Abstract
Cancer, the second leading cause of death worldwide, demands the identification of modifiable risk factors to optimize its prevention. Diet has emerged as a pivotal focus in current research efforts. This literature review aims to enhance the ACS guidelines on diet and cancer by integrating the latest findings and addressing unresolved questions. The methodology involved an advanced PubMed search with specific filters relevant to the research topic. Topics covered include time-restricted diet, diet quality, acid load, counseling, exercise and diet combination, Mediterranean diet, vegetarian and pescetarian diets, weight loss, dairy consumption, coffee and tea, iron, carbohydrates, meat, fruits and vegetables, heavy metals, micronutrients, and phytoestrogens. The review highlights the benefits of the Mediterranean diet in reducing cancer risk. Adherence to overnight fasting or carbohydrate consumption may contribute to cancer prevention, but excessive fasting may harm patients’ quality of life. A vegetarian/pescetarian diet is associated with lower risks of general and colorectal cancer compared to a carnivorous diet. High heme and total iron intake are linked to increased lung cancer risk, while phytoestrogen intake is associated with reduced risk. Coffee and tea have a neutral impact on cancer risk. Finally, the roles of several preventive micronutrients and carcinogenic heavy metals are discussed.
Keywords:
diet; nutrition; cancer; risk; prevention; Mediterranean diet; dairy; vitamin D; diet quality; carbohydrates 1. Introduction
Traditionally, eating habits have been conceived as a matter of balancing caloric intake and the types of macronutrients consumed [1,2,3]. This vision is centered on the role of diet in maintaining body weight and meeting the body’s physiological needs. However, contemporary research has brought about a shift aiming to understand the influence of diet in health and disease. Indeed, diet is now widely recognized not only as a determinant of physical well-being but also as a modifiable risk factor linked to the prevalence and prognosis of various health conditions [4,5,6]. In fact, dietary patterns are associated with chronic diseases such as cardiovascular disorders [4], obesity [5], type 2 diabetes, and cancer [6]. Consequently, food choices are no longer considered isolated decisions in terms of calories or macronutrients, but rather as integral components of complex interactions between diet and general overall health.
Worldwide, cancer remains one of the leading causes of death, with an estimated 19.3 million new cases and nearly 10.0 million deaths only in 2020 [7]. Among the factors that contribute to the global burden of cancer, diet has been proposed as a modifiable risk factor with potential roles in cancer prevention, recurrence, and overall survival. Diet can also improve other health-related outcomes, such as survival and quality of life [8,9,10,11].
In recent decades, the association between diet and cancer has sparked the interest of investigators. In 2020, the American Cancer Society (ACS) published diet and physical activity guidelines for cancer prevention [12]. Summaries of the ACS’s recommendations are shown in Table 1 and Table 2.

Table 1.
Modified from ACS recommendations on breast, colorectal, lung and prostate cancer [12,13].

Table 2.
General health recommendations [12,13].
This review aims to deliver an update on these recommendations by summarizing novel evidence and highlighting areas that require further investigation. Some of the discussed topics include associations between cancer risk and time-restricted eating, consumption of dairy, vegetarian and pescatarian diets, coffee and tea, iron, meat, fruits and vegetables, acid charge, vitamin D, and phytoestrogens. We also include the potential role of professional advice, dietary patterns (such as the Mediterranean diet (MD)), and diet quality in cancer risk [13]. Diet quality levels are based on the application of specific indexes, outlined in Table 3, which have shown a moderate predictive capacity for the incidence of chronic diseases and other health determinants [14].

Table 3.
Assessment indexes that can be used to assess diet quality [15].
This literature review aims to update known knowledge with new research published and highlight areas that still require further study. The main points of discussion will be time-restricted feeding, dairy, vegetarian and pescetarian diets, coffee and tea, iron, meat, fruits and vegetables, acid charge, vitamin D, phytoestrogens, professional advice, dietary patterns (such as Mediterranean diet (MD)), and diet quality, which refers to both the quantity of nutrients and the absorption of specific nutrients from food [14]. These indexes have shown a moderate capacity to predict the incidence of chronic diseases and other health determinants, highlighting their relevance [15] to prostate, breast, lung, colon, and the overall risk of cancer.
2. Materials and Methods
2.1. Search Strategy
The initial phase of our study (visualized in Figure 1) began with the development of an effective search strategy to capture as much relevant literature as possible. This was achieved through a combination of general and advanced search query parameters: “‘cancer’ AND ‘nutrition’” and, alternatively, “‘cancer’ AND ‘diet’”, along with Boolean operators (’AND’, ’OR’, ’NOT’) to expand or limit the search as needed, and specific filters (such as the selection of randomized clinical trials and a publication period within 6 years). This was done on several academic databases, including, but not limited to, Google Scholar, PubMed, and ScienceDirect. Within this set of resources, priority was given to PubMed Advanced Search Results as our main source of information.
