Prospecting of the Antioxidant Activity from Extracts Obtained from Chañar (Geoffroea decorticans) Seeds Evaluated In Vitro and In Vivo Using the Tenebrio molitor Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material
2.3. Extraction of Ethanolic and Aqueous Extracts from Geoffroea decorticans Seeds
2.4. Phytochemical Characterization
2.4.1. Total Phenolic Compound Determination
2.4.2. Quantification of Total Flavonoid Content
2.4.3. Identification of Phenolic Compounds by HPLC Followed by GC-MS/SIM
2.5. Evaluation of In Vitro Antioxidant Activity
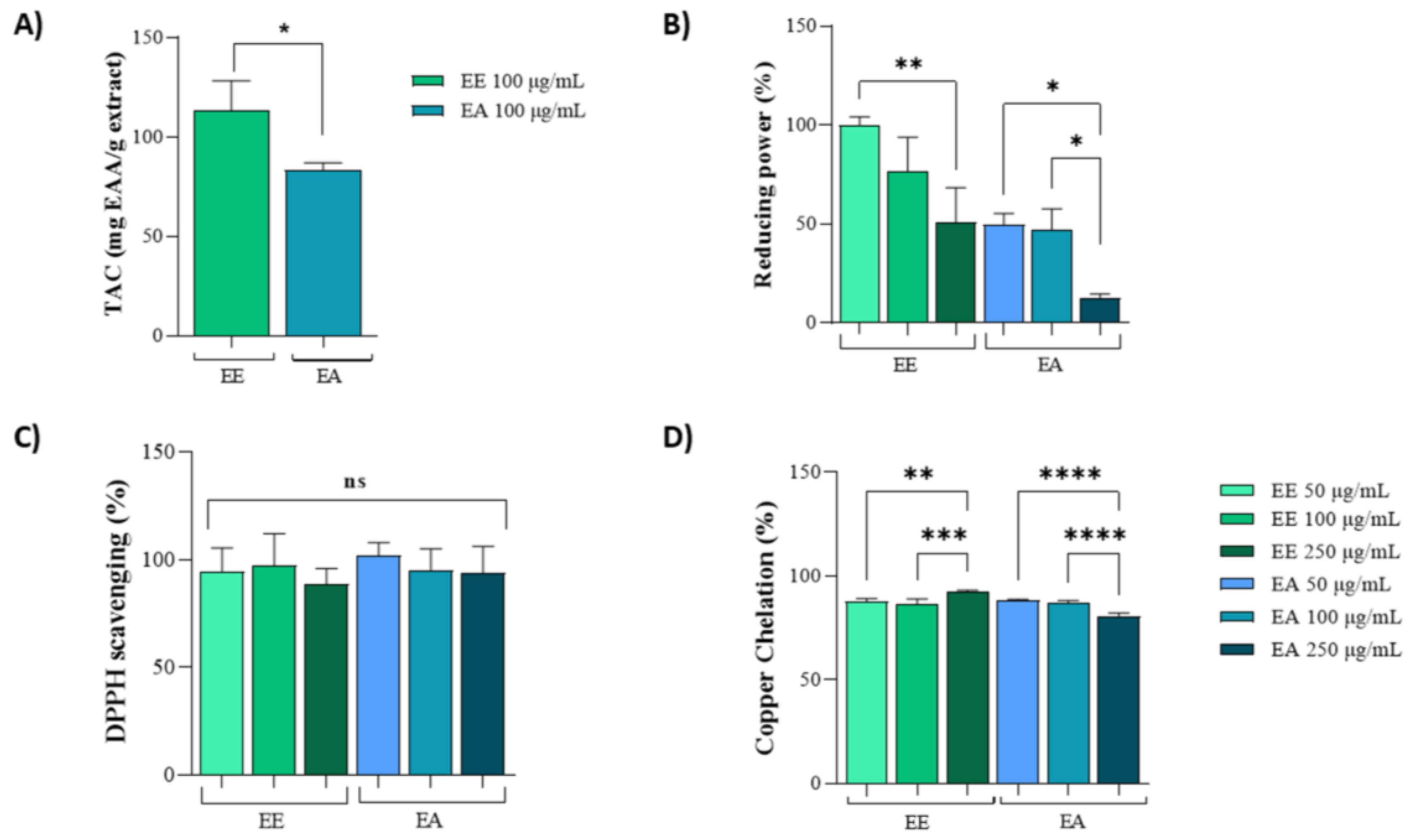
2.5.1. Total Antioxidant Capacity (TAC)
2.5.2. Reducing Power
2.5.3. DPPH Radical Scavenging Assay
2.5.4. Copper Chelation
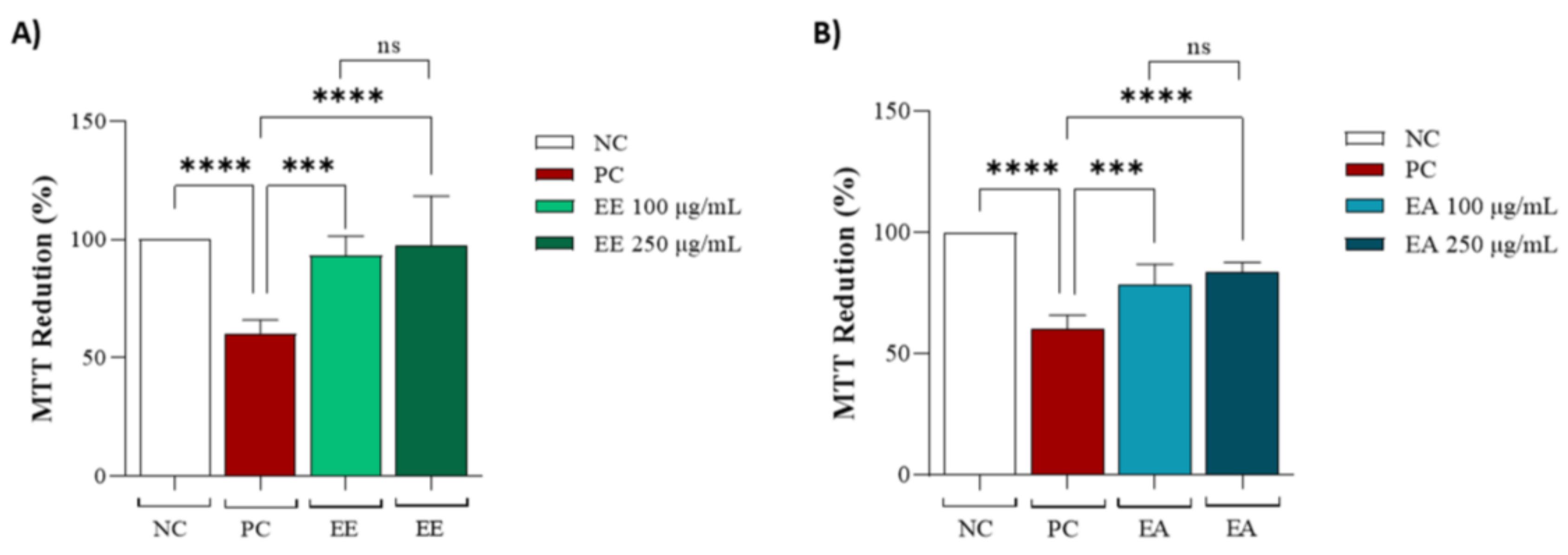
2.6. Evaluation of Cytotoxic Activity on 3T3 Cell Line
2.6.1. MTT Assay (3T3)
2.6.2. In Vitro Wound Healing Assay in 3T3 Cells
2.6.3. Copper Sulfate (CuSO4)-Induced Oxidative Stress Assay in 3T3 Cellular Model
2.7. Evaluation of In Vivo Toxicity and Antioxidant Capacity
2.7.1. Animal Model—Tenebrio molitor
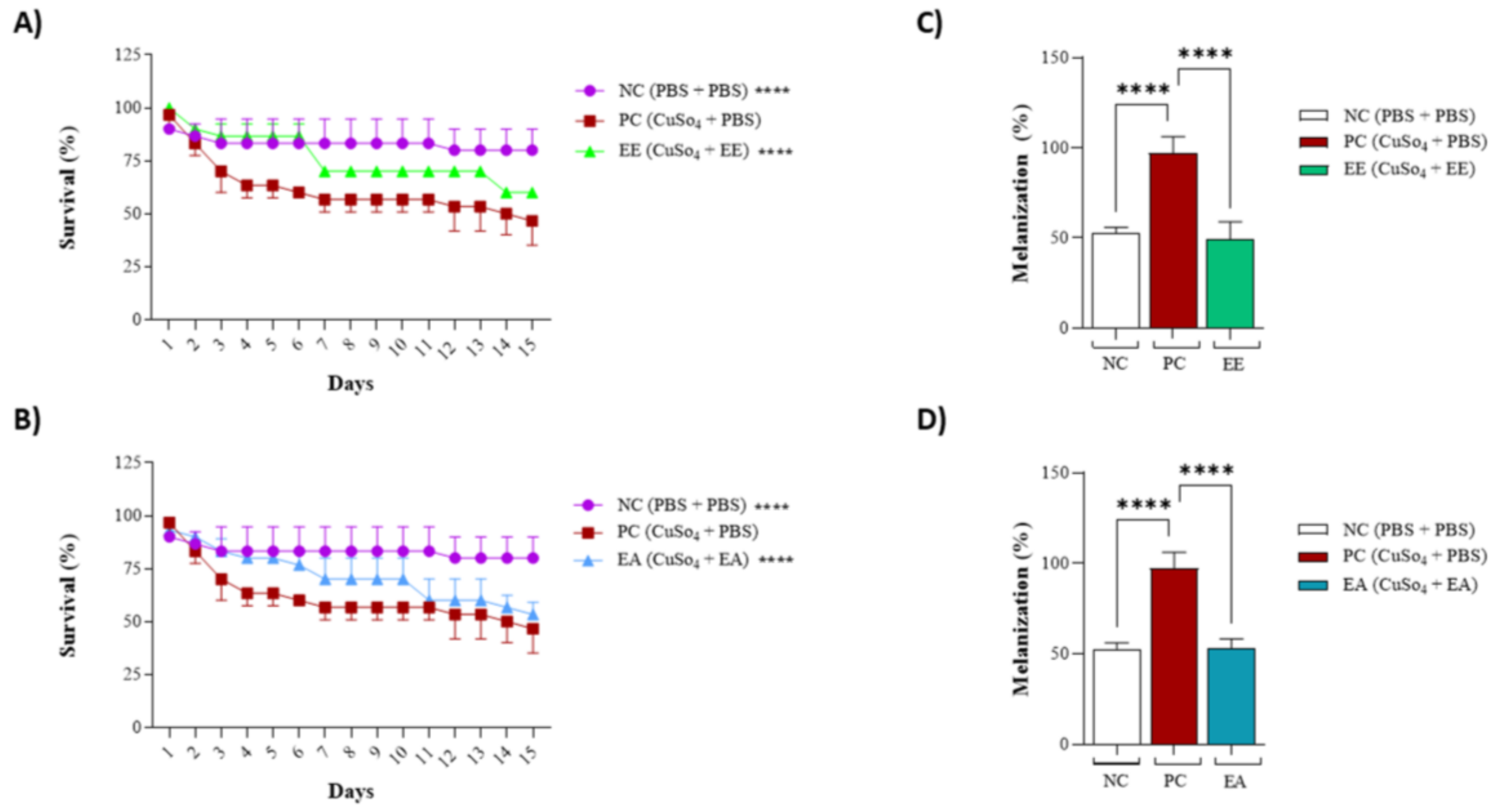
2.7.2. Toxicity Assay of Extracts in T. molitor Larvae
2.7.3. Copper Sulfate (CuSO4)-Induced Oxidative Stress Assay in T. molitor
2.7.4. Melanization Assay Following Copper Sulfate (CuSO4)-Induced Oxidative Stress in T. molitor
