The Association of Dietary Diversity with Hyperuricemia among Community Inhabitants in Shanghai, China: A Prospective Research
Abstract
1. Introduction
2. Materials and Methods
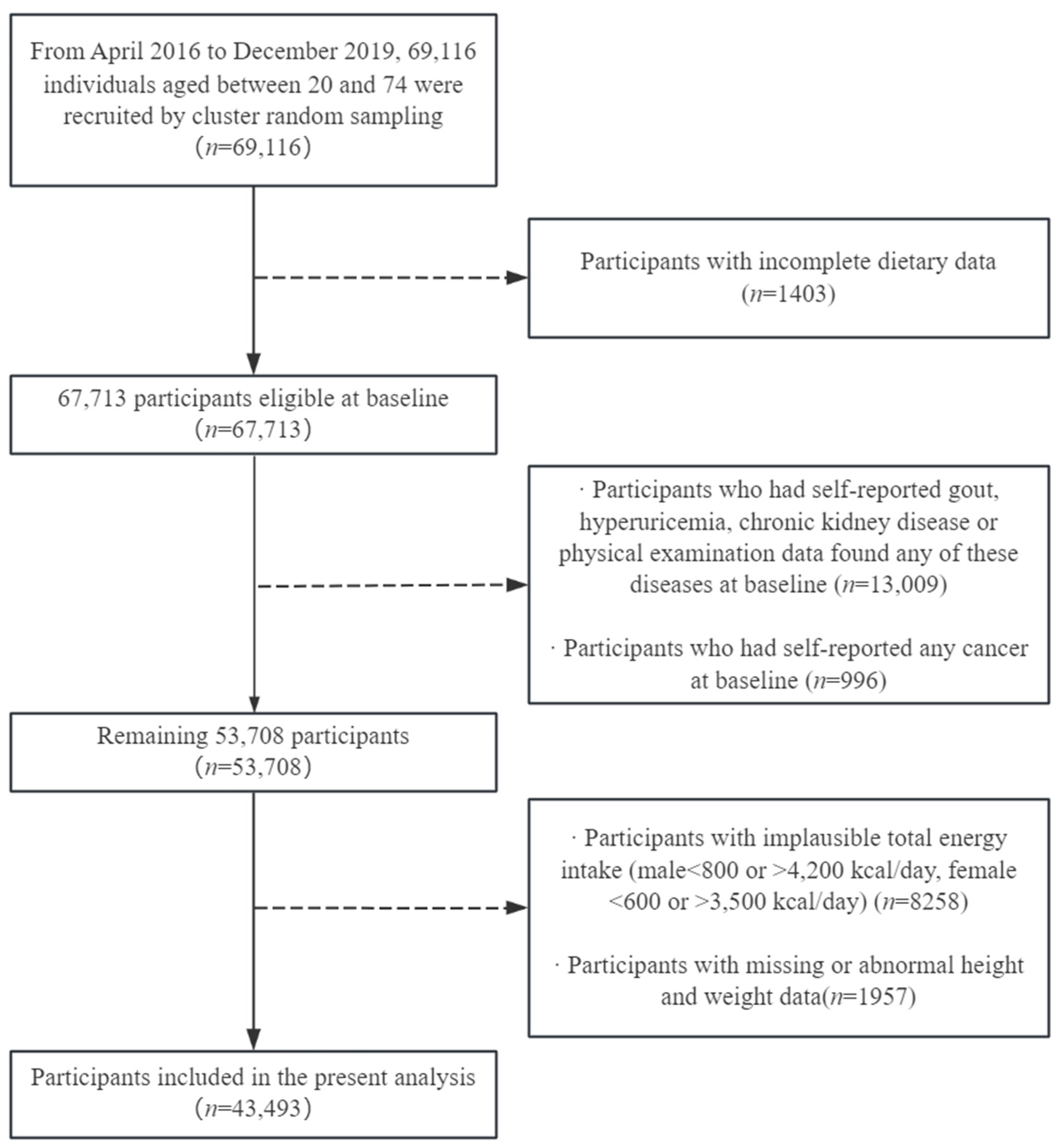
2.1. Study Design and Population
2.2. Evaluation of Dietary Diversity
2.3. Follow-Up and Ascertainment of Hyperuricemia
2.4. Assessment of Covariates
2.5. Statistical Analyses
3. Results
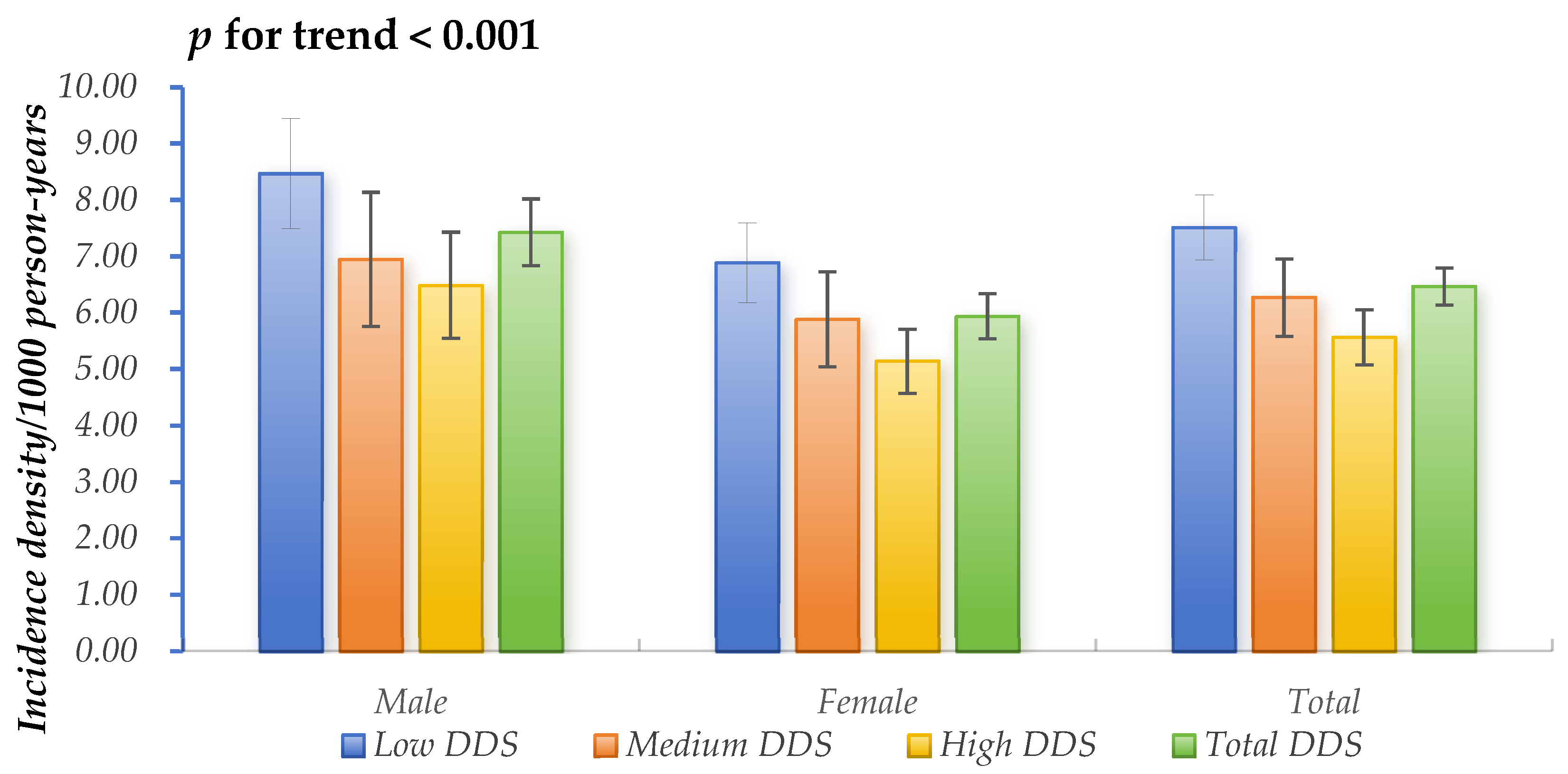
3.1. Baseline Characteristics and Hyperuricemia Incidence
3.2. Association of Dietary Diversity Score with Hyperuricemia
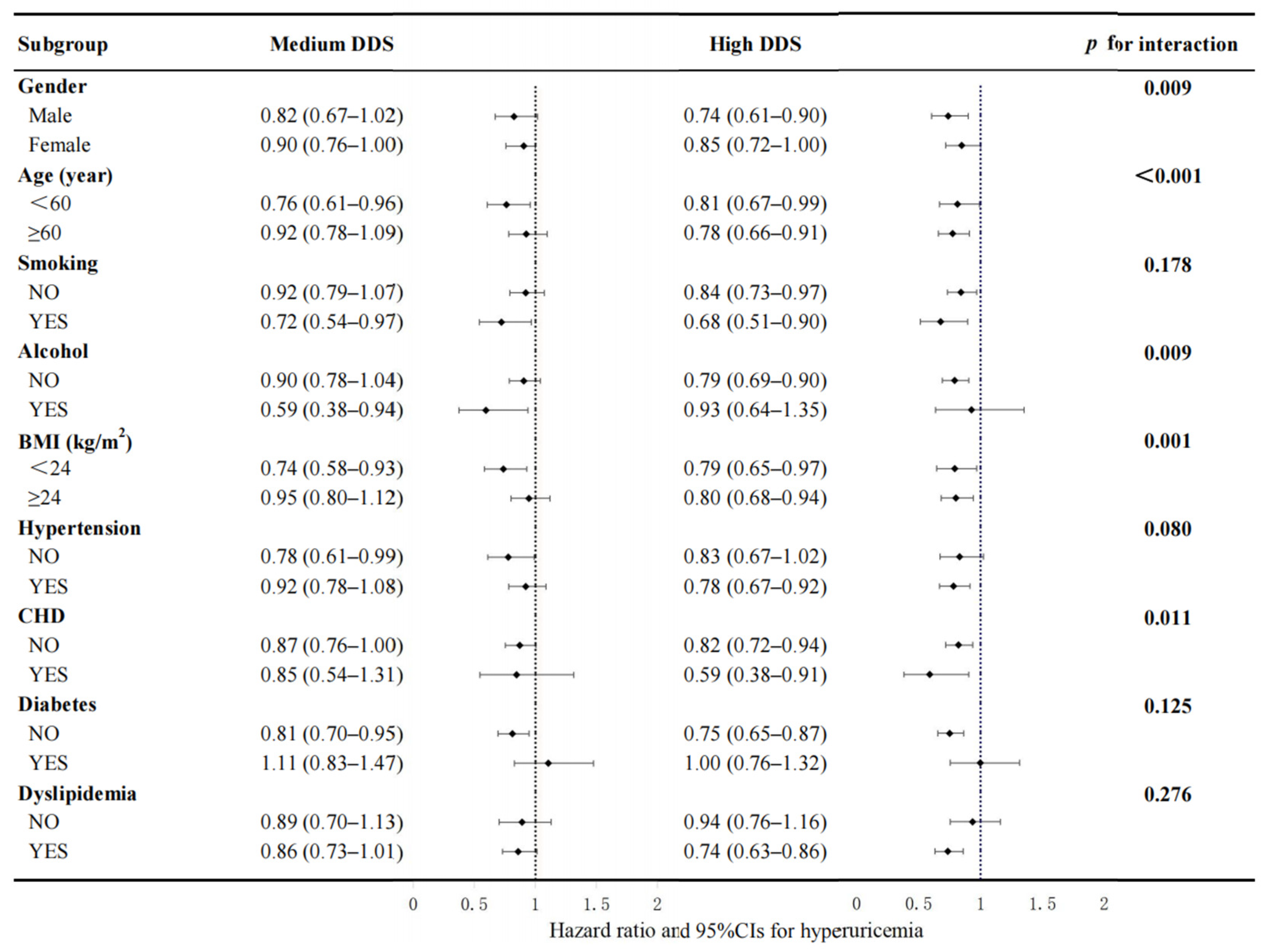
3.3. Subgroup Analyses
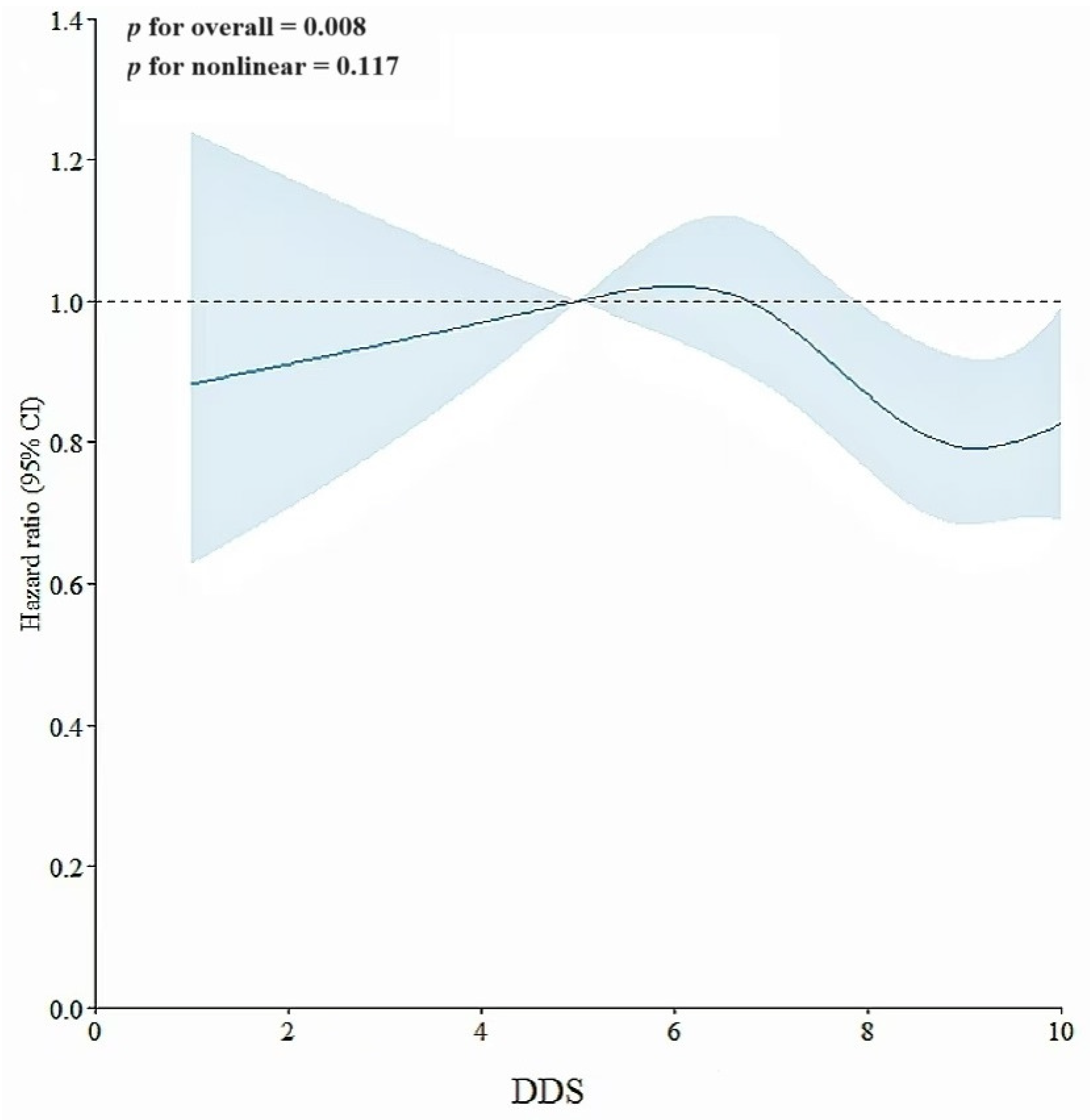
3.4. Dose–Response Analysis of Dietary Diversity Score with Hyperuricemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DASH | Dietary approaches to stop hypertension |
| DDS | Dietary Diversity Score |
| SSACB | Shanghai Suburban Adult Cohort and Biobank |
| EMR | Electronic Medical Record System |
| CDM | Chronic Disease Management System |
| CR | Cancer Registry System |
| CDSS | Cause-of-Death Surveillance System |
| FFQ | Food frequency questionnaire |
| ICD-10 | International Classification of Diseases tenth revision |
| PA | Physical activity |
| IPAQ | International Physical Activity Questionnaire |
| BMI | Body mass index |
| CHD | Coronary heart disease |
| COPD | Chronic obstructive pulmonary disease |
| HbA1C | Hemoglobin type A1C |
| FPG | Fasting blood glucose |
| HR | Hazard ratio |
| 95% CIs | 95% confidence intervals |
| RCS | Restricted cubic splines |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| OTC | Over the counter |
References
- Dalbeth, N.; Gosling, A.L.; Gafo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef]
- Mortada, I. Hyperuricemia, type 2 diabetes mellitus, and hypertension: An emerging association. Curr. Hypertens. Rep. 2017, 19, 1–5. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, M.J.; Choi, H.G.; Lee, S.J.; Kim, S.W.; Kim, J.H.; Kwon, B.C.; Lee, J.W. The association between hyperuricemia and cardiovascular disease history: A cross-sectional study using KoGES HEXA data. Medicine 2022, 101, e32338. [Google Scholar] [CrossRef] [PubMed]
- Prasad Sah, O.S.; Qing, Y.X. Associations between hyperuricemia and chronic kidney disease: A review. Nephrourol Mon. 2015, 7, e27233. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Kawamoto, R.; Ninomiya, D.; Kumagi, T. Hyperuricemia is associated with all-cause mortality among males and females: Findings from a study on Japanese community-dwelling individuals. Metabol. Open 2022, 14, 100186. [Google Scholar] [CrossRef]