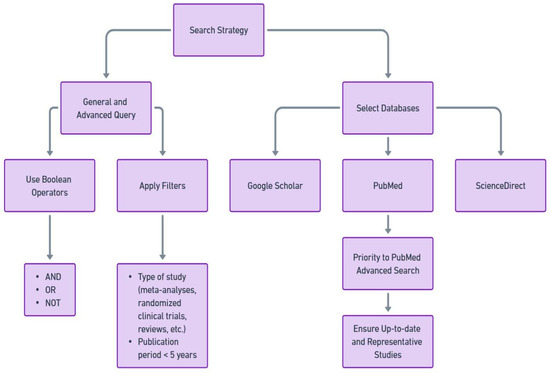
Figure 1.
Search strategy employed to find publications involved in our area of interest.
2.2. Screening and Selection
The search results underwent a screening and selection process. First, we selected an internationally renowned and relevant medical association’s guideline and used it as the basis for our review (the ACS guideline for diet and physical activity for cancer prevention and cancer survivors [12,13]). After this we found studies published shortly before and after our selected guideline. Only studies published between 2018 and 2024 were considered. These articles were revised to assess their relevance to the research question. Articles that were reviews of other studies were discarded. Exceptionally, a review was considered to discuss micronutrients in more depth, which is cited in the Section 3. The remaining articles were reviewed by our team to ensure they provided new insight into the selected review topic, or else were entirely new topics not previously covered. The ACS guideline references were reviewed with Google Scholar to ensure the literature found was not previously cited. Thus, studies that were not cited by any guideline are presented in the Section 3.
2.3. Synthesis and Reference Verification
Once we selected the final group of articles, we implemented two measures to ensure the reliability and robustness of our paper. First, as already mentioned, we verified the citations of each article using Google Scholar and its advanced search function, making sure that none of the selected articles had been previously cited in an earlier guideline. Subsequently, we proceeded to draft a synthesis of each article with the purpose of more effectively organizing the conclusions obtained from the selected research. With all of this, we had 24 original articles left that had not been referenced and therefore could be considered as new or novel.
3. Results
A total of 33 studies (summarized in Table 4) were included in our analysis. Most relevant conclusions for these studies are shown below.

Table 4.
Summary of studies on dietary interventions and cancer risk.
3.1. Time-Restricted Eating
A case-control study [16] suggested that prolonged nighttime fasting and an early breakfast could be associated with a lower risk of prostate cancer.
3.2. Mediterranean Diet
A case-control study conducted in Italy [17] with over 3000 patients found that higher adherence to the MD reduced the risk of breast cancer. A Polish study suggested that pro-healthy dietary patterns, including the Mediterranean pattern, may favor a lower risk of lung cancer in moderate smokers, although this was not confirmed in heavy smokers [18]. However, a dietary intervention involving 115 healthy individuals [19] found that the Mediterranean diet did not reduce trimethylamine N-oxide (TMAO) concentrations, a compound that has been identified as a risk factor for numerous cardiovascular diseases and colon cancer [20] The above shows a lack of knowledge of the mechanisms of how this diet works.
3.3. Dairy
Individual-level data from over 1 million women show that there is no clear association between the consumption of specific dairy products, dietary calcium, and total calcium, and the risk of overall breast cancer [21].
3.4. Vegetarians and Pescetarians
Vegetarians and pescetarians had a lower risk of overall cancer and colon cancer compared to carnivores [22].
3.5. Coffee and Tea
No evidence of an association between total coffee or tea consumption, with or without caffeine, and the risk of overall prostate cancer or cancer by stage, grade, or mortality in this large cohort exists [23].
3.6. Iron
Heme iron intake was moderately associated with the risk of lung cancer, and non-heme iron showed an inverse association that was corrected after adjusting for smoking history [24]. The genetic level of iron was not causally associated with the risk of lung cancer [25].
By contrast, a Uruguayan study [26] found that total iron, animal, heme, and non-heme animal iron were positively associated with lung cancer, while plant and non-heme fraction in total iron were inversely associated with lung cancer incidence. Furthermore, stratified analyses showed higher ORs for heavy smokers and heavy maté drinkers.