3. Results
3.1. Antioxidant Potential by Biochemistry Assay
3.2. Extract Composition
3.3. In Vitro Assays Using NHI/3T3 Cell Line
3.4. In Vivo Assays Using Tenebrio molitor Animal Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- El-Guezzane, C.; El-Moudden, H.; Harhar, H.; Chahboun, N.; Tabyaoui, M.; Zarrouk, A. A comparative study of the antioxidant activity of two Moroccan prickly pear cultivars collected in different regions. Chem. Data Collect. 2021, 31, 100637. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, X.; Cao, T.; Chen, E.; Li, Y.; Lei, W.; Hu, Y.; He, B.; Liu, S. Endoplasmic Reticulum Stress and Oxidative Stress in Inflammatory Diseases. DNA Cell Biol. 2022, 41, 924–934. [Google Scholar] [CrossRef]
- Assavarittirong, C.; Samborski, W.; Grygiel-Górniak, B. Oxidative Stress in Fibromyalgia: From Pathology to Treatment. Oxid. Med. Cell. Longev. 2022, 2022, 1582432. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Kianmehr, M.; Anaeigoudari, A. Effects of Medicinal Plants and Flavonoids on Parkinson’s Disease: A Review on Basic and Clinical Evidences. Adv. Pharm. Bull. 2021, 11, 224–232. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.H. Natural anti-ageing skincare: Role and potential. Biogerontologia 2020, 21, 293–310. [Google Scholar] [CrossRef]