- Otaki, Y.; Konta, T.; Ichikawa, K.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Narita, I.; Kondo, M.; et al. Possible burden of hyperuricaemia on mortality in a community-based population: A large-scale cohort study. Sci. Rep. 2021, 11, 8999. [Google Scholar] [CrossRef] [PubMed]
- Yokose, C.; McCormick, N.; Choi, H.K. The role of diet in hyperuricemia and gout. Curr. Opin. Rheumatol. 2021, 33, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Kumar, A.U.A.; Browne, L.D.; Li, X.; Adeeb, F.; Perez-Ruiz, F.; Fraser, A.D.; Stack, A.G. Temporal trends in hyperuricaemia in the Irish health system from 2006-2014: A cohort study. PLoS ONE 2018, 13, e0198197. [Google Scholar] [CrossRef]
- Pathmanathan, K.; Robinson, P.C.; Hill, C.L.; Keen, H.I. The prevalence of gout and hyperuricaemia in Australia: An updated systematic review. Semin. Arthritis Rheum. 2021, 51, 121–128. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, X.; Wu, J.; Huang, Z.; Zhao, Z.; Zhang, X.; Xue, Y.; Wan, W.; Li, C.; Zhang, W.; et al. Prevalence of Hyperuricemia Among Chinese Adults: Findings from Two Nationally Representative Cross-Sectional Surveys in 2015-16 and 2018-19. Front. Immunol. 2022, 12, 791983. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Jia, X.; Fang, M.; Yang, Q.; Gong, Z. Assessment of the temporal trend and daily profiles of the dietary purine intake among Chinese residents during 2014 to 2021. Front. Nutr. 2023, 10, 1259053. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Fukuuchi, T.; Aoki, Y.; Mizuta, E.; Ouchi, M.; Kurajoh, M.; Maruhashi, T.; Tanaka, A.; Morikawa, N.; Nishimiya, K.; et al. Exploring the Multifaceted Nexus of Uric Acid and Health: A Review of Recent Studies on Diverse Diseases. Biomolecules 2023, 13, 1519. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, K.; Li, C. Dietary factors and risk of gout and hyperuricemia: A meta-analysis and systematic review. Asia Pac. J. Clin. Nutr. 2018, 27, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cui, L.F.; Sun, Y.Y.; Yang, W.H.; Wang, J.R.; Wu, S.L.; Gao, X. Adherence to the dietary approaches to stop hypertension diet and hyperuricemia: A cross-sectional study. Arthritis Care Res. 2021, 73, 603–611. [Google Scholar] [CrossRef]
- Yokose, C.; McCormick, N.; Choi, H.K. Dietary and Lifestyle-Centered Approach in Gout Care and Prevention. Curr. Rheumatol. Rep. 2021, 23, 51. [Google Scholar] [CrossRef]
- Yi, K.; Cui, S.; Tang, M.; Wu, Y.; Xiang, Y.; Yu, Y.; Tong, X.; Jiang, Y.; Zhao, Q.; Zhao, G. Adherence to DASH Dietary Pattern and Its Association with Incident Hyperuricemia Risk: A Prospective Study in Chinese Community Residents. Nutrients 2022, 14, 4853. [Google Scholar] [CrossRef]