3.7. Carbohydrates
A Japanese study [27], included in the Korean guidelines for gastric cancer [28], concluded that low-carbohydrate diets are associated with a higher risk of overall incidence of colorectal and lung cancer but reduce the risk of gastric cancer.
3.8. Diet Quality
A study based on data from the Women’s Health Initiative Observational Study concluded that, although overall diet quality was not associated with lung cancer in general, a high-quality diet showed an inverse relationship with the incidence of squamous cell lung cancer [29]. These results were independent of the patient’s age and smoking habit. These findings are similar to another study conducted in a multiethnic group, which also indicated that high-quality diets are associated with a lower risk of lung cancer, especially the squamous cell subtype [30]. On the other hand, another study with Iranian adults found that a proinflammatory diet (using the Dietary Inflammatory Index) increased the likelihood of lung cancer in adults, mainly in men [31]. The Golestan Cohort Study concluded that higher DASH-Fung and HEI-2015, each respectively, were inversely associated with lung cancer risk [32].
A Finnish cohort [33] had a 33% decrease in postmenopausal breast cancer risk for those with the highest diet quality compared with those with the lowest quality according to the Nordic diet; (95% CI 0.48–1.01). While diet quality measured by mMEDI and mAHEI were not associated with postmenopausal breast cancer risk.
3.9. Meat
A large prospective cohort study conducted in the UK [34] with over 400,000 patients demonstrated that a high intake of red and processed meat was associated with an increased risk of lung cancer.
3.10. Fruits and Vegetables
The same large UK study demonstrated that the consumption of fruits, vegetables, breakfast cereals, and dietary fiber were inversely associated with the risk of lung cancer [34]. This study [35] supports cancer prevention strategies with a diet rich in vegetables, fruits, fish, and whole grains.
3.11. Acid Charge
The net endogenous acid production (NEAP) score, directly associated with meat consumption and inversely associated with vegetable consumption, was significantly related to an increased risk of all types of lung cancer, except for small cell cancers [36].
3.12. Phytoestrogens
The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) [37] found that the intake of phytoestrogens (PE) was associated with a reduced risk of lung cancer.
3.13. Importance of Professional Advice
The Oslo diet and the anti-smoking study [38] showed that 5-year counseling for a healthy lifestyle did not reduce overall long-term cancer risk. However, in the first 25 years, counseling reduced the risk of relevant cancer types in overweight/obese subjects and smokers.
3.14. Alternative Diet Advice
A large Canadian study [35] showed that high adherence to a prudent eating pattern (characterized by a high intake of vegetables, fruits, and lean meat from fish and other seafood) was associated with a lower risk of lung cancer.
In 2019, the EAT-Lancet commission proposed a universal dietary pattern that was analyzed in 2022 [39]. It was found that adherence to the EAT-Lancet diet was not associated with a significantly lower risk of cancer, and only among those with low alcohol consumption was a decrease in cancer risk observed.
The influence of the paleolithic diet, centered on vegetables, fruits, nuts, fish, and lean meat [40], revealed that women with a high adherence to this diet experienced a 17% lower risk of developing breast cancer. This benefit was sustained when analyzing the different subtypes of breast cancer, evidencing a consistent pattern of association.
3.15. Heavy Metals
A recent review shows an association between exposure to heavy metals and the risk of developing cancer. Elevated levels of metals such as copper, lead, and zinc are associated with an increased risk of breast cancer [41]. In particular, a marginal positive relationship has been identified between dietary intake of cadmium and breast cancer, although this relationship was not observed when exposure was assessed through urinary cadmium excretion [42]. In addition, it has been reported that edible mushrooms may be a significant source of toxic heavy metals, which could have a negative impact on long-term health [43].
In terms of dietary interventions, some studies have investigated strategies to mitigate heavy metal exposure through diet. For example, daily rice bran intake for six months in Nicaraguan infants did not result in significant differences in the levels of trace elements and heavy metals in serum and feces, suggesting controlled exposure [44]. In addition, the use of organic residue treatments in crop soils has been shown to be effective in reducing heavy metal levels in corn kernels, which could offer health benefits by reducing exposure to these contaminants [45].
3.16. Micronutrients
Low levels of zinc (Zn) in women with the BRCA1 mutation is weakly associated with an increased risk of ovarian cancer, although there was no similar association with breast cancer [46]. A high zinc/copper (Zn/Cu) ratio could reduce cancer risk in this same population [47], suggesting the importance of optimizing these minerals for cancer prevention in BRCA1 carriers [47].
In prostate cancer patients, low levels of selenium (Se) and Zn are associated with shorter 5-year survival, reinforcing the importance of maintaining adequate levels of both elements to improve prognosis [48]. In addition, high levels of Zn and Se are associated with longer survival in several types of cancer, whereas high levels of copper (Cu) may increase mortality in breast, prostate, lung, and laryngeal cancers [49].
