calorie supplement*” or “oral nutrition*” or ((oral or orally) N10 nutrition* N10 supplement*) or ((oral or orally) and (“enteral nutrition*” or “enteral supplement*” or “enteral feeding*”) NOT (“tube feeding*” ))) OR TI (“oral nutritional supplement*” or “oral nutrition supplement*” or “young child formula*” or PediaSure or Sustagen or NUTREN or “Boost Kid Essentials” or Kindercal or “Bright Beginnings” or “Peptamen Junior” or “S-26 PE Gold” or “Junior Horlicks” or Bournvita or Complan or Fortisip or Fortini or “Protinex Junior” or “Nutrilite Kids” or Dinoshake or “Nutraherbs Kids” or “Amul Pro” or Wyeth or “NG Solutions” or “Mead Johnson” or Nestle or Nutricia or ((nutrition or nutritional or nutrient or nutrients) N10 (“oral supplement*” or “dietary supplement*” or “diet supplement*”)) or “supplementary oral nutrition” or “supplemented oral nutrition” or “supplementary enteral nutrition” or “supplemented enteral nutrition” or “supplemental enteral nutrition” or ((oral or orally) and (“enteral formula*” or “enteric formula*” or “polymeric formula*” or “polymeric diet*”)) or “oral nutritional intervention*” or “sip feed*” or “nutrition drink” or “nutrition drinks” or “nutritional drink*” or “milk drink” or “milk drinks” or “fortified milk*” or “enriched milk*” or “fortified beverage*” or “liquid nutrition* supplement*” or “oral enteral nutrition*” or “oral enteric nutrition*” or “oral enteral feeding*” or “protein energy supplement*” or “protein-energy supplement*” or “protein and energy supplement*” or “protein calorie supplement*” or “protein-calorie supplement*” or “protein and calorie supplement*” or “oral nutrition*” or ((oral or orally) N10 nutrition* N10 supplement*) or ((oral or orally) and (“enteral nutrition*” or “enteral supplement*” or “enteral feeding*”) NOT (“tube feeding*” ))) ) AND ( AB (child* or schoolchild* or “school child*” or kid or kids or toddler* or adoles* or preadolescen* or pre-adolescen* or preteen* or teen* or boy* or girl* or minors or underage* or “under ag*” or juvenile* or youth* or kindergar* or puber* or pubescen* or prepubescent* or prepuberty* or pediatric* or paediatric* or peadiatric* or schools or “nursery school*” or preschool* or “pre school*” or “primary school*” or “secondary school*” or “elementary school*” or “high school*” or highschool* or “school age” or schoolage or “school age*” or schoolage* or student* or youngster*) OR TI (child* or schoolchild* or “school child*” or kid or kids or toddler* or adoles* or preadolescen* or pre-adolescen* or preteen* or teen* or boy* or girl* or minors or underage* or “under ag*” or juvenile* or youth* or kindergar* or puber* or pubescen* or prepubescent* or prepuberty* or pediatric* or paediatric* or peadiatric* or schools or “nursery school*” or preschool* or “pre school*” or “primary school*” or “secondary school*” or “elementary school*” or “high school*” or highschool* or “school age” or schoolage or “school age*” or schoolage* or student* or youngster*) ) AND ( AB (stunted or stunting or underweight or “under-weight” or wasting or wasted or “failure to thrive” or “growth retardation” or “growth faltering” or “growth failure” or “failure to grow” or “delayed growth*” or “restricted growth*” or “suboptimal growth*” or “sub-optimal growth*” or “catch-up growth*” or “catch-up growth*” or malnutrition or malnourishment or malnourished or “mal-nourished” or “under-nourished” or undernourishment or undernutrition or “under-nutrition” or undernourished or “picky eating” or “picky eater*” or “fussy eater*” or “feeding disorder*” or “infantile anorexia”) OR TI (stunted or stunting or underweight or “under-weight” or wasting or wasted or “failure to thrive” or “growth retardation” or “growth faltering” or “growth failure” or “failure to grow” or “delayed growth*” or “restricted growth*” or “suboptimal growth*” or “sub-optimal growth*” or “catch-up growth*” or “catch-up growth*” or malnutrition or malnourishment or malnourished or “mal-nourished” or “under-nourished” or undernourishment or undernutrition or “under-nutrition” or undernourished or “picky eating” or “picky eater*” or “fussy eater*” or “feeding disorder*” or “infantile anorexia”) ) |
| Web of Science |
| ( AB = (“oral nutritional supplement*” or “oral nutrition supplement*” or “young child formula*” or PediaSure or Sustagen or NUTREN or “Boost Kid Essentials” or Kindercal or “Bright Beginnings” or “Peptamen Junior” or “S-26 PE Gold” or “Junior Horlicks” or Bournvita or Complan or Fortisip or Fortini or “Protinex Junior” or “Nutrilite Kids” or Dinoshake or “Nutraherbs Kids” or “Amul Pro” or Wyeth or “NG Solutions” or “Mead Johnson” or Nestle or Nutricia or ((nutrition or nutritional or nutrient or nutrients) NEAR/10 (“oral supplement*” or “dietary supplement*” or “diet supplement*”)) or “supplementary oral nutrition” or “supplemented oral nutrition” or “supplementary enteral nutrition” or “supplemented enteral nutrition” or “supplemental enteral nutrition” or ((oral or orally) and (“enteral formula*” or “enteric formula*” or “polymeric formula*” or “polymeric diet*”)) or “oral nutritional intervention*” or “sip feed*” or “nutrition drink” or “nutrition drinks” or “nutritional drink*” or “milk drink” or “milk drinks” or “fortified milk*” or “enriched milk*” or “fortified beverage*” or “liquid nutrition* supplement*” or “oral enteral nutrition*” or “oral enteric nutrition*” or “oral enteral feeding*” or “protein energy supplement*” or “protein-energy supplement*” or “protein and energy supplement*” or “protein calorie supplement*” or “protein-calorie supplement*” or “protein and calorie supplement*” or “oral