- Phillips, J.A. Dietary Guidelines for Americans, 2020–2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef]
- Springmann, M.; Spajic, L.; Clark, M.A.; Poore, J.; Herforth, A.; Webb, P.; Rayner, M.; Scarborough, P. The healthiness and sustainability of national and global food based dietary guidelines: Modelling study. BMJ 2020, 370, m2322. [Google Scholar] [CrossRef]
- O’Hearn, M.; Erndt-Marino, J.; Gerber, S.; Lauren, B.N.; Economos, C.; Wong, J.B.; Blumberg, J.B.; Mozaffarian, D. Validation of Food Compass with a healthy diet, cardiometabolic health, and mortality among U.S. adults, 1999–2018. Nat. Commun. 2022, 13, 7066. [Google Scholar] [CrossRef]
- Gardner, C.D.; Vadiveloo, M.K.; Petersen, K.S.; Anderson, C.A.M.; Springfield, S.; Van Horn, L.; Khera, A.; Lamendola, C.; Mayo, S.M.; Joseph, J.J.; et al. Popular Dietary Patterns: Alignment With American Heart Association 2021 Dietary Guidance: A Scientific Statement from the American Heart Association. Circulation 2023, 147, 1715–1730. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; A Lear, S.; Wei, L.; Diaz, R.; et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023, 44, 2560–2579. [Google Scholar] [CrossRef]
- Kennedy, G.; Ballard, T.; Dop, M.-C. Guidelines for Measuring Household and Individual Dietary Diversity; FAO: Rome, Italy, 2011. [Google Scholar]
- Foote, J.A.; Murphy, S.P.; Wilkens, L.R.; Basiotis, P.P.; Carlson, A. Dietary variety increases the probability of nutrient adequacy among adults. J. Nutr. 2004, 134, 1779–1785. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Anderson, C.A.M.; Dearborn, J.L.; Ferranti, E.P.; Mozaffarian, D.; Rao, G.; Wylie-Rosett, J.; Lichtenstein, A.H.; American Heart Association Behavioral Change for Improving Health Factors Committee of the Council on Lifestyle and Cardiometabolic Health and Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; et al. Dietary Diversity: Implications for Obesity Prevention in Adult Populations: A Science Advisory from the American Heart Association. Circulation 2018, 138, e160–e168, Correction in Circulation 2018, 138, e712. [Google Scholar] [CrossRef]
- Zheng, G.; Xia, H.; Shi, H.; Zheng, D.; Wang, X.; Ai, B.; Tian, F.; Lin, H. Effect modification of dietary diversity on the association of air pollution with incidence, complications, and mortality of type 2 diabetes: Results from a large prospective cohort study. Sci. Total Environ. 2024, 908, 168314. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.F.; Song, W.Q.; Wang, X.M.; Li, Z.H.; Shen, D.; Liu, D.; Zhang, P.D.; Shen, Q.Q.; Liang, F.; Nan, Y.; et al. Dietary Diversity Changes and Cognitive Frailty in Chinese Older Adults: A Prospective Community-Based Cohort Study. Nutrients 2023, 15, 3784. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhong, W.F.; Li, Z.H.; Chen, P.L.; Zhang, Y.J.; Ren, J.J.; Liu, D.; Shen, Q.Q.; Yang, P.; Song, W.Q.; et al. Dietary diversity and frailty among older Chinese people: Evidence from the Chinese Longitudinal Healthy Longevity Study. Am. J. Clin. Nutr. 2023, 117, 383–391. [Google Scholar] [CrossRef]
- Lv, Y.; Kraus, V.B.; Gao, X.; Yin, Z.; Zhou, J.; Mao, C.; Duan, J.; Zeng, Y.; Brasher, M.S.; Shi, W.; et al. Higher dietary diversity scores and protein-rich food consumption were associated with lower risk of all-cause mortality in the oldest old. Clin. Nutr. 2020, 39, 2246–2254. [Google Scholar] [CrossRef]
- Torres-Collado, L.; García-de la Hera, M.; Cano-Ibañez, N.; Bueno-Cavanillas, A.; Vioque, J. Association between Dietary Diversity and All-Cause Mortality: A Multivariable Model in a Mediterranean Population with 18 Years of Follow-Up. Nutrients 2022, 14, 1583. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, B.; Wang, R.; Zhu, M.; Shao, Y.; Wang, N.; Liu, X.; Zhang, T.; Jiang, F.; Wang, W.; et al. Cohort profile: Protocol and baseline survey for the Shanghai Suburban Adult Cohort and Biobank (SSACB) study. BMJ Open 2020, 10, e035430. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; An, Q.; Rao, J. The effects of dietary diversity on health status among the older adults: An empirical study from China. BMC Public Health 2024, 24, 674. [Google Scholar] [CrossRef]
- Duan, Y.; Qi, Q.; Cui, Y.; Yang, L.; Zhang, M.; Liu, H. Effects of dietary diversity on frailty in Chinese older adults: A 3-year cohort study. BMC Geriatr. 2023, 23, 141. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents (2022); People’s Medical Publishing House: Beijing, China, 2022. (In Chinese) [Google Scholar]
- Zhang, J.; Liang, D.; Zhao, A. Dietary Diversity and the Risk of Fracture in Adults: A Prospective Study. Nutrients 2020, 12, 3655. [Google Scholar] [CrossRef]
- Borghi, C.; Domienik-Karłowicz, J.; Tykarski, A.; Widecka, K.; Filipiak, K.J.; Jaguszewski, M.J.; Narkiewicz, K.; Mancia, G. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk: 2021 update. Cardiol. J. 2021, 28, 1–14. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, G.Y.; Pan, X.C. (Eds.) China Food Composition Table, 2nd ed.; Beijing Medical University Press: Beijing, China, 2009. [Google Scholar]