As for vitamins, vitamin C can enhance the ability of NK cells to kill tumor cells [50,51]. Vitamin D, identified as key in the immune system because of its receptor on macrophages, dendritic cells, and activated T and B lymphocytes, plays an important role in immune modulation [50,52]. However, a Finnish study showed that vitamin D3 supplementation does not reduce the incidence of cancer in individuals with adequate levels of vitamin D3 [53].
On the other hand, vitamin E, a potent antioxidant, protects cell membranes and enhances immunity [50,54]. Retinoic acid (RA), derived from vitamin A, regulates cytokine release and could prevent cancer by influencing Th1/Th2 immune pathway differentiation [50,55].
4. Discussion
In line with the ACS recommendations [12] that emphasize the importance of a healthy diet in the prevention of disease, several studies highlight the protective role of the MD, along with abundant consumption of fruits and vegetables, against cancer [17,34,35]. Studies also point out the potentially harmful effects of excessive red meat consumption, which could be at least partly explained by a higher acid load, as evidenced by the high NEAP Score [36]. On the other hand, the fact that lower levels of TMAO do not correlate with MD adherence [19] warrants further investigation into other potential physiological mechanisms, beyond oxidative stress, that could explain its protective role.
Although some studies have attributed specific benefits to certain diets, such as the prudent eating pattern or the paleolithic diet [35,40], the scientific evidence that supports the cancer prevention properties of the MD is overwhelming. Therefore, adherence to this dietary pattern as a cancer preventive strategy should be encouraged.
Objective assessments of diet quality using validated indices, such as the HEI-2015, AHEI-2010, aMED Score, DII, and DASH Score are valuable tools for the prevention of chronic, cardiovascular, and neoplastic diseases. Therefore, in our opinion, these indices should be included in specialized clinical guidelines, allowing a personalized quantification of risk by health professionals according to patients’ dietary habits.
Regarding time-restricted eating strategies, we consider the evidence on its effects and potential risks as still uncertain; therefore, more studies are required to incorporate these strategies into specialized guidelines. In contrast, the evidence that supports the beneficial role of phytoestrogens in cancer prevention is more consistent, particularly after the multicenter cancer screening study by Wang et al. that involved >100,000 individuals [37], suggesting that the consumption of phytoestrogens deserves to be considered in specialized guidelines.
Although previous reports by the NCCN suggested that higher consumption of cows’ milk was associated with an increased BC risk [56], a more recent publication by You Wu [21] contradicts this report, suggesting that additional research is required to confirm or discard this association. Similarly, many studies have demonstrated a lack of significant effects in cancer prevention of certain foods like coffee and tea [10,23]. Considering this information, it would be useful to dedicate a formal section in specialized guidelines emphasizing the lack of effect on cancer risk of these foods. For example, a study by Hong Qin [25], based on a European database, determined a lack of association between genetic iron levels and lung cancer risk. Interestingly, this study was based on a specific population, which opens an important field of research regarding the influence of genetic factors in specific ethnic groups.
Another cancer preventive recommendation by the ACS is to avoid highly refined carbohydrates [12]. While certain carbohydrates may be associated with carcinogenesis, others are linked to protective effects, suggesting more research is needed to determine the specific amounts that balance cancer risk versus benefits.
The analyzed literature suggests that professional counseling should continue lifelong for the lifestyle of both patients and healthy subjects, and should not be limited to specific interventions.
As opposed to the MD and its benefits, exposure to heavy metals poses a significant cancer risk factor, particularly for BC. Therefore, periodical monitoring of copper, lead, and zinc levels, along with a strict diet avoiding the exposure to these metals, should be recommended to the general population, especially individuals at a higher for BC [41]. Although the association between BC and dietary intake of cadmium is marginal and dependent on assessment methods, these findings warrant further investigation into the potential underlying mechanism(s) [42]. Evidence also suggests that certain foods, such as mushrooms, can be a significant source of toxic heavy metals, highlighting the importance of careful food selection and dietary interventions [43]. In this regard, interventions such as daily rice bran did not show a significant impact on heavy metal levels in the body, suggesting that some dietary strategies may be insufficient to reduce exposure [44]. In contrast, agricultural practices that reduce the concentration of heavy metals in food, such as the use of organic residues in soils, offer a promising intervention that could reduce cancer risk by limiting dietary exposure to these contaminants [45]. Evidently, this is another topic that demands further research in the near future.
