NMDA Suppresses Pancreatic ABCA1 Expression through the MEK/ERK/LXR Pathway in Pancreatic Beta Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Pancreatic Islet Culture
2.3. Western Blot Analysis
2.4. Real-Time Polymerase Chain Reaction (PCR)
2.5. Luciferase Reporter Gene Assay
2.6. Glucose-Stimulated Insulin Secretion
2.7. Cholesterol and Triglyceride Content Assay
2.8. Oil Red O Stain
2.9. Statistical Analysis
3. Results
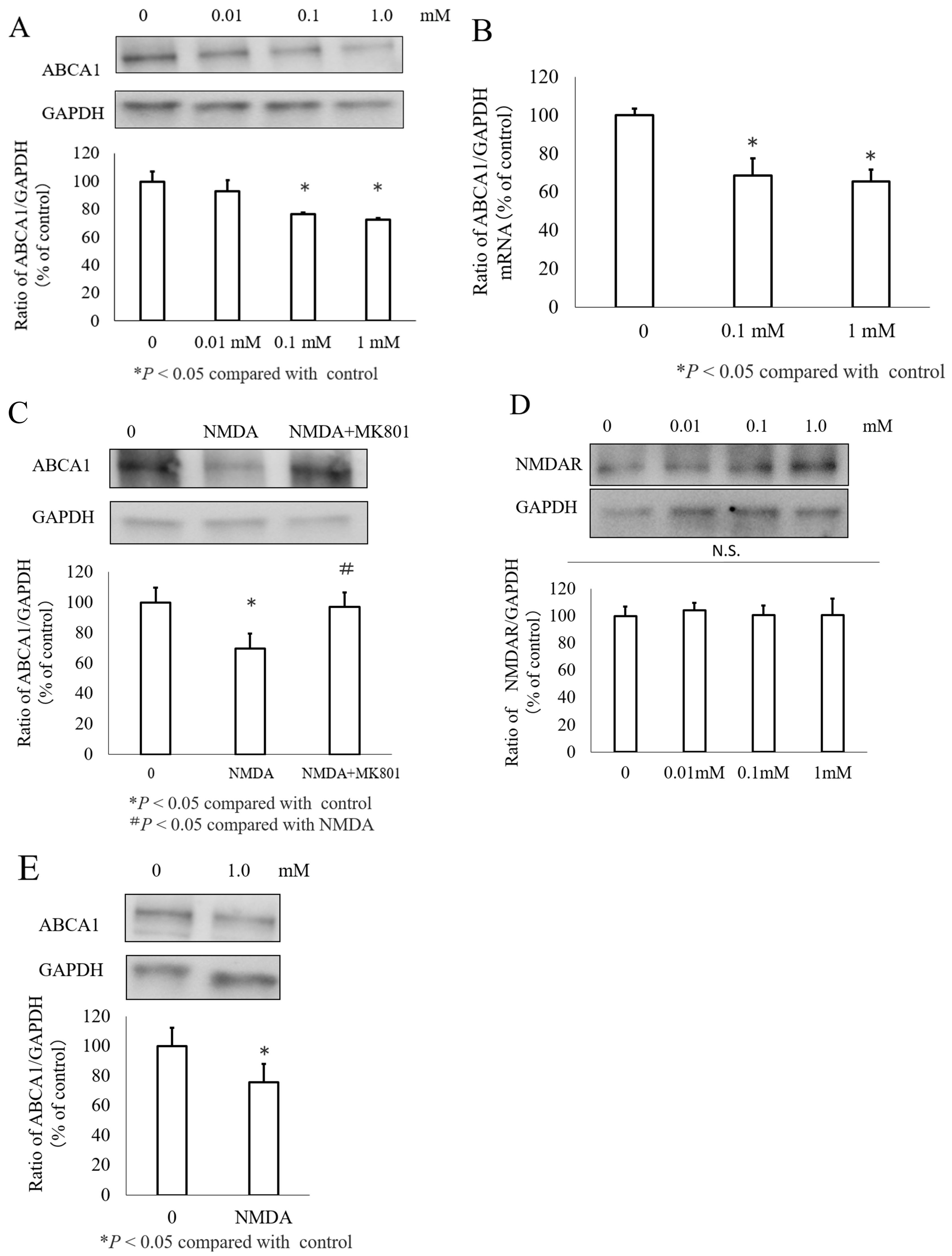
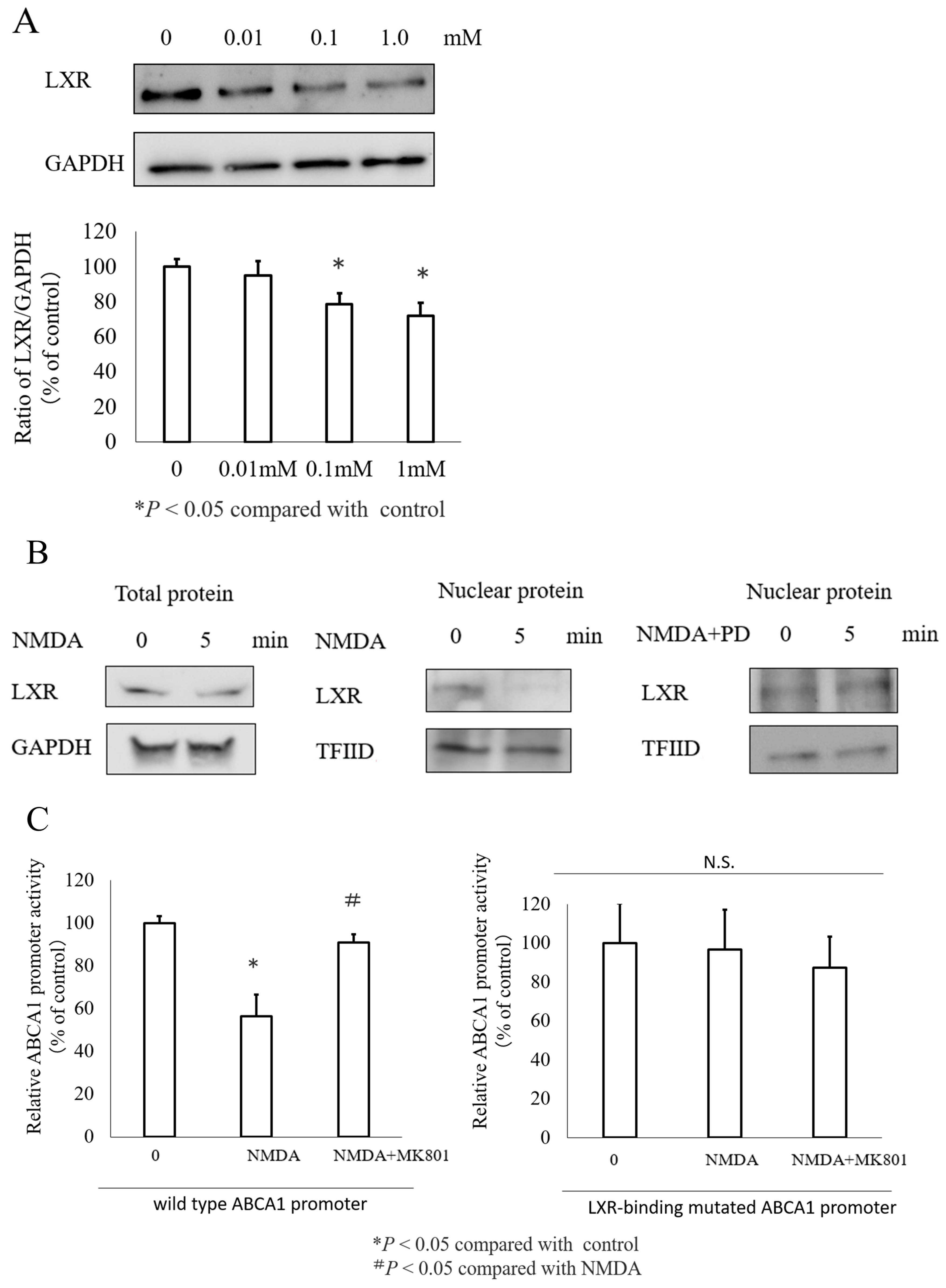
3.1. NMDA Decreased ABCA1 Expression in INS-1 Cells and Islets Cells
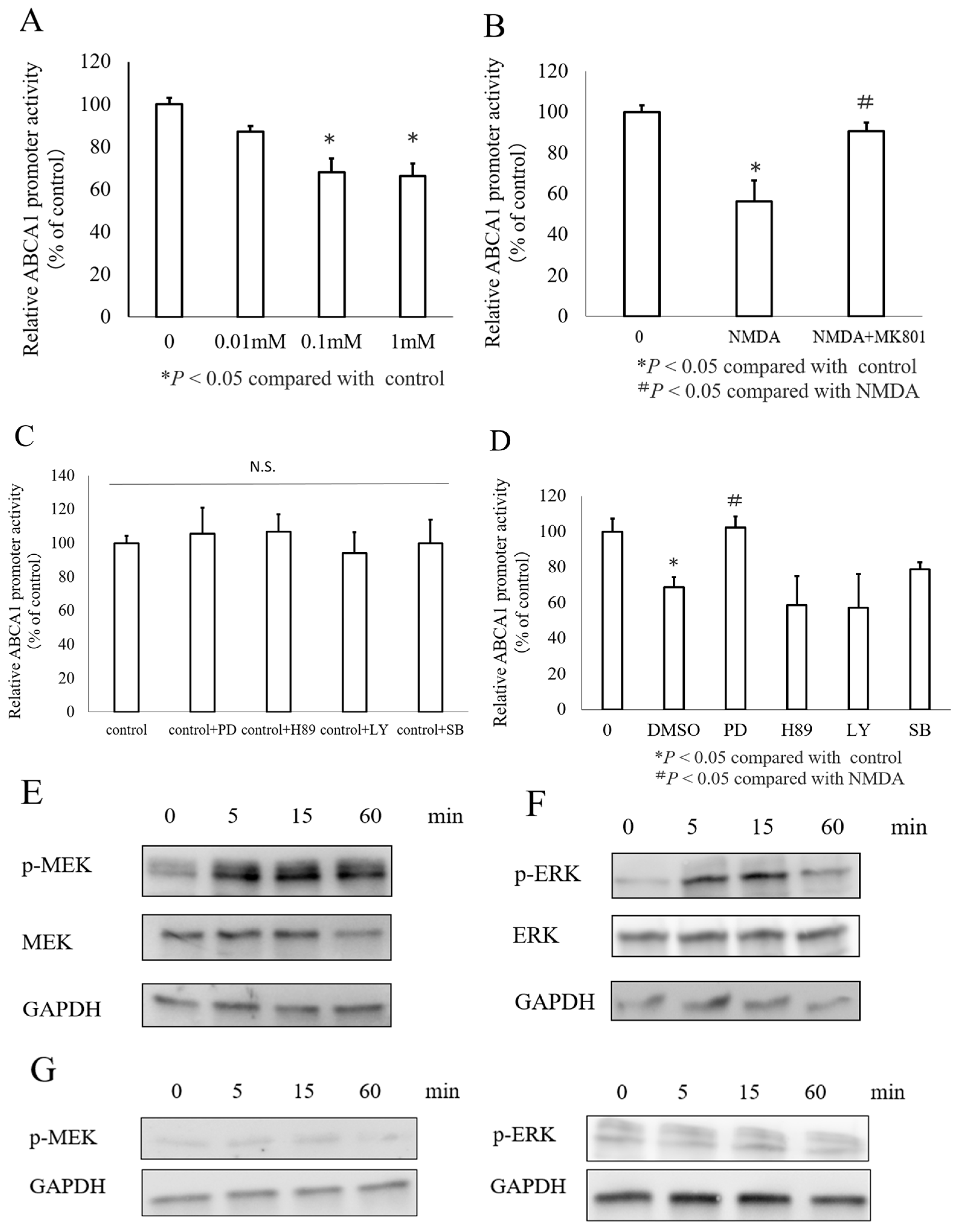
3.2. NMDA Decreased ABCA1 Transcription in INS-1 Cells via the MEK/ERK Signaling Pathway
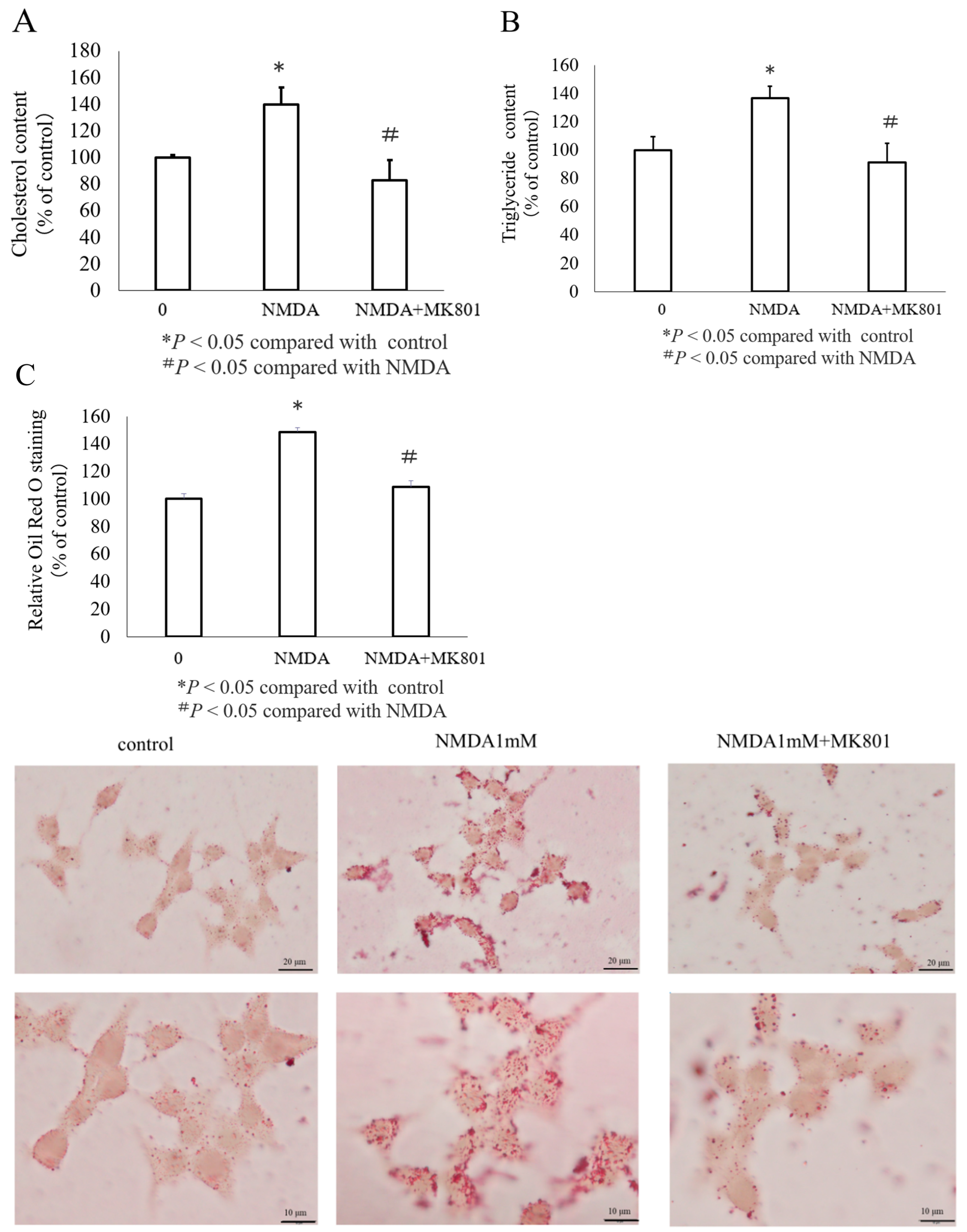
3.3. Effects of NMDA on Lipid Accumulation in INS-1 Cells
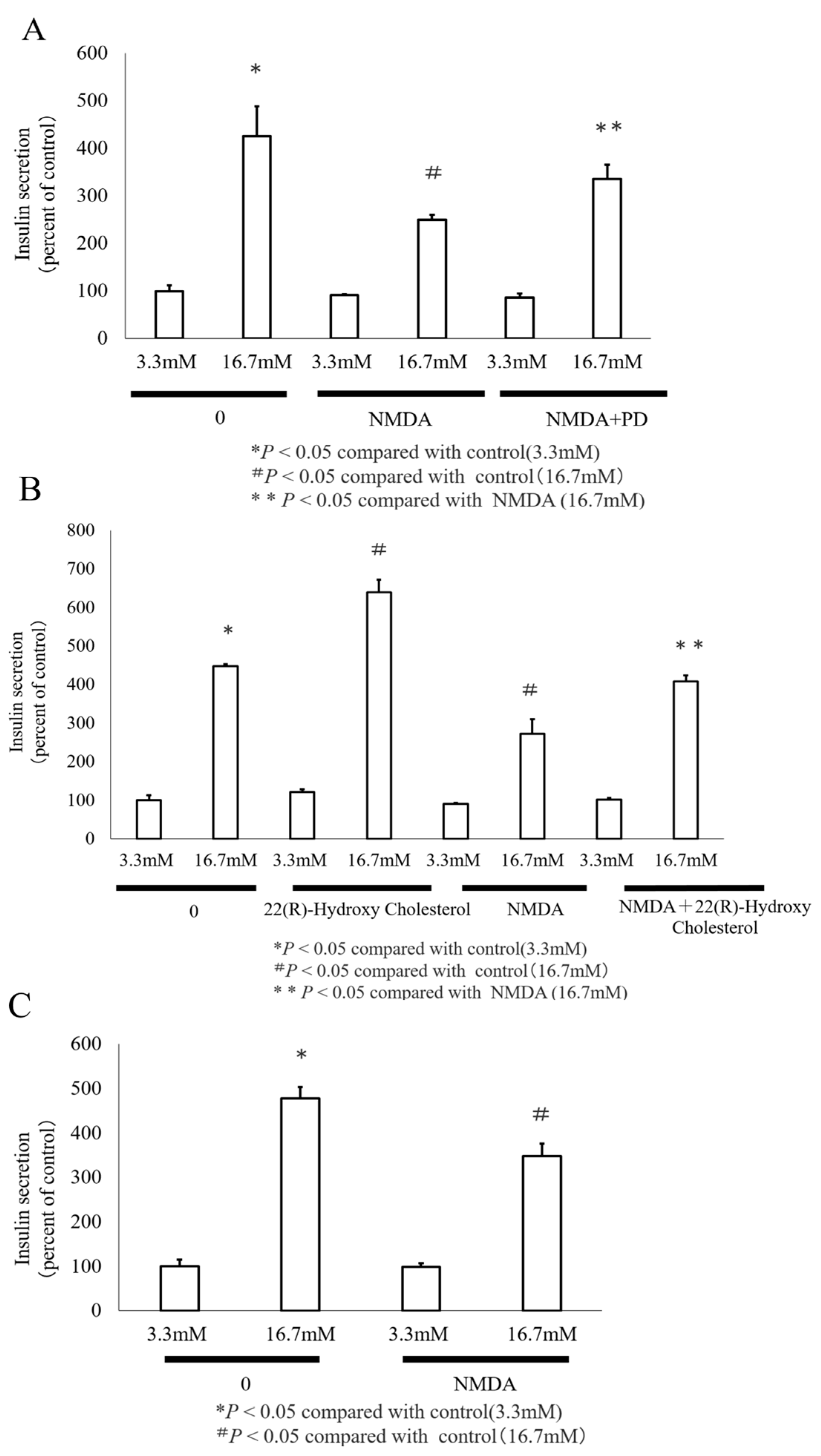
3.4. GSIS Is Reduced by NMDA
3.5. The Transcription Factor LXR Mediated NMDA Induced ABCA1 Inhibition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.P.; Grill, V. Long Term Exposure to Fatty Acids and Ketones Inhibits B-Cell Functions in Human Pancreatic Islets of Langerhans. J. Clin. Endocrinol. Metab. 1995, 80, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Singaraja, R.R.; Brunham, L.R.; Visscher, H.; Kastelein, J.J.P.; Hayden, M.R. Efflux and Atherosclerosis: The Clinical and Biochemical Impact of Variations in the ABCA1 Gene. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1322–1332. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Timmins, J.M.; Reuwer, A.Q.; Vasanji, Z.; Marsh, B.J.; Rodrigues, B.; Johnson, J.D.; Parks, J.S.; et al. Beta-Cell ABCA1 Influences Insulin Secretion, Glucose Homeostasis and Response to Thiazolidinedione Treatment. Nat. Med. 2007, 13, 340–347. [Google Scholar] [CrossRef]
- Lyu, J.; Imachi, H.; Fukunaga, K.; Sato, S.; Ibata, T.; Kobayashi, T.; Dong, T.; Yoshimoto, T.; Yonezaki, K.; Nagata, H.; et al. Angiotensin II Induces Cholesterol Accumulation and Impairs Insulin Secretion by Regulating ABCA1 in Beta Cells. J. Lipid Res. 2018, 59, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Imachi, H.; Lyu, J.; Miyai, Y.; Fukunaga, K.; Dong, T.; Ibata, T.; Kobayashi, T.; Yoshimoto, T.; Kikuchi, F.; et al. Effect of TNF-α on the Expression of ABCA1 in Pancreatic β-Cells. J. Mol. Endocrinol. 2018, 61, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Fukunaga, K.; Imachi, H.; Sato, S.; Kobayashi, T.; Saheki, T.; Ibata, T.; Yoshimura, T.; Iwama, H.; Murao, K. Oxidized LDL Downregulates ABCA1 Expression via MEK/ERK/LXR Pathway in INS-1 Cells. Nutrients 2021, 13, 3017. [Google Scholar] [CrossRef]
- Li, J.; Murao, K.; Imachi, H.; Masugata, H.; Iwama, H.; Tada, S.; Zhang, G.-X.; Kobayashi, R.; Ishida, T.; Tokumitsu, H. Exendin-4 Regulates Pancreatic ABCA1 Transcription via CaMKK/CaMKIV Pathway. J. Cell Mol. Med. 2010, 14, 1083–1087. [Google Scholar] [CrossRef][Green Version]