- Bassett, D.R., Jr. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1396. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Lan, X.; Qin, X.; Huang, Y.; Wang, J.; Luo, P.; Wen, Z.; Li, Y.; Kong, Y.; et al. Low BMI and high waist-to-hip ratio are associated with mortality risk among hemodialysis patients: A multicenter prospective cohort study. Clin. Kidney J. 2022, 16, 167–175. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980, Erratum in Lancet 2022, 399, 520. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Chang, Y.; Zhang, Y.; Kim, S.G.; Cho, J.; Son, H.J.; Shin, H.; Guallar, E. A cohort study of hyperuricemia in middle-aged South Korean men. Am. J. Epidemiol. 2012, 175, 133–143. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, J.; Yu, H.; Ding, L.; Liu, F.; Wang, J. Incidence density of hyperuricemia and association between metabolism-related predisposing risk factors and serum urate in Chinese adults: A cohort study. Front. Endocrinol. 2023, 14, 1253470. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Lu, X.; Chen, C.; Du, H.; Zhang, R. Risk factors for the development of hyperuricemia: A STROBE-compliant cross-sectional and longitudinal study. Medicine 2019, 98, e17597. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, X.; Wang, J.; Liu, H.; Kwong, J.S.; Chen, H.; Li, L.; Chung, S.C.; Shah, A.; Chen, Y.; et al. Diagnosis and treatment for hyperuricemia and gout: A systematic review of clinical practice guidelines and consensus statements. BMJ Open 2019, 9, e026677. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.; Shin, Y.; Cho, J.H.; Lee, D.; Kim, Y. Association between Dietary Diversity Score and Metabolic Syndrome in Korean Adults: A Community-Based Prospective Cohort Study. Nutrients 2022, 14, 5298. [Google Scholar] [CrossRef] [PubMed]
- Karimbeiki, R.; Pourmasoumi, M.; Feizi, A.; Abbasi, B.; Hadi, A.; Rafie, N.; Safavi, S.M. Higher dietary diversity score is associated with obesity: A case-control study. Public Health 2018, 157, 127–134. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef]
- Zheng, G.; Cai, M.; Liu, H.; Li, R.; Qian, Z.; Howard, S.W.; Keith, A.E.; Zhang, S.; Wang, X.; Zhang, J.; et al. Dietary Diversity and Inflammatory Diet Associated with All-Cause Mortality and Incidence and Mortality of Type 2 Diabetes: Two Prospective Cohort Studies. Nutrients 2023, 15, 2120. [Google Scholar] [CrossRef]
- Moraeus, L.; Lindroos, A.K.; Warensjö Lemming, E.; Mattisson, I. Diet diversity score and healthy eating index in relation to diet quality and socio-demographic factors: Results from a cross-sectional national dietary survey of Swedish adolescents. Public Health Nutr. 2020, 23, 1754–1765. [Google Scholar] [CrossRef]