Regarding micronutrients, such as vitamins, minerals, and antioxidants, these play a vital role in the maintenance of cellular health and the reduction of oxidative stress. Although preliminary studies did not find an association between serum Zn levels and cancer risk in BRCA1 mutation carriers [46], the Zn/Cu ratio may be a biomarker in this subset of women [47]. Therefore, it is necessary to consider the optimization of serum levels of both Zn and Cu to have a more useful reference in clinical practice. In this context, studies demonstrate the impact of Se and Zn levels on the survival of prostate cancer patients [48]. Furthermore, a trial seeking to confirm these claims by systematically measuring serum levels of these microelements is currently underway.
The results of our literature search confirm and extend our current understanding of the relationship between vitamins and cancer risk, emphasizing their role as immune modulators that also protect our DNA. Within this context, the activating effect of vitamin C on NK cell function and its inhibitory effect on tumor cell migration suggests a potential role in the prevention of cancer spread and metastasis. Obviously, its potential clinical use in the future will require further research to optimize doses and conditions [50,51]. As for vitamin D, although its receptor has been identified on various immune cells, suggesting a broad immunomodulatory role, a Finnish study indicates that additional supplementation in individuals with adequate vitamin D levels does not reduce cancer incidence, suggesting the benefit is limited to deficient populations, which also highlights the importance of assessing basal levels prior to supplementation measures or recommendations [50,52,54].
Besides its potent antioxidant activity, vitamin E enhances T-cell function and inhibits COX-2, which may act as an immunomodulatory mechanism, stimulating antitumor immunity. Unfortunately, the evidence for this mechanism is mostly based on experimental studies, and the confirmation of potential clinical benefits in humans demands further investigation. In this regard, retinoic acid, an active form of vitamin A, has a dual effect in being both proinflammatory and anti-inflammatory, reflecting the complexity of immune modulation. Its ability to influence Th1/Th2 pathway differentiation and regulate cytokine production suggests a therapeutic potential in modulating specific immune responses in the context of cancer. However, its role in cancer prevention and treatment should be approached with caution, as the effects may vary according to the inflammatory context and the type of neoplasm. Overall, these findings point to more personalized vitamin supplementation, balancing benefits and risks for every individual.
5. Limitations and Future Direction of Work
Our literature search had several limitations. Firstly, selecting studies from the last six years may introduce temporal bias. Secondly, the search was conducted on PubMed, Google Scholar, and ScienceDirect, potentially omitting relevant studies published in other databases. Thirdly, our review was based on published literature, possibly omitting studies with negative or non-significant results. Finally, the multifactorial nature of the relationship between diet and cancer development makes it challenging to fully address the complexity of this association, leaving room for incomplete exploration of the interaction between various genetic and environmental factors.
6. Conclusions
Dietary habits are not only a key determinant of physical well-being, but also a modifiable risk factor directly associated with chronic diseases such as obesity, type 2 diabetes, hypertension, and cancer. Our findings support the ACS recommendations and highlight the need for deeper research in specific areas, such as time-restricted diets, structured physical activity programs, the impact of alternative diets (not MD) on cancer risk, and the effect of unprocessed carbohydrates.
Author Contributions
Conceptualization, T.M. and C.S.; data curation, Á.T., F.Q. and E.B.; investigation, Á.T., F.Q. and E.B.; methodology, Á.T., F.Q. and E.B.; project administration, T.M. and B.W.; resources, T.M.; supervision, T.M., F.A. and E.B.; visualization, Á.T., F.Q. and E.B.; writing—original draft, Á.T., F.Q. and E.B.; writing—review and editing, Á.T., F.Q. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACS | The American Cancer Society |
| MD | Mediterranean diet |
| MCC | Multicase control |
| CC | Case-control |
| ND | No data |
| ES | Epidemiological study |
| PS | Prospective study |
| Ma | Meta-analysis |
| OS | Observational study |
| RCT | Randomized controlled trial |
| PC | Prostate cancer |
| BC | Breast cancer |
| LC | Lung cancer |
| NC | No cancer |
| C | Controls |
| FU | Follow-up |
| CC | Colon cancer |
| OC | Overall cancer |
| I | Intervention |
| TMAO | Trimethylamine N-oxide |
| NEAP | Net endogenous acid production |
| PLCO | Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial phytoestrogens |
| Zn | Zinc |
| Zn/Cu | Zinc/copper ratio |
| Cu | Copper |
| Se | Selenium |
| DC | Dendritic cells |
| RA | Retinoic acid |
References
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Willett, W.C.; Koplan, J.P.; Nugent, R.; Dusenbury, C.; Puska, P.; Gaziano, T.A. Prevention of chronic disease by means of diet and lifestyle changes. In Disease Control Priorities in Developing Countries, 2nd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Feingold, K.R. The effect of diet on cardiovascular disease and lipid and lipoprotein levels. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Malik, V.S.; Hu, F.B. Obesity prevention. Disease control priorities. In Cardiovascular, Respiratory, and Related Disorders, 3rd ed.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017; p. 5. [Google Scholar]
- National Research Council (US) Committee on Diet, Nutrition, and Cancer. Diet, Nutrition, and Cancer; National Academies Press: Washington, DC, USA, 1982. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Diet Quality. 2018. Available online: https://www.iaea.org/topics/diet-quality#:~:text=Diet%20quality%20refers%20to%20both,activity%20and%20protection%20against%20infection (accessed on 10 November 2023).