nutrition*” or (((oral or orally) NEAR/10 nutrition*) NEAR/10 supplement*) or ((oral or orally) and (“enteral nutrition*” or “enteral supplement*” or “enteral feeding*”) NOT (“tube feeding*” ))) OR TI = (“oral nutritional supplement*” or “oral nutrition supplement*” or “young child formula*” or PediaSure or Sustagen or NUTREN or “Boost Kid Essentials” or Kindercal or “Bright Beginnings” or “Peptamen Junior” or “S-26 PE Gold” or “Junior Horlicks” or Bournvita or Complan or Fortisip or Fortini or “Protinex Junior” or “Nutrilite Kids” or Dinoshake or “Nutraherbs Kids” or “Amul Pro” or Wyeth or “NG Solutions” or “Mead Johnson” or Nestle or Nutricia or ((nutrition or nutritional or nutrient or nutrients) NEAR/10 (“oral supplement*” or “dietary supplement*” or “diet supplement*”)) or “supplementary oral nutrition” or “supplemented oral nutrition” or “supplementary enteral nutrition” or “supplemented enteral nutrition” or “supplemental enteral nutrition” or ((oral or orally) and (“enteral formula*” or “enteric formula*” or “polymeric formula*” or “polymeric diet*”)) or “oral nutritional intervention*” or “sip feed*” or “nutrition drink” or “nutrition drinks” or “nutritional drink*” or “milk drink” or “milk drinks” or “fortified milk*” or “enriched milk*” or “fortified beverage*” or “liquid nutrition* supplement*” or “oral enteral nutrition*” or “oral enteric nutrition*” or “oral enteral feeding*” or “protein energy supplement*” or “protein-energy supplement*” or “protein and energy supplement*” or “protein calorie supplement*” or “protein-calorie supplement*” or “protein and calorie supplement*” or “oral nutrition*” or (((oral or orally) NEAR/10 nutrition*) NEAR/10 supplement*) or ((oral or orally) and (“enteral nutrition*” or “enteral supplement*” or “enteral feeding*”) NOT (“tube feeding*” ))) ) AND ( AB = (child* or schoolchild* or “school child*” or kid or kids or toddler* or adoles* or preadolescen* or pre-adolescen* or preteen* or teen* or boy* or girl* or minors or underage* or “under ag*” or juvenile* or youth* or kindergar* or puber* or pubescen* or prepubescent* or prepuberty* or pediatric* or paediatric* or peadiatric* or schools or “nursery school*” or preschool* or “pre school*” or “primary school*” or “secondary school*” or “elementary school*” or “high school*” or highschool* or “school age” or schoolage or “school age*” or schoolage* or student* or youngster*) OR TI = (child* or schoolchild* or “school child*” or kid or kids or toddler* or adoles* or preadolescen* or pre-adolescen* or preteen* or teen* or boy* or girl* or minors or underage* or “under ag*” or juvenile* or youth* or kindergar* or puber* or pubescen* or prepubescent* or prepuberty* or pediatric* or paediatric* or peadiatric* or schools or “nursery school*” or preschool* or “pre school*” or “primary school*” or “secondary school*” or “elementary school*” or “high school*” or highschool* or “school age” or schoolage or “school age*” or schoolage* or student* or youngster*) ) AND ( AB = (stunted or stunting or underweight or “under-weight” or wasting or wasted or “failure to thrive” or “growth retardation” or “growth faltering” or “growth failure” or “failure to grow” or “delayed growth*” or “restricted growth*” or “suboptimal growth*” or “sub-optimal growth*” or “catch-up growth*” or “catch-up growth*” or malnutrition or malnourishment or malnourished or “mal-nourished” or “under-nourished” or undernourishment or undernutrition or “under-nutrition” or undernourished or “picky eating” or “picky eater*” or “fussy eater*” or “feeding disorder*” or “infantile anorexia”) OR TI = (stunted or stunting or underweight or “under-weight” or wasting or wasted or “failure to thrive” or “growth retardation” or “growth faltering” or “growth failure” or “failure to grow” or “delayed growth*” or “restricted growth*” or “suboptimal growth*” or “sub-optimal growth*” or “catch-up growth*” or “catch-up growth*” or malnutrition or malnourishment or malnourished or “mal-nourished” or “under-nourished” or undernourishment or undernutrition or “under-nutrition” or undernourished or “picky eating” or “picky eater*” or “fussy eater*” or “feeding disorder*” or “infantile anorexia”) ) |
| Scopus | Scopus | TITLE-ABS-KEY( (“oral nutritional supplement*” or “oral nutrition supplement*” or “young child formula*” or PediaSure or Sustagen or NUTREN or “Boost Kid Essentials” or Kindercal or “Bright Beginnings” or “Peptamen Junior” or “S-26 PE Gold” or “Junior Horlicks” or Bournvita or Complan or Fortisip or Fortini or “Protinex Junior” or “Nutrilite Kids” or Dinoshake or “Nutraherbs Kids” or “Amul Pro” or Wyeth or “NG Solutions” or “Mead Johnson” or Nestle or Nutricia or ((nutrition or nutritional or nutrient or nutrients) W/10 (“oral supplement*” or “dietary supplement*” or “diet supplement*”)) or “supplementary oral nutrition” or “supplemented oral nutrition” or “supplementary enteral nutrition” or “supplemented enteral nutrition” or “supplemental enteral nutrition” or ((oral or orally) and (“enteral formula*” or “enteric formula*” or “polymeric formula*” or “polymeric diet*”)) or “oral nutritional intervention*” or “sip feed*” or “nutrition drink” or “nutrition drinks” or “nutritional drink*” or “milk drink” or “milk drinks” or “fortified milk*” or “enriched milk*” or “fortified beverage*” or “liquid nutrition* supplement*” or “oral enteral nutrition*” or “oral enteric nutrition*” or “oral enteral feeding*” or “protein energy supplement*” or “protein-energy supplement*” or “protein and energy supplement*” or “protein calorie supplement*” or “protein-calorie supplement*” or “protein and calorie supplement*” or “oral