- Conklin, A.I.; Monsivais, P.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Dietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study. PLoS Med. 2016, 13, e1002085, Erratum in PLoS Med. 2016, 13, e1002123. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, H.; Zhang, B.; Zhang, J.; Ma, Q.; Sun, H. Association between dyslipidaemia and the risk of hyperuricaemia: A six-year longitudinal cohort study of elderly individuals in China. Ann. Med. 2022, 54, 2402–2410. [Google Scholar] [CrossRef]
- Lai, H.M.; Chen, C.J.; Su, B.Y.; Chen, Y.C.; Yu, S.F.; Yen, J.H.; Hsieh, M.C.; Cheng, T.T.; Chang, S.J. Gout and type 2 diabetes have a mutual inter-dependent effect on genetic risk factors and higher incidences. Rheumatology 2012, 51, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hsieh, M.C.; Chang, S.J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Lu, Y.; Hu, F.; Feng, X.; Zhang, Y.; Li, S.; Zhang, C.; Zhang, H.; Zeng, Y.; Yao, Y.; et al. Dietary diversity and possible sarcopenia among older people in China: A nationwide population-based study. Front. Nutr. 2023, 10, 1218453. [Google Scholar] [CrossRef]
- Oliveira Otto, M.C.D.; Padhye, N.S.; Bertoni, A.G.; Jacobs, D.R., Jr.; Mozaffarian, D. Everything in Moderation—Dietary Diversity and Quality, Central Obesity and Risk of Diabetes. PLoS ONE 2015, 10, e0141341. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China; China National Center for Food Safety Risk Assessment. Dietary guidelines for adult Hyperuricemia and gout (2024). J. Hyg. Res. 2024, 53, 352–356. (In Chinese) [Google Scholar]
- Sun, Y.; Sun, J.; Zhang, P.; Zhong, F.; Cai, J.; Ma, A. Association of dietary fiber intake with hyperuricemia in U.S. adults. Food Funct. 2019, 10, 4932–4940. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Li, H.; Ding, X.; Li, X.; Jing, X.; Chen, J.; Liu, G.; Lin, Y.; Jiang, C.; et al. Inulin supplementation ameliorates hyperuricemia and modulates gut microbiota in Uox-knockout mice. Eur. J. Nutr. 2021, 60, 2217–2230. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, Z.; Lu, Y. The potential of probiotics in the amelioration of hyperuricemia. Food Funct. 2022, 13, 2394–2414. [Google Scholar] [CrossRef]
- Lian, Y.; Gu, L.; Yang, L.; Wang, L.; Li, H. The Reasonableness and Spatial Differences of the Food Consumption Structure of Urban and Rural Residents in China, 2015–2021. Foods 2023, 12, 1997. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.G.; Attard, S.M.; Herring, A.H.; Wang, H.; Du, S.; Gordon-Larsen, P. Socioeconomic gradients in the Westernization of diet in China over 20 years. SSM Popul. Health 2021, 16, 100943. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | Low DDS (0–7) | Medium DDS (8) | High DDS (9–10) | p-Value |