- Kourlaba, G.; Panagiotakos, D.B. Dietary quality indices and human health: A review. Maturitas 2009, 62, 1–8. [Google Scholar] [CrossRef]
- Palomar-Cros, A.; Espinosa, A.; Straif, K.; Pérez-Gómez, B.; Papantoniou, K.; Gómez-Acebo, I.; Molina-Barceló, A.; Olmedo-Requena, R.; Alguacil, J.; Fernández-Tardón, G.; et al. The association of nighttime fasting duration and prostate cancer risk: Results from the multicase-control (MCC) study in Spain. Nutrients 2021, 13, 2662. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean diet and breast cancer risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Hawrysz, I.; Wadolowska, L.; Slowinska, M.A.; Czerwinska, A.; Golota, J.J. Adherence to prudent and Mediterranean dietary patterns is inversely associated with lung cancer in moderate but not heavy male Polish smokers: A case-control study. Nutrients 2020, 12, 3788. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.E.; Djuric, Z.; Angiletta, C.J.; Mitchell, C.M.; Baugh, M.E.; Davy, K.P.; Neilson, A.P. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct. 2019, 10, 2138–2147. [Google Scholar] [CrossRef]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301. [Google Scholar]
- Wu, Y.; Huang, R.; Wang, M.; Bernstein, L.; Bethea, T.N.; Chen, C.; Chen, Y.; Eliassen, A.H.; Freedman, N.D.; Gaudet, M.M.; et al. Dairy foods, calcium, and risk of breast cancer overall and for subtypes defined by estrogen receptor status: A pooled analysis of 21 cohort studies. Am. J. Clin. Nutr. 2021, 114, 450–461. [Google Scholar] [CrossRef]
- Parra-Soto, S.; Ahumada, D.; Petermann-Rocha, F.; Boonpoor, J.; Gallegos, J.L.; Anderson, J.; Sharp, L.; Malcomson, F.C.; Livingstone, K.M.; Mathers, J.C.; et al. Association of meat, vegetarian, pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer: Findings from the UK Biobank prospective cohort study and meta-analysis. BMC Med. 2022, 20, 79. [Google Scholar] [CrossRef]
- Sen, A.; Papadimitriou, N.; Lagiou, P.; Perez-Cornago, A.; Travis, R.C.; Key, T.J.; Murphy, N.; Gunter, M.; Freisling, H.; Tzoulaki, I.; et al. Coffee and tea consumption and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2019, 144, 240–250. [Google Scholar] [CrossRef]
- Ward, H.A.; Whitman, J.; Muller, D.C.; Johansson, M.; Jakszyn, P.; Weiderpass, E.; Palli, D.; Fanidi, A.; Vermeulen, R.; Tjønneland, A.; et al. Haem iron intake and risk of lung cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Eur. J. Clin. Nutr. 2019, 73, 1122–1132. [Google Scholar] [CrossRef]
- Qin, H.; Zeng, W.; Lou, Y. Mendelian randomization study indicates lack of causal associations between iron status and lung cancer. Medicine 2022, 101, e29879. [Google Scholar] [CrossRef]
- Ronco, A.L.; Lasalvia-Galante, E.; Calderon, J.M.; Espinosa, E. Dietary iron source and lung cancer risk: A case-control study in Uruguayan men. Multidiscip. Cancer Investig. 2019, 3, 20–36. [Google Scholar] [CrossRef]
- Cai, H.; Sobue, T.; Kitamura, T.; Ishihara, J.; Nanri, A.; Mizoue, T.; Iwasaki, M.; Yamaji, T.; Inoue, M.; Tsugane, S.; et al. Low- carbohydrate diet and risk of cancer incidence: The Japan Public Health Center-based prospective study. Cancer Sci. 2022, 113, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, I.H.; Kang, S.J.; Choi, M.; Kim, B.H.; Eom, B.W.; Kim, B.J.; Min, B.H.; Choi, C.I.; Shin, C.M.; et al. Korean practice guidelines for gastric cancer 2022: An evidence-based, multidisciplinary approach. J. Gastric Cancer 2023, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Myneni, A.A.; Giovino, G.A.; Millen, A.E.; LaMonte, M.J.; Wactawski-Wende, J.; Neuhouser, M.L.; Zhao, J.; Shikany, J.M.; Mu, L. Indices of diet quality and risk of lung cancer in the Women’s Health Initiative Observational Study. J. Nutr. 2021, 151, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Boushey, C.J.; Shvetsov, Y.B.; Wirth, M.D.; Shivappa, N.; Hébert, J.R.; Haiman, C.A.; Wilkens, L.R.; Le Marchand, L. Diet quality and risk of lung cancer in the multiethnic cohort study. Nutrients 2021, 13, 1614. [Google Scholar] [CrossRef]