- Lyu, J.; Imachi, H.; Fukunaga, K.; Sato, S.; Kobayashi, T.; Dong, T.; Saheki, T.; Matsumoto, M.; Iwama, H.; Zhang, H.; et al. Role of ATP-Binding Cassette Transporter A1 in Suppressing Lipid Accumulation by Glucagon-like Peptide-1 Agonist in Hepatocytes. Mol. Metab. 2020, 34, 16–26. [Google Scholar] [CrossRef]
- Welters, A.; Lammert, E.; Mayatepek, E.; Meissner, T. Need for Better Diabetes Treatment: The Therapeutic Potential of NMDA Receptor Antagonists. Klin. Padiatr. 2017, 229, 14–20. [Google Scholar] [CrossRef]
- Moriguchi, S.; Ishizuka, T.; Yabuki, Y.; Shioda, N.; Sasaki, Y.; Tagashira, H.; Yawo, H.; Yeh, J.Z.; Sakagami, H.; Narahashi, T.; et al. Blockade of the KATP Channel Kir6.2 by Memantine Represents a Novel Mechanism Relevant to Alzheimer’s Disease Therapy. Mol. Psychiatry 2018, 23, 211–221. [Google Scholar] [CrossRef]
- Huang, X.-T.; Li, C.; Peng, X.-P.; Guo, J.; Yue, S.-J.; Liu, W.; Zhao, F.-Y.; Han, J.-Z.; Huang, Y.-H.; Yang-Li; et al. An Excessive Increase in Glutamate Contributes to Glucose-Toxicity in β-Cells via Activation of Pancreatic NMDA Receptors in Rodent Diabetes. Sci. Rep. 2017, 7, 44120. [Google Scholar] [CrossRef] [PubMed]