nutrition*” or (((oral or orally) W/10 nutrition*) W/10 supplement*) or ((oral or orally) and (“enteral nutrition*” or “enteral supplement*” or “enteral feeding*”) AND NOT (“tube feeding*” ))) AND (child* or schoolchild* or “school child*” or kid or kids or toddler* or adoles* or preadolescen* or pre-adolescen* or preteen* or teen* or boy* or girl* or minors or underage* or “under ag*” or juvenile* or youth* or kindergar* or puber* or pubescen* or prepubescent* or prepuberty* or pediatric* or paediatric* or peadiatric* or schools or “nursery school*” or preschool* or “pre school*” or “primary school*” or “secondary school*” or “elementary school*” or “high school*” or highschool* or “school age” or schoolage or “school age*” or schoolage* or student* or youngster*) AND (stunted or stunting or underweight or “under-weight” or wasting or wasted or “failure to thrive” or “growth retardation” or “growth faltering” or “growth failure” or “failure to grow” or “delayed growth*” or “restricted growth*” or “suboptimal growth*” or “sub-optimal growth*” or “catch-up growth*” or “catch-up growth*” or malnutrition or malnourishment or malnourished or “mal-nourished” or “under-nourished” or undernourishment or undernutrition or “under-nutrition” or undernourished or “picky eating” or “picky eater*” or “fussy eater*” or “feeding disorder*” or “infantile anorexia”) |
References
- Ssentongo, P.; Ssentongo, A.; Ba, D.; Ericson, J.; Na, M.; Gao, X.; Fronterrè, C.; Chinchilli, V.; Schiff, S. Global, regional and national epidemiology and prevalence of child stunting, wasting and underweight in low- and middle-income countries, 2006–2018. Sci. Rep. 2021, 11, 5204. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R. Adolescent undernutrition: Global burden, physiology, and nutritional risks. Ann. Nutr. Metab. 2018, 72, 316–328. [Google Scholar] [CrossRef] [PubMed]
- United Nations Children’s Fund (UNICEF); World Health Organization (WHO); International Bank for Reconstruction and Development/The World Bank. Levels and Trends in Child Malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2023 Edition; UNICEF, WHO, and World Bank: New York, NY, USA, 2023. [Google Scholar]
- United Nations Children’s Fund (UNICEF). Childhood Malnutrition. Available online: https://data.unicef.org/topic/nutrition/malnutrition/ (accessed on 1 May 2023).
- World Health Organization. The Double Burden of Malnutrition: Policy Brief. Available online: https://iris.who.int/bitstream/handle/10665/255413/WHO-NMH-NHD-17.3-eng.pdf?sequence=1 (accessed on 27 September 2023).
- Walson, J.L.; Berkley, J.A. The impact of malnutrition on childhood infections. Curr. Opin. Infect. Dis. 2018, 31, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, S.; Ovchinnikov, R. The Relationship between Nutrition and Infectious Diseases: A Review. Biomed. Biotechnol. Res. J. 2018, 2, 168–172. [Google Scholar]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Govers, C.; Calder, P.; Savelkoul, H.; Albers, R.; Neerven, R. Ingestion, Immunity, and Infection: Nutrition and Viral Respiratory Tract Infections. Front. Immunol. 2022, 13, 841532. [Google Scholar] [CrossRef]
- Savino, W.; Durães, J.; Maldonado-Galdeano, C.; Perdigón, G.; Mendes-da-Cruz, D.; Cuervo, P. Thymus, Undernutrition, and Infection: Approaching Cellular and Molecular Interactions. Front. Nutr. 2022, 9, 948488. [Google Scholar] [CrossRef]
- Prendergast, A.J. Malnutrition and vaccination in developing countries. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140141. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.; Jones, K.; Prendergast, A. Current Understanding of Innate Immune Cell Dysfunction in Childhood Undernutrition. Front. Immunol. 2019, 10, 1728. [Google Scholar] [CrossRef]
- Scrimshaw, N.S.; SanGiovanni, J.P. Synergism of nutrition, infection, and immunity: An overview. Am. J. Clin. Nutr. 1997, 66, 464S–477S. [Google Scholar] [CrossRef]
- Phelan, A.L.; Katz, R.; Gostin, L.O. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA 2020, 323, 709–710. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Bhuiyan, F.R.; Emon, T.H.; Hasan, M. Prospects of nutritional interventions in the care of COVID-19 patients. Heliyon 2021, 7, e06285. [Google Scholar] [CrossRef]
- United Nations International Children’s Emergency Fund (UNICEF). Nutrition in Middle Childhood and Adolescence: Preventing Malnutrition in School-Age Children and Adolescents. 2021. Available online: https://www.unicef.org/nutrition/middle-childhood-and-adolescence (accessed on 27 September 2023).
- Golden, M.H. Proposed recommended nutrient densities for moderately malnourished children. Food Nutr. Bull. 2009, 30, S267–S342. [Google Scholar] [CrossRef] [PubMed]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The influence of nutritional factors on immunological outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Chang, H.; Yang, Y.; Lin, T.; Lai, W.; Lin, Y.; Chang, C. The Role of Micronutrient and Immunomodulation Effect in the Vaccine Era of COVID-19. J. Chin. Med. Assoc. 2021, 84, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.B.M.; van de Steeg, E.; van Bilsen, J.; Meijerink, M. Mechanisms and immunomodulatory properties of pre- and probiotics. Benef. Microbes 2019, 10, 225–236. [Google Scholar] [CrossRef]
- Sanders, M.; Merenstein, D.; Reid, G.; Gibson, G.; Rastall, R. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- The British Association for Parenteral and Enteral Nutrition. Oral Nutritional Supplements (ONS). Available online: http://www.bapen.org.uk/nutrition-support/nutrition-by-mouth/oral-nutritional-supplements (accessed on 27 September 2023).