- Sadeghi, A.; Parastouei, K.; Seifi, S.; Khosravi, A.; Salimi, B.; Zahedi, H.; Sadeghi, O.; Rasekhi, H.; Amini, M. Inflammatory potential of diet and odds of lung cancer: A case-control study. Nutr. Cancer 2022, 74, 2859–2867. [Google Scholar] [CrossRef]
- Wang, Q.; Hashemian, M.; Sepanlou, S.G.; Sharafkhah, M.; Poustchi, H.; Khoshnia, M.; Gharavi, A.; Pourshams, A.; Malekshah, A.F.; Kamangar, F.; et al. Dietary quality using four dietary indices and lung cancer risk: The Golestan Cohort Study (GCS). Cancer Causes Control 2021, 32, 493–503. [Google Scholar] [CrossRef]
- Männistö, S.; Harald, K.; Härkänen, T.; Maukonen, M.; Eriksson, J.G.; Heikkinen, S.; Jousilahti, P.; Kaartinen, N.E.; Kanerva, N.; Knekt, P.; et al. Association between overall diet quality and postmenopausal breast cancer risk in five Finnish cohort studies. Sci. Rep. 2021, 11, 16718. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, C.; Ji, M.; Fan, J.; Xie, J.; Huang, Y.; Jiang, X.; Xu, J.; Yin, R.; Du, L.; et al. Diet and risk of incident lung cancer: A large prospective cohort study in UK biobank. Am. J. Clin. Nutr. 2021, 114, 2043–2051. [Google Scholar] [CrossRef]
- Willemsen, R.F.; McNeil, J.; Heer, E.; Johnson, S.T.; Friedenreich, C.M.; Brenner, D.R. Dietary patterns with combined and site-specific cancer incidence in Alberta’s Tomorrow Project cohort. Eur. J. Clin. Nutr. 2022, 76, 360–372. [Google Scholar] [CrossRef]
- Ronco, A.L.; Martínez-López, W.; Calderón, J.M.; Golomar, W. Dietary acid load and lung cancer risk: A case-control study in men. Cancer Treat. Res. Commun. 2021, 28, 100382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ru, M.; Zhang, Y.; Kurbanova, T.; Boffetta, P. Dietary phytoestrogen intake and lung cancer risk: An analysis of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Carcinogenesis 2021, 42, 1250–1259. [Google Scholar] [CrossRef]
- Botteri, E.; de Lange, T.; Tonstad, S.; Berstad, P. Exploring the effect of a lifestyle intervention on cancer risk: 43-year follow-up of the randomized Oslo diet and antismoking study. J. Intern. Med. 2018, 284, 282–291. [Google Scholar] [CrossRef]
- Berthy, F.; Brunin, J.; Allès, B.; Fezeu, L.K.; Touvier, M.; Hercberg, S.; Galan, P.; Pointereau, P.; Lairon, D.; Baudry, J.; et al. Association between adherence to the EAT-Lancet diet and risk of cancer and cardiovascular outcomes in the prospective NutriNet-Santé cohort. Am. J. Clin. Nutr. 2022, 116, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Mahamat-Saleh, Y.; Hajji-Louati, M.; Correia, E.; Oulhote, Y.; Boutron-Ruault, M.C.; Laouali, N. Palaeolithic diet score and risk of breast cancer among postmenopausal women overall and by hormone receptor and histologic subtypes. Eur. J. Clin. Nutr. 2023, 77, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Liu, C.; Luo, Y.; Chen, J.; Fu, Y.; Xu, Y.; Wu, H.; Li, X.; Wang, H. Relationships Between Biological Heavy Metals and Breast Cancer: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 838762. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Naska, A.; Kasdagli, M.; Malavolti, M.; Orsini, N.; Vinceti, M. Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ. Int. 2020, 142, 105879. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, M.; Sobhi, H.; Esrafili, A.; Farzadkia, M.; Yeganeh, M. Heavy metals content in edible mushrooms: A systematic review, meta-analysis and health risk assessment. Trends Food Sci. Technol. 2021, 109, 527–535. [Google Scholar] [CrossRef]