- Lupton, M.K.; Proitsi, P.; Lin, K.; Hamilton, G.; Daniilidou, M.; Tsolaki, M.; Powell, J.F. The Role of ABCA1 Gene Sequence Variants on Risk of Alzheimer’s Disease. J. Alzheimers Dis. 2014, 38, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-B.; Murray, K.D.; Jones, E.G. Switching of NMDA Receptor 2A and 2B Subunits at Thalamic and Cortical Synapses during Early Postnatal Development. J. Neurosci. 2004, 24, 8885–8895. [Google Scholar] [CrossRef]
- Nagao, S.; Murao, K.; Imachi, H.; Cao, W.-M.; Yu, X.; Li, J.; Matsumoto, K.; Nishiuchi, T.; Ahmed, R.A.M.; Wong, N.C.W.; et al. Platelet Derived Growth Factor Regulates ABCA1 Expression in Vascular Smooth Muscle Cells. FEBS Lett. 2006, 580, 4371–4376. [Google Scholar] [CrossRef]
- Costet, P.; Luo, Y.; Wang, N.; Tall, A.R. Sterol-Dependent Transactivation of the ABC1 Promoter by the Liver X Receptor/Retinoid X Receptor. J. Biol. Chem. 2000, 275, 28240–28245. [Google Scholar] [CrossRef]
- Nishiuchi, Y.; Murao, K.; Imachi, H.; Nishiuchi, T.; Iwama, H.; Ishida, T. Transcriptional Factor Prolactin Regulatory Element-Binding Protein-Mediated Gene Transcription of ABCA1 via 3′,5′-Cyclic Adenosine-5′-Monophosphate. Atherosclerosis 2010, 212, 418–425. [Google Scholar] [CrossRef]
- Morel, E.; Demignot, S.; Chateau, D.; Chambaz, J.; Rousset, M.; Delers, F. Lipid-Dependent Bidirectional Traffic of Apolipoprotein B in Polarized Enterocytes. Mol. Biol. Cell 2004, 15, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, N.; Kuromi, H.; Gonoi, T.; Okamoto, Y.; Ishida, H.; Seino, Y.; Kaneko, T.; Iwanaga, T.; Seino, S. Expression and Role of Ionotropic Glutamate Receptors in Pancreatic Islet Cells. FASEB J. 1995, 9, 686–691. [Google Scholar] [CrossRef]