- Kleinman, R.E.; Greer, F.R. American Academy of Pediatrics Committee on Nutrition. In Pediatric Nutrition, 7th ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2013. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- FAO. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; FAO Food and Nutrition Paper; FAO: Rome, Italy, 2010; Volume 91, pp. 1–166. [Google Scholar]
- Young Child Nutrition Working Group: Formulation Subgroup. Formulations for fortified complementary foods and supplements: Review of successful products for improving the nutritional status of infants and young children. Food Nutr. Bull. 2009, 30, S239–S255. [Google Scholar] [CrossRef]
- Stratton, R.J.; Green, C.J.; Elia, M. Disease-Related Malnutrition: An Evidence-Based Approach to Treatment, 1st ed.; CABI: Wallingford, UK, 2003. [Google Scholar]
- Zhang, Z.; Li, F.; Hannon, B.A.; Hustead, D.S.; Aw, M.M.; Liu, Z.; Chuah, K.A.; Low, Y.L.; Huynh, D.T.T. Effect of oral nutritional supplementation on growth in children with undernutrition: A systematic review and meta-analysis. Nutrients 2021, 13, 3036. [Google Scholar] [CrossRef]
- Ow, M.Y.L.; Tran, N.T.; Berde, Y.; Nguyen, T.S.; Tran, V.K.; Jablonka, M.J.; Baggs, G.E.; Huynh, D.T.T. Oral nutritional supplementation with dietary counseling improves linear catch-up growth and health outcomes in children with or at risk of undernutrition: A randomized controlled trial. Front. Nutr. 2024, 11, 1341963. [Google Scholar] [CrossRef]
- Cawood, A.L.; Smith, C.; Kinnear, F.J.; Upton, L.; Trace, S.; O’Connor, G.; Stratton, R.J. Effect of oral nutritional supplements on outcomes in children presenting with, or at risk of, faltering growth in clinical settings: A systematic review and meta-analysis. J. Child Health Care 2023, 13674935231185181. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2011. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) Design as a Framework to Formulate Eligibility Criteria in Systematic Reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The WHO Child Growth Standards. Available online: https://www.who.int/childgrowth/standards/en/ (accessed on 27 September 2023).
- Mehta, N.M.; Corkins, M.R.; Lyman, B.; Malone, A.; Goday, P.S.; Carney, L.N.; Monczka, J.L.; Plogsted, S.W.; Schwenk, W.F.; American Society for Parenteral and Enteral Nutrition Board of Directors. Defining pediatric malnutrition: A paradigm shift toward etiology-related definitions. JPEN J. Parenter. Enter. Nutr. 2013, 37, 460–481. [Google Scholar] [CrossRef]
- Nutrition Landscape Information System (NLiS) Country Profile Indicators: Interpretation Guide, 2nd ed.; World Health Organization: Geneva, Switzerland, 2019.
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Akram, D.S.; Bharmal, F.Y.; Hussain, T. PediaSure in the treatment of severe malnutrition in Pakistani children. J. Pak. Med. Assoc. 2000, 50, 377–380. [Google Scholar]
- Alarcon, P.A.; Lin, L.H.; Noche, M., Jr.; Hernandez, V.C.; Cimafranca, L.; Lam, W.; Comer, G.M. Effect of oral supplementation on catch-up growth in picky eaters. Clin. Pediatr. 2003, 42, 209–217. [Google Scholar] [CrossRef]
- Fisberg, M.; Maulen-Radovan, I.E.; Tormo, R.; Carrascoso, M.T.; Giner, C.P.; Martin, F.A.; Belinchon, P.P.; Costa, C.M.; Perez, M.P.; Caro, J.G.; et al. Effects of oral nutritional supplementation with or without synbiotics on sickness and catch-up growth in preschool children. Int. Pediatr. 2002, 17, 216–222. [Google Scholar]
- Ghosh, A.K.; Kishore, B.; Shaikh, I.; Satyavrat, V.; Kumar, A.; Shah, T.; Pote, P.; Shinde, S.; Berde, Y.; Low, Y.L. Effect of oral nutritional supplementation on growth and recurrent upper respiratory tract infections in picky eating children at nutritional risk: A randomized, controlled trial. J. Int. Med. Res. 2018, 46, 2186–2201. [Google Scholar] [CrossRef]
- Han, J.C.; Damaso, L.; Welch, S.; Balagopal, P.; Hossain, J.; Mauras, N. Effects of growth hormone and nutritional therapy in boys with constitutional growth delay: A randomized controlled trial. J. Pediatr. 2011, 158, 427–432. [Google Scholar] [CrossRef]
- Huynh, D.T.; Estorninos, E.; Capeding, R.Z.; Oliver, J.S.; Low, Y.L.; Rosales, F.J. Longitudinal growth and health outcomes in nutritionally at-risk children who received long-term nutritional intervention. J. Hum. Nutr. Diet. 2015, 28, 623–635. [Google Scholar] [CrossRef]
- Kansu, A.; Durmaz Ugurcan, O.; Arslan, D.; Unalp, A.; Celtik, C.; Sarıoglu, A.A.; ANTK Study Group. High-fibre enteral feeding results in improved anthropometrics and favorable gastrointestinal tolerance in malnourished children with growth failure. Acta Paediatr. 2018, 107, 1036–1042. [Google Scholar] [CrossRef]
- Khadilkar, A.; Dhongade, A.; Agrawal, N. Impact of oral nutritional supplement on growth, nutrient intake and frequency of illness in children aged 4-6 years: A randomized controlled study. J. Clin. Diagn. Res. 2021, 15, 11–16. [Google Scholar] [CrossRef]