|---|---|---|---|---|---|
| (n = 43,493) | (n = 16,014) | (n = 9805) | (n = 17,674) | ||
| Newly developed hyperuricemia (%) | 1460 (3.36) | 647 (4.04) | 320 (3.26) | 493 (2.79) | <0.001 |
| Male (%) | 15,727 (36.16) | 6361 (39.72) | 3666 (37.39) | 5700 (32.25) | <0.001 |
| Age (year) | 58 (50–65) | 59 (52–65) | 58 (50–65) | 57 (48–64) | <0.001 |
| Age (group) | <0.001 | ||||
| 20–39 | 4892 (11.25) | 1343 (8.39) | 1096 (11.18) | 2453 (13.88) | |
| 40–49 | 5556 (12.77) | 1812 (11.32) | 1290 (13.16) | 2454 (13.88) | |
| 50–59 | 13,438 (30.90) | 5078 (31.71) | 3060 (31.21) | 5300 (29.99) | |
| 60–69 | 15,557 (35.77) | 6158 (38.45) | 3502 (35.71) | 5897 (33.37) | |
| 70–74 | 4050 (9.31) | 1623 (10.13) | 857 (8.74) | 1570 (8.88) | |
| Educational attainment (%) | <0.001 | ||||
| Primary school or below | 13,810 (31.75) | 6884 (42.99) | 3135 (31.97) | 3791 (21.45) | |
| Junior high school | 16,991 (39.07) | 5944 (37.12) | 3962 (40.41) | 7085 (40.09) | |
| Senior high school or above | 12,692 (29.18) | 3186 (19.89) | 2708 (27.62) | 6798 (38.46) | |
| Marriage situation (%) | |||||
| Unmarried | 830 (1.91) | 273 (1.70) | 193 (1.97) | 364 (2.06) | <0.001 |
| Married | 39,867 (91.66) | 14,579 (91.04) | 8952 (91.3) | 16,336 (92.43) | |
| Divorced and other | 2796 (6.43) | 1162 (7.26) | 660 (6.73) | 974 (5.51) | |
| Retirement (%) | 26,547 (61.04) | 10,004 (62.47) | 5881 (59.98) | 10,662 (60.33) | <0.001 |
| Smoking (%) | 8656 (19.90) | 3968 (24.78) | 1999 (20.39) | 2689 (15.21) | <0.001 |
| Alcohol consumption (%) | 4525 (10.40) | 2000 (12.49) | 1072 (10.93) | 1453 (8.22) | <0.001 |
| Tea intake (%) | 12,845 (29.53) | 4596 (28.70) | 2994 (30.54) | 5255 (29.73) | 0.005 |
| PA level (%) | <0.001 | ||||
| Low | 24,803 (57.03) | 10,504 (65.59) | 5611 (57.23) | 8688 (49.16) | |
| Moderate | 14,496 (33.33) | 4354 (27.19) | 3276 (33.41) | 6866 (38.85) | |
| High | 4194 (9.64) | 1156 (7.22) | 918 (9.36) | 2120 (11.99) | |
| Sleeping time (%) | <0.001 | ||||
| <5 h | 2067 (4.75) | 931 (5.82) | 434 (4.43) | 702 (3.97) | |
| 5–8 h | 33,501 (77.03) | 11,910 (74.37) | 7554 (77.04) | 14,037 (79.42) | |
| ≥8 h | 7925(18.22) | 3173 (19.81) | 1817 (18.53) | 2935 (16.61) | |
| BMI (kg/m2) | 23.95 ± 3.25 | 24.09 ± 3.29 | 23.98 ± 3.21 | 23.82 ± 3.22 | <0.001 |
| BMI (%) | <0.001 | ||||
| Underweight | 1453 (3.34) | 515 (3.22) | 324 (3.31) | 614 (3.47) | |
| Normal Weight | 21,577(49.61) | 7672 (47.91) | 4842 (49.38) | 9063 (51.28) | |
| Overweight | 15,948 (36.67) | 6023 (37.61) | 3623 (36.95) | 6302 (35.66) | |