- Zambrana, L.; Weber, A.; Borresen, E.; Zarei, I.; Perez, J.; Pérez, C.; Rodriguez, I.; Becker-Dreps, S.; Yuan, L.; Vilchez, S.; et al. Daily Rice Bran Consumption for 6 Months Influences Serum Glucagon-Like Peptide 2 and Metabolite Profiles without Differences in Trace Elements and Heavy Metals in Weaning Nicaraguan Infants at 12 Months of Age. Curr. Dev. Nutr. 2021, 5, nzab101. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Antoniadis, V.; Wang, K.; Huang, Y.; Tian, H. Assessment of heavy metal(loid)s contamination risk and grain nutritional quality in organic waste-amended soil. J. Hazard. Mater. 2020, 399, 123095. [Google Scholar] [CrossRef]
- Matuszczak, M.; Kiljańczyk, A.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryśkiewicz, M.; Sun, P.; Cheriyan, A.; Cybulski, C.; et al. Zinc and Its Antioxidant Properties: The Potential Use of Blood Zinc Levels as a Marker of Cancer Risk in BRCA1 Mutation Carriers. Antioxidants 2024, 13, 609. [Google Scholar] [CrossRef]
- Matuszczak, M.; Kiljańczyk, A.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryśkiewicz, M.; Cybulski, C.; Dębniak, T.; Gronwald, J.; et al. Antioxidant Properties of Zinc and Copper—Blood Zinc-to Copper-Ratio as a Marker of Cancer Risk BRCA1 Mutation Carriers. Antioxidants 2024, 13, 841. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, S.; Marciniak, W.; Derkacz, R.; Matuszczak, M.; Kiljańczyk, A.; Baszuk, P.; Bryśkiewicz, M.; Sikorski, A.; Gronwald, J.; Słojewski, M.; et al. Correlation between Selenium and Zinc Levels and Survival among Prostate Cancer Patients. Nutrients 2024, 16, 527. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, J.; Lener, S.; Marciniak, W.; Pietrzak, S.; Derkacz, R.; Cybulski, C.; Gronwald, J.; Dębniak, T.; Jakubowska, A.; Huzarski, T.; et al. Elementos esenciales del suero y supervivencia tras el diagnóstico de cáncer. Nutrientes 2023, 15, 2611. [Google Scholar] [CrossRef]
- Mitra, S.; Paul, S.; Roy, S.; Sutradhar, H.; Bin Emran, T.; Nainu, F.; Khandaker, M.U.; Almalki, M.; Wilairatana, P.; Mubarak, M.S. Exploring the Immune-Boosting Functions of Vitamins and Minerals as Nutritional Food Bioactive Compounds: A Comprehensive Review. Molecules 2022, 27, 555. [Google Scholar] [CrossRef] [PubMed]
- Toliopoulos, I.K.; Simos, Y.V.; Daskalou, T.A.; Verginadis, I.I.; Evangelou, A.M.; Karkabounas, S.C. Inhibition of platelet aggregation and immunomodulation of NK lymphocytes by administration of ascorbic acid. Indian J. Exp. Biol. 2011, 49, 904–908. [Google Scholar]
- Bikle, D.D. Vitamin D and immune function: Understanding common pathways. Curr. Osteoporos. Rep. 2009, 7, 58–63. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Nurmi, T.; Aro, A.; Bertone-Johnson, E.R.; Hyppönen, E.; Kröger, H.; Lamberg-Allardt, C.; Manson, J.E.; Mursu, J.; Mäntyselkä, P.; et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish Vitamin D Trial: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 1300–1310. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Roy, S.; Awasthi, A. Vitamin A and the Immune System. In Nutrition and Immunity; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Yang, B.; McCullough, M.L.; Gapstur, S.M.; Jacobs, E.J.; Bostick, R.M.; Fedirko, V.; Flanders, W.D.; Campbell, P.T. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: The Cancer Prevention Study-II Nutrition Cohort. J. Clin. Oncol. 2014, 32, 2335–2343. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).