- Pham, D.T.; Ninh, N.T.; Hoang, T.N.; Pham, C.T.K.; Nguyen, L.H.; Tran, T.Q.; Huynh, D.T.T. The effectiveness of oral nutritional supplements improves the micronutrient deficiency of Vietnamese children with stunting. Arch. Pharm. Pract. 2020, 11, 7–13. [Google Scholar]
- Sheng, X.; Tong, M.; Zhao, D.; Leung, T.F.; Zhang, F.; Hays, N.P.; Ge, J.; Ho, W.M.; Northington, R.; Terry, D.; et al. Randomized controlled trial to compare growth parameters and nutrient adequacy in children with picky eating behaviors who received nutritional counseling with or without an oral nutritional supplement. Nutr. Metab. Insights 2014, 7, S15097. [Google Scholar] [CrossRef]
- Soliman, A.; Sanctis, V.; Elsiddig, S.; Alyafei, F.; Alaaraj, N.; Itani, M.; Jour, C.; Elawwa, A. Impact of oral nutritional supplements (ONS) on growth outcomes and IGF-1 level in underweight older children and young adolescents (5–14 years) with short stature and no systemic disease: High versus normal calories density formula. Acta Biomed. 2021, 92, e2021320. [Google Scholar]
- Vijayalakshmi, P.; Premakumari, S.; Haripriya, S. Supplementation of milk-based health drink enriched with micronutrients—Part-I impact on growth and haemoglobin status of 7–12 year old children. Ind. J. Nutr. Dietet. 2008, 45, 449–465. [Google Scholar]
- Vijayalakshmi, P.; Premakumari, S.; Haripriya, S. Supplementation of milk-based health drink enriched with micronutrients Part II–impact on clinical and morbidity picture, physical performance and cognitive development of 7–12 year old children. Ind. J. Nutr. Dietet. 2008, 45, 495–505. [Google Scholar]
- Shim, J.O.; Kim, S.; Choe, B.H.; Seo, J.H.; Yang, H.R. Effect of nutritional supplement formula on catch-up growth in young children with nonorganic faltering growth: A prospective multicenter study. Nutr. Res. Pract. 2020, 14, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional modulation of immune function: Analysis of evidence, mechanisms, and clinical relevance. Front. Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Nutrition and immunity: Lessons for COVID-19. Nutr. Diabetes 2021, 11, 19. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Zambruni, M.; Melby, C.L.; Melby, P.C. Impact of childhood malnutrition on host defense and infection. Clin. Microbiol. Rev. 2017, 30, 919–971. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.V.; Dinh, D.M.; Ward, H.D. Childhood malnutrition and the intestinal microbiome. Pediatr. Res. 2015, 77, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Gwela, A.; Mupere, E.; Berkley, J.A.; Lancioni, C. Undernutrition, host immunity and vulnerability to infection among young children. Pediatr. Infect. Dis. J. 2019, 38, e175–e177. [Google Scholar] [CrossRef]
- Iddrisu, I.; Monteagudo-Mera, A.; Poveda, C.; Pyle, S.; Shahzad, M.; Andrews, S.; Walton, G.E. Malnutrition and gut microbiota in children. Nutrients 2021, 13, 2727. [Google Scholar] [CrossRef]
- Tommaso, N.; Gasbarrini, A.; Ponziani, F. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Cecchi, N.; Romanelli, R.; Ricevuti, F.; Carbone, M.G.; Dinardo, M.; Cesarano, E.; De Michele, A.; Messere, G.; Morra, S.; Scognamiglio, A.; et al. Bioactives in Oral Nutritional Supplementation: A Pediatric Point of View. Nutrients 2024, 16, 2067. [Google Scholar] [CrossRef]
- Jones, K.D.; Thitiri, J.; Ngari, M.; Berkley, J.A. Childhood malnutrition: Toward an understanding of infections, inflammation, and antimicrobials. Food Nutr. Bull. 2014, 35, S64–S70. [Google Scholar] [CrossRef] [PubMed]
- Picó, C.; Serra, F.; Rodríguez, A.; Keijer, J.; Palou, A. Biomarkers of Nutrition and Health: New Tools for New Approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Niforou, A.; Konstantinidou, V.; Naska, A. Genetic Variants Shaping Inter-Individual Differences in Response to Dietary Intakes—A Narrative Review of the Case of Vitamins. Front. Nutr. 2020, 7, 558598. [Google Scholar] [CrossRef]
- Jenab, M.; Slimani, N.; Bictash, M.; Ferrari, P.; Bingham, S.A. Biomarkers in nutritional epidemiology: Applications, needs and new horizons. Hum. Genet. 2009, 125, 507–525. [Google Scholar] [CrossRef]
- Rytter, M.J.H.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The immune system in children with malnutrition—A systematic review. PLoS ONE 2014, 9, e105017. [Google Scholar] [CrossRef]
- Wu, B.; Luo, S.; Xu, C.; Yang, T.; Chen, Y. Influence factors for upper respiratory tract infection in Chinese rural children: A cross-sectional study. Front Pediatr. 2022, 10, 954363. [Google Scholar] [CrossRef]
| Study ID | Author, Year | Country | Undernutrition Status | Study Design | Sample Size | Age Range | Mean Age ± SD | % Boys | Grade Quality |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Akram, 2000 [41] | Pakistan | weight-for-age < −3 SD | Pre-post | 30 | 1–5 y | 22.0 ± 8.6 m | 50 | Low |