- Gonoi, T.; Mizuno, N.; Inagaki, N.; Kuromi, H.; Seino, Y.; Miyazaki, J.; Seino, S. Functional Neuronal Ionotropic Glutamate Receptors Are Expressed in the Non-Neuronal Cell Line MIN6. J. Biol. Chem. 1994, 269, 16989–16992. [Google Scholar] [CrossRef]
- Marquard, J.; Otter, S.; Welters, A.; Stirban, A.; Fischer, A.; Eglinger, J.; Herebian, D.; Kletke, O.; Klemen, M.S.; Stožer, A.; et al. Characterization of Pancreatic NMDA Receptors as Possible Drug Targets for Diabetes Treatment. Nat. Med. 2015, 21, 363–372. [Google Scholar] [CrossRef]
- Huang, X.-T.; Yang, J.-X.; Wang, Z.; Zhang, C.-Y.; Luo, Z.-Q.; Liu, W.; Tang, S.-Y. Activation of N-Methyl-D-Aspartate Receptor Regulates Insulin Sensitivity and Lipid Metabolism. Theranostics 2021, 11, 2247–2262. [Google Scholar] [CrossRef] [PubMed]
- Endoplasmic Reticulum Stress Contributes to NMDA-Induced Pancreatic β-Cell Dysfunction in a CHOP-Dependent Manner—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31260687/ (accessed on 8 May 2023).
- Wu, Y.; Fortin, D.A.; Cochrane, V.A.; Chen, P.-C.; Shyng, S.-L. NMDA Receptors Mediate Leptin Signaling and Regulate Potassium Channel Trafficking in Pancreatic β-Cells. J. Biol. Chem. 2017, 292, 15512–15524. [Google Scholar] [CrossRef] [PubMed]