| Obese | 4515 (10.38) | 1804 (11.26) | 1016 (10.36) | 1695 (9.59) | |
| Energy intake (kcal/d) | 1142.79 (909.34–1482.55) | 1008.75 (830.18–1292.32) | 1133.62 (912.03–1450.63) | 1280.47 (1022.72–1634.33) | <0.001 |
| History of chronic diseases (%) | |||||
| Hypertension | 20,715 (47.63) | 8053 (50.29) | 4708 (48.02) | 7954 (45.00) | <0.001 |
| CHD | 1972 (4.53) | 699 (4.36) | 468 (4.77) | 805 (4.55) | 0.306 |
| Diabetes | 6431 (14.79) | 2587 (16.15) | 1454 (14.83) | 2390 (13.52) | <0.001 |
| Dyslipidemia | 24,835 (57.10) | 9113 (56.91) | 5613 (57.25) | 10,109 (57.20) | 0.819 |
| COPD | 241 (0.55) | 87 (0.54) | 54 (0.55) | 100 (0.57) | 0.961 |
| Chronic bronchitis | 2969 (6.83) | 1240 (7.74) | 663 (6.76) | 1066 (6.03) | <0.001 |
| Asthma | 1033 (2.38) | 416 (2.60) | 250 (2.55) | 367 (2.08) | 0.003 |
| Per Unit Increase in DDS | DDS Group | p for Trend | |||
|---|---|---|---|---|---|
| Low (0–7) | Medium (8) | High (9–10) | |||
| Non-Adjusted Model | 0.94 (0.91–0.97) * | 1.00 | 0.85 (0.74–0.97) * | 0.76 (0.68–0.86) * | <0.001 |
| Adjusted Model 1 | 0.96 (0.93–0.99) * | 1.00 | 0.88 (0.77–1.01) | 0.82 (0.72–0.92) * | 0.001 |
| Adjusted Model 2 | 0.95 (0.92–0.99) * | 1.00 | 0.87 (0.76–0.99) * | 0.79 (0.70–0.90) * | <0.001 |
| Adjusted Model 3 | 0.95 (0.92–0.99) * | 1.00 | 0.87 (0.76–0.99) * | 0.80 (0.70–0.91) * | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; He, M.; Zhao, G.; Liu, X.; Liu, X.; Xu, H.; Cheng, Y.; Jiang, Y.; Peng, Q.; Shi, J.; et al. The Association of Dietary Diversity with Hyperuricemia among Community Inhabitants in Shanghai, China: A Prospective Research. Nutrients 2024, 16, 2968. https://doi.org/10.3390/nu16172968
Xu X, He M, Zhao G, Liu X, Liu X, Xu H, Cheng Y, Jiang Y, Peng Q, Shi J, et al. The Association of Dietary Diversity with Hyperuricemia among Community Inhabitants in Shanghai, China: A Prospective Research. Nutrients. 2024; 16(17):2968. https://doi.org/10.3390/nu16172968
Chicago/Turabian StyleXu, Xiaoli, Mengru He, Genming Zhao, Xing Liu, Xiaohua Liu, Huilin Xu, Yuping Cheng, Yonggen Jiang, Qian Peng, Jianhua Shi, and et al. 2024. "The Association of Dietary Diversity with Hyperuricemia among Community Inhabitants in Shanghai, China: A Prospective Research" Nutrients 16, no. 17: 2968. https://doi.org/10.3390/nu16172968
APA StyleXu, X., He, M., Zhao, G., Liu, X., Liu, X., Xu, H., Cheng, Y., Jiang, Y., Peng, Q., Shi, J., & He, D. (2024). The Association of Dietary Diversity with Hyperuricemia among Community Inhabitants in Shanghai, China: A Prospective Research. Nutrients, 16(17), 2968. https://doi.org/10.3390/nu16172968