| 2 | Fisberg, 2002 [43] | Brazil, Mexico, Portugal, Spain | −1 SD < weight-for-height < −3 SD | RCT | 626 | 1–6 y | Treatment arm: 3.5 ± 2.0 y Control arm: 3.5 ± 2.3 y | 53 | Moderate |
| 3 | Alarcon, 2003 [42] | Philippines, Taiwan | weight-for-height < 25th percentile | RCT | 104 | 3–5 y | 48.5 m | 58 | High |
| 4 | Vijayalakshmi, 2008a [52] | India | N/A | RCT | 842 | 7–12 y | N/A | 50 | Low |
| 5 | Vijayalakshmi, 2008b [53] | India | N/A | RCT | 842 | 7–12 y | N/A | 50 | Low |
| 6 | Han, 2011 [45] | USA | height < −2 SD, bone age < 10 y and delayed by > 1 y | RCT | 20 | 7–11 y | 9.3 ± 1.3 y | 100 | High |
| 7 | Sheng, 2014 [50] | China | weight-for-height < 25th percentile | RCT | 142 | 3–5 y | Treatment arm: 3.8 ± 0.7 y Control arm: 3.6 ± 0.7 y | 46.5 | High |
| 8 | Huynh, 2015 [46] | Philippines | 5th percentile < weight-for-height < 25th percentile | Pre-post | 199 | 3–4 y | 41.2 ± 3.6 m | 49.7 | Low |
| 9 | Ghosh, 2018 [44] | India | −2 < weight-for-age z-scores < −1 | RCT | 255 | 2–6 y | 44.0 ± 14.3 m | 62.7 | High |
| 10 | Kansu, 2018 [47] | Turkey | weight-for-age < −2 SD, height-for-age < −2 SD | Observational | 345 | 1–10 y | 4.8 ± 2.7 y | 48.4 | Low |
| 11 | Pham, 2020 [49] | Vietnam | height-for-age z-scores < −2, weight-for-height z-scores < −1 | Pre-post | 106 | 2–4 y | N/A | 47.2 | Low |
| 12 | Shim, 2020 [54] | Korea | weight-for-height z-score < −2 | Pre-post | 82 | 1–3 y | 21.6 ± 7.3 m | 61 | Low |
| 13 | Khadilkar, 2021 [48] | India | −2 < weight-for-age z-scores < −1 | RCT | 216 | 4–6 y | Treatment group: 4.5 ± 0.6 y Control group: 4.6 ± 0.6 y | 46.3 | High |
| 14 | Soliman, 2021 [51] | Qatar | height-for-age < −2, 2 SD < standardized BMI < −1 SD | RCT | 34 | 5–14 y | 10.2 y | N/A | Moderate |
| Study ID | Author, Year | Intervention Type | Intervention Setting | Intervention Duration | Arm-Specific Sample Size |
|---|---|---|---|---|---|
| 1 | Akram, 2000 [41] | Pre: no ONS Post: ONS | Hospital/clinics | 0.5 m | Single arm: 30 |
| 2 | Fisberg, 2002 [43] | Treatment arm: ONS + synbiotics Control arm: ONS | Daycare center | 4 m | Treatment arm: 310 Control arm: 316 |
| 3 | Alarcon, 2003 [42] | Treatment arm: NC + ONS Control arm: NC | Study site | 3 m | Treatment arm: 53 Control arm: 51 |
| 4 | Vijayalakshmi, 2008a [52] | Treatment arm 1: ONS (milk-based micronutrient supplement) Treatment arm 2: water-based micronutrient supplement Control group: no ONS | School | 12 m | Treatment arm 1: 270 Treatment arm 2: 291 Control arm: 281 |
| 5 | Vijayalakshmi, 2008b [53] | Treatment arm 1: ONS (milk-based micronutrient supplement) Treatment arm 2: water-based micronutrient supplement Control group: no ONS | School | 12 m | Treatment arm 1: 270 Treatment arm 2: 291 Control arm: 281 |
| 6 | Han, 2011 [45] | Treatment arm: 6 m ONS + 12 m daily G.H. therapy Control group: 6 m observation + 12 m daily GH therapy | Hospital/clinics | 18 m | Treatment arm: 10 Control arm: 10 |
| 7 | Sheng, 2014 [50] | Treatment arm: NC + ONS Control arm: NC | Hospital/clinics | 4 m | Treatment arm: 75 Control arm: 67 |
| 8 | Huynh, 2015 [46] | Pre: no ONS Post: ONS | Hospital/clinics | 12 m | Single arm: 199 |
| 9 | Ghosh, 2018 [44] | Treatment arm: NC + ONS Control arm: NC | Hospital/clinics | 3 m | Treatment arm: 127 Control arm: 128 |
| 10 | Kansu, 2018 [47] | All subjects: ONS (high-fiber) | Hospital/clinics | 6 m | Single arm: 345 Baseline: 345 1st follow-up visit (2–3 m): 126 2nd follow-up visit (4–6 m): 138 |
| 11 | Pham, 2020 [49] | Pre: no ONS Post: ONS | School | 6 m | Single arm: 106 |
| 12 | Shim, 2020 [54] | Good consumption group: consumed ≥ 60% of the recommended dose of the formula) Poor consumption group: consumed < 60% of the recommended dose of the formula) | Hospital/clinics | 6 m | Good consumption group: 38 Poor consumption group: 44 |
| 13 | Khadilkar, 2021 [48] | Treatment arm: NC + ONS Control arm: NC | Hospital/clinics | 3 m | Treatment arm: 108 Control arm: 108 |
| 14 | Soliman, 2021 [51] | Treatment arm: ONS (energy-dense) Control arm: ONS (standard) | Hospital/clinics | 12 m | Treatment arm: 22 Control arm: 12 |
| Study ID | Author, Year | ONS Product Characteristics | ONS Daily Dose | Nutritional Biomarkers and Other Biochemical Indices | Health-Related Outcomes | Intervention Effectiveness |
|---|---|---|---|---|---|---|
| 1 | Akram, 2000 [41] | PediaSure (Abbott): 1 kcal/mL, 3 g of protein/kcal, 49.8 g of total fat/L, and 109.7 g of carbohydrate/L | The quantity of PediaSure administered varied by child according to demand and tolerance. The minimum energy administered for each child was at least 50% of the total requirement based on weight-for-age. | Serum electrolyte, sodium, potassium, chloride, BUN creatinine, calcium, phosphorus, total protein, fasting/random glucose, albumin, cholesterol, triglyceride, alkaline phosphatase, uric acid, total bilirubin, HDL, WBC, Hb, platelet, hematocrit, neutrophil, lymphocyte, monocyte, and eosinophil. |