- Maltais-Payette, I.; Allam-Ndoul, B.; Pérusse, L.; Vohl, M.-C.; Tchernof, A. Circulating Glutamate Level as a Potential Biomarker for Abdominal Obesity and Metabolic Risk. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-T.; Yue, S.-J.; Li, C.; Huang, Y.-H.; Cheng, Q.-M.; Li, X.-H.; Hao, C.-X.; Wang, L.-Z.; Xu, J.-P.; Ji, M.; et al. A Sustained Activation of Pancreatic NMDARs Is a Novel Factor of β-Cell Apoptosis and Dysfunction. Endocrinology 2017, 158, 3900–3913. [Google Scholar] [CrossRef]
- Takahashi, H.; Yokoi, N.; Seino, S. Glutamate as Intracellular and Extracellular Signals in Pancreatic Islet Functions. Proc. Jpn. Acad. Ser. B 2019, 95, 246–260. [Google Scholar] [CrossRef]
- Soumian, S.; Albrecht, C.; Davies, A.H.; Gibbs, R.G.J. ABCA1 and Atherosclerosis. Vasc. Med. 2005, 10, 109–119. [Google Scholar] [CrossRef]
- Cheng, H.; Cheng, Q.; Bao, X.; Luo, Y.; Zhou, Y.; Li, Y.; Hua, Q.; Liu, W.; Tang, S.; Feng, D.; et al. Over-Activation of NMDA Receptors Promotes ABCA1 Degradation and Foam Cell Formation. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158778. [Google Scholar] [CrossRef]
- Crews, C.M.; Alessandrini, A.; Erikson, R.L. The Primary Structure of MEK, a Protein Kinase That Phosphorylates the ERK Gene Product. Science 1992, 258, 478–480. [Google Scholar] [CrossRef]
- Mulay, V.; Wood, P.; Manetsch, M.; Darabi, M.; Cairns, R.; Hoque, M.; Chan, K.C.; Reverter, M.; Alvarez-Guaita, A.; Rye, K.-A.; et al. Inhibition of Mitogen-Activated Protein Kinase Erk1/2 Promotes Protein Degradation of ATP Binding Cassette Transporters A1 and G1 in CHO and HuH7 Cells. PLoS ONE 2013, 8, e62667. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K. Liver X Receptor in Cholesterol Metabolism. J. Endocrinol. 2010, 204, 233–240. [Google Scholar] [CrossRef]
- Green, C.D.; Jump, D.B.; Olson, L.K. Elevated Insulin Secretion from Liver X Receptor-Activated Pancreatic Beta-Cells Involves Increased de Novo Lipid Synthesis and Triacylglyceride Turnover. Endocrinology 2009, 150, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Kawana, Y.; Imai, J.; Morizawa, Y.M.; Ikoma, Y.; Kohata, M.; Komamura, H.; Sato, T.; Izumi, T.; Yamamoto, J.; Endo, A.; et al. Optogenetic Stimulation of Vagal Nerves for Enhanced Glucose-Stimulated Insulin Secretion and β Cell Proliferation. Nat. Biomed. Eng. 2024, 8, 808–822. [Google Scholar] [CrossRef] [PubMed]
- EASD 2023—Glucose-Dependent Insulin Production and Insulin-Independence in Patients with Type 1 Diabetes Infused with Stem Cell-Derived, Fully Differentiated Islet Cells (VX-880). Available online: https://cattendee.abstractsonline.com/meeting/10899/presentation/1140 (accessed on 16 August 2024).
- Marquard, J.; Stirban, A.; Schliess, F.; Sievers, F.; Welters, A.; Otter, S.; Fischer, A.; Wnendt, S.; Meissner, T.; Heise, T.; et al. Effects of Dextromethorphan as Add-on to Sitagliptin on Blood Glucose and Serum Insulin Concentrations in Individuals with Type 2 Diabetes Mellitus: A Randomized, Placebo-Controlled, Double-Blinded, Multiple Crossover, Single-Dose Clinical Trial. Diabetes Obes. Metab. 