There was no statistically significant change for all other outcome measures before and after the intervention. | |
| 2 | Fisberg, 2002 [43] | PediaSure (Abbott) + synbiotics: FOS (0.5g/L after reconstitution) and probiotic bacteria bifidobacterium and acidophilus, each at the level of 3 × 107 CFU/g. | Subjects were expected to consume between 375 and 750 mL/d of their assigned supplement for at least 80% of the 4 m study period. | Sick days, diarrhea, upper and lower respiratory tract infection, stool frequency and consistency. | Within-arm comparisons: Treatment arm:
Between-arm comparisons (treatment arm vs. control arm):
Subgroup comparisons:
| |
| 3 | Alarcon, 2003 [42] | PediaSure (Abbott): lactose-free, providing 1.0 kcal/mL (12% protein, 43.8% carbohydrate, and 44.8% fat). | Subjects in the treatment arm were to consume 40 mL/kg/d of the supplement in addition to their regular diet and not to consume any similar products during the study. | Serum albumin, iron, ferritin, and zinc. | Gastrointestinal symptoms include diarrhea, constipation, nausea, vomiting or regurgitation, abdominal distention, belching or burping, and flatulence. | Between-arm comparisons (treatment arm vs. control arm):
|
| 4 | Vijayalakshmi, 2008a [52] | Treatment arm 1: 66 g of micronutrient supplement mixed in 300 mL of milk. Treatment arm 2: 66 g of micronutrient supplement mixed in 300 mL of water. | Twice a day for 6 d in a week for a duration of 12 m except Sundays and long holidays. | Hb | Within-arm comparisons: Treatment arm 1:
↑ 1.40 for 8 years old ↑ 1.44 for 9 years old ↑ 1.40 for 10 years old ↑ 1.42 for 11 years old ↑ 1.46 for 12 years old Treatment arm 2:
↑ 0.96 for 8 years old ↑ 0.90 for 9 years old ↑ 0.94 for 10 years old ↑ 0.94 for 11 years old ↑ 0.98 for 12 years old Control arm:
↑ 0.80 for 8 years old ↑ 0.76 for 9 years old ↑ 0.38 for 10 years old ↑ 0.88 for 11 years old ↑ 0.14 for 12 years old Between-arm comparisons (treatment arm vs. control arm)
| |
| 5 | Vijayalakshmi, 2008b [53] | Treatment arm 1: 66 g of micronutrient supplement mixed in 300 mL of milk. Treatment arm 2: 66 g of micronutrient supplement mixed in 300 mL of water. | Twice a day for 6 d in a week for a duration of 12 m except Sundays and long holidays. | Morbidity patterns | Within-arm comparisons: Treatment arm 1:
Treatment arm 2:
Control arm:
| |
| 6 | Han, 2011 [45] | PediaSure (Abbott): 237 kcal and 7 g protein/8-oz can. G.H. therapy: dosed at 0.3 mg/kg/w administered subcutaneously once daily (Nutropin AQ, Genentech). | The children were prescribed, on average, 10.4 ± 2.5 oz ONS daily (i.e., energy 13.4 kcal/kg/d and protein 0.4 g/kg/d), with subsequent adjustments based on individual weight and energy needs (6 m: 16.8 ± 2.0 oz/d; 12 m: 20.0 ± 1.9 oz/d; 18 m: 22.0 ± 2.1 oz/d). GH therapy: GH was dosed at 0.3 mg/kg/w and administered subcutaneously once daily. | Growth hormones, pre-albumin, lipids, transferrin, IGF-1, IGFBP-3, pre-albumin, acylated ghrelin, fasting insulin, and glucose. | Within-arm comparisons:
Between-arm comparisons (treatment arm vs. control arm):
| |
| 7 | Sheng, 2014 [50] | The ONS was a milk-based powder (S-26 PE GOLD, Wyeth Nutrition, Singapore). The ONS provided 200 kcal/serving (14% protein, 54% carbohydrate, and 32%. fat) and micronutrients. Amount per serving (230 mL): energy 200 kcal, protein 7 g, fat 7 g, arachidonic acid 5.2 mg, docosahexaenoic acid 3.6 mg, carbohydrates 27 g, nucleotides 5.2 mg, taurine 9.4 mg, l-carnitine 3.4 mg, lutein 40 mcg, vitamin A 200 mcg, carotenes 42 mcg, vitamin D 3.3 mcg, vitamin E 2.2 mg, vitamin K 13 mcg, vitamin B1 0.26 mg, vitamin B2 320 mcg, vitamin B6 250 mcg, vitamin B12 0.5 mcg, niacin 1388 mcg, folic acid 19 mcg, pantothenic acid 1000 mcg, biotin 4.9 mcg, vitamin C 24 mg, choline 60 mg, inositol 15 mg, calcium 260 mg, phosphorus 170 mg, magnesium 28 mg, iron 3.8 mg, zinc 2.4 mg, manganese 225 mcg, copper 175 mcg, iodine 19 mcg, sodium 118 mg, potassium 475 mg, and chloride 275 mg. | At least two 230 mL servings per day. | Diarrhea, and upper and lower respiratory tract infections. | Between-arm comparisons (treatment arm vs. control arm):
| |
| 8 | Huynh, 2015 [46] | PediaSure (Abbott) ONS (450 mL): energy 450 kcal, protein 13.5 g, vitamin A 270 mcg, vitamin C 45 mg, vitamin B1 1.4 mg, vitamin B2 0.9 mg, niacin 6.8 mg, vitamin B6 1.2 mg, vitamin B12 1.4 mcg, vitamin D 9.0 mcg, vitamin E 7.2 mg, folate 112.5 mcg, calcium 432 mg, iron 6.3 mg, iodine 43.7 mcg, magnesium 89.1 mg, phosphorus 373.5 mg, zinc 3.0 mg, selenium 14.4 mcg, manganese 0.7 mg, lactobacillus acidophilus 3.9 × 107 CFU, bifidobacterium 2.45 × 106 CFU, and FOS 1.98 g. | Two servings of ONS per day for 48 w, the ONS provided 450 kcal, 13.5 g of high-quality protein, 17.7 g of easily-digested fat and 59.4 g of carbohydrate, and 28 minerals and vitamins (450 mL in total). | Sick days and number of acute illnesses. |