2016, 18, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Journey, J.D.; Agrawal, S.; Stern, E. Dextromethorphan Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saheki, T.; Imachi, H.; Fukunaga, K.; Sato, S.; Kobayashi, T.; Yoshimura, T.; Saheki, N.; Murao, K. NMDA Suppresses Pancreatic ABCA1 Expression through the MEK/ERK/LXR Pathway in Pancreatic Beta Cells. Nutrients 2024, 16, 2865. https://doi.org/10.3390/nu16172865
Saheki T, Imachi H, Fukunaga K, Sato S, Kobayashi T, Yoshimura T, Saheki N, Murao K. NMDA Suppresses Pancreatic ABCA1 Expression through the MEK/ERK/LXR Pathway in Pancreatic Beta Cells. Nutrients. 2024; 16(17):2865. https://doi.org/10.3390/nu16172865
Chicago/Turabian StyleSaheki, Takanobu, Hitomi Imachi, Kensaku Fukunaga, Seisuke Sato, Toshihiro Kobayashi, Takafumi Yoshimura, Nao Saheki, and Koji Murao. 2024. "NMDA Suppresses Pancreatic ABCA1 Expression through the MEK/ERK/LXR Pathway in Pancreatic Beta Cells" Nutrients 16, no. 17: 2865. https://doi.org/10.3390/nu16172865
APA StyleSaheki, T., Imachi, H., Fukunaga, K., Sato, S., Kobayashi, T., Yoshimura, T., Saheki, N., & Murao, K. (2024). NMDA Suppresses Pancreatic ABCA1 Expression through the MEK/ERK/LXR Pathway in Pancreatic Beta Cells. Nutrients, 16(17), 2865. https://doi.org/10.3390/nu16172865