| |
| 9 | Ghosh, 2018 [44] | PediaSure (Abbott), ONS per serving (45.5 g): energy 213 kcal, protein 6.4 g, fat 10.6 g, carbohydrate 22.8 g, vitamin A 138.8 mcg, vitamin C 20.0 mg, vitamin B1 0.41 mg, vitamin B2 0.46 mg, niacin 3.19 mg, vitamin B6 0.46 mg, vitamin B12 0.68 mg, pantothenic acid 1.41 mg, biotin 7.28 mcg, choline 53.7 mg, vitamin D2 1.43 mcg, vitamin E 5.01 mg, vitamin K 7.96 mcg, folic acid 45.5 mcg, calcium 175.6 mg, iron 2.5 mg, iodine 17.3 mcg, magnesium 89.1 mg, phosphorus 109.2 mg, zinc 1.59 mg, selenium 5.60 mcg, and manganese 0.45 mg. | Children 24–48 m: at least 224 mL ONS; Children 48–72 m: 448 mL of ONS. | Sick days from URTI and number of acute URTI episodes. |
| |
| 10 | Kansu, 2018 [47] | PediaSure Fiber version (Abbott): protein (11.2%), carbohydrate (43.6%), fat (44.7%) and dietary fiber and short-chain FOS (0.5%). Fortini 1.0 Multi Fibre (Nutricia): carbohydrates (10.0%), fat (47%), and dietary fiber (3.0%). | High-fiber regimens were used as ONS based on an average of 2 packages/d in the majority of patients that provided daily calorie intakes of at least 40 kcal/kg, daily fiber intake of at least 300 mg/kg and daily water intake of at least 25 mL/kg in half of the patients. | Comorbidity, gastrointestinal symptoms, and defecation habits. |
| |
| 11 | Pham, 2020 [49] | PediaSure (Abbott): Each cup consisted of 190 mL of cooled boiled water (<37 °C) mixed with 5 tablespoons brushed-across PediaSure powder (equivalent to 49 g of flour) and stirred 225 mL (1 mL is equivalent to 1 kcal). | Each child was given 2 glasses of PediaSure per d continuously for 6 m. | Hb, albumin, zinc, CRP, and AGP. |
Subgroup comparisons:
| |
| 12 | Shim, 2020 [54] | Pediapowder (MDwell, Seoul, Korea) (400 mL): calorie 400 kcal, protein 12 g, lipid 14 g, carbohydrate 56 g, vitamin A 250 μg, vitamin D 4 μg, vitamin E 10 mg, vitamin K 26 μg, vitamin C 40 mg, vitamin B1 1 mg, vitamin B2 0.84 mg, vitamin B6 1 mg, vitamin B12 2.48 μg, Niacin 3.32 mg, Biotin 7.2 μg, folic acid 100 μg, pantothenic acid 4.16 mg, inositol 33.2 mg, calcium 422 mg, phosphorus 340 mg, potassium 516 mg, iron 4.48 mg, magnesium 44 mg, sodium 120 mg, zinc 4.4 mg, manganese 0.68 mg, and copper 0.44 mg. | Children were instructed to consume 2 sachets of the formula, Pediapowder® (MDwell, Seoul, Korea), mixed with 400 mL of water per day. | Blood urea nitrogen concentration, serum concentration of calcium, iron, and total iron binding capacity, ferritin, uric acid, total bilirubin, hemoglobin, albumin, and creatinine. | Within-arm comparisons: Good consumption group
Poor consumption group
Between-arm comparisons:
| |
| 13 | Khadilkar, 2021 [48] | ONS per serving (45 g): energy 201 kcal, protein 8 g, carbohydrates 27 g, and total fat 7 g. | 45 g of ONS daily. | Incidences of fever, vomiting, cold, diarrhea, cough, URTI, and rashes. | Between-arm comparisons (treatment arm vs. control arm):
| |
| 14 | Soliman, 2021 [51] | Energy-dense ONS per 200 mL bottle: energy 300 kcal, energy density 1.5 kcal/mL, protein 6.8 g, carbohydrates 37.6 g, and fat 13.6 g. Standard ONS per 200 mL bottle: energy 200 kcal, energy density 1 kcal/mL, protein 4.8 g, carbohydrates 23.6 g, and fat 9 g. | Treatment arm (energy-dense ONS): 1.5 kcal/mL. Control arm (standard ONS): 1 kcal/ml. | IGF-1 | Within-arm comparisons: Treatment arm
Control arm
Between-arm comparisons (treatment arm vs. control arm):
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, R.; Shen, J.; Zhang, Z.; Lim, M.T.; Huynh, D.T.T. Effect of Oral Nutritional Supplementation on Health-Related Outcomes and Nutritional Biomarkers in Children and Adolescents with Undernutrition: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2970. https://doi.org/10.3390/nu16172970
An R, Shen J, Zhang Z, Lim MT, Huynh DTT. Effect of Oral Nutritional Supplementation on Health-Related Outcomes and Nutritional Biomarkers in Children and Adolescents with Undernutrition: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(17):2970. https://doi.org/10.3390/nu16172970
Chicago/Turabian StyleAn, Ruopeng, Jing Shen, Zhiying Zhang, Meng Thiam Lim, and Dieu T. T. Huynh. 2024. "Effect of Oral Nutritional Supplementation on Health-Related Outcomes and Nutritional Biomarkers in Children and Adolescents with Undernutrition: A Systematic Review and Meta-Analysis" Nutrients 16, no. 17: 2970. https://doi.org/10.3390/nu16172970
APA StyleAn, R., Shen, J., Zhang, Z., Lim, M. T., & Huynh, D. T. T. (2024). Effect of Oral Nutritional Supplementation on Health-Related Outcomes and Nutritional Biomarkers in Children and Adolescents with Undernutrition: A Systematic Review and Meta-Analysis. Nutrients, 16(17), 2970. https://doi.org/10.3390/nu16172970