- Gouws, C.; Hamman, J.H. What are the dangers of drug interactions with herbal medicines? Expert Opin. Drug Metab. Toxicol. 2020, 16, 65–167. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Araujo, L.R.L.; Viana, F.C.V.; Caimiro, V.C.; Nogueira, T.B.S.S.; Souza, M.N.A. 2016 Antioxidants in preventing cancer in the elderly. J. Med. Health Promot. 2024, 1, 18–26. [Google Scholar]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics, com foco na supressão de doenças crônicas: Uma revisão. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Hachkova, H.; Nagalievska, M.; Soliljak, Z.; Kanyuka, O.; Kucharska, A.; Sokół-Łętowska, A.; Belonovskaya, E.; Buko, V.; Sybirna, N. Medicinal Plants Galega officinalis L. and Yacon Leaves as Potential Sources of Antidiabetic Drugs. Antioxidants 2021, 10, 1362. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, Z.X.; Cai, J.; Li, R.; Deng, K.Q.; Ji, Y.X.; Lei, F.; Li, H.P.; Lu, Z.; Li, H. Energy substrate metabolism and oxidative stress in metabolic cardiomyopathy. J. Mol. Med. 2022, 100, 1721–1739. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Si, H.; Liu, D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr Biochem. 2014, 25, 581–591. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Bjorklund, G.; Dadar, M.; Martins, N.; Chirumbolo, S.; Goh, B.H.; Smetanina, K.; Lysiuk, R. Brief Challenges in medicinal plants: A revealing look at age-related disorders. Basic Clin. Pharmacol. Toxicol. 2018, 122, 539–558. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in ageing and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Liu, J.K. Anti-aging agents: Safe interventions to delay aging and extend healthy life. Nat. Prod. Bioprospect. 2022, 12, 18. [Google Scholar] [CrossRef]
- Ugalde, P.C.; McRostie, V.; Gayo, E.M.; García, M.; Latorre, C.; Santoro, C.M. 13,000 years of sociocultural plant use in the Atacama Desert of northern Chile. Veget. Hist. Archaeobot. 2021, 30, 213–230. [Google Scholar] [CrossRef]
- Reynoso, M.A.; Vera, N.; Aristimuño, M.E.; Daud, A.; Sánchez Riera, A. Antinociceptive activity of fruits extracts and Geoffroea decorticans (Chañar). J. Ethnopharmacol. 2013, 145, 355–362. [Google Scholar] [CrossRef]
- Somaini, G.C.; Aybar, M.J.; Vera, N.R.; Tríbulo, C. Geoffroea decorticans fruit extracts inhibit the wnt/β-catenin pathway, a therapeutic target in cancer. Biochem. Biophys. Res. Commun. 2021, 546, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Isla, M.I.; Ezquer, M.E.; Leal, M.; Moreno, M.A.; Zampini, I.C. Flower beverages of native medicinal plants from Argentina (Acacia caven, Geoffroea decorticans and Larrea divaricata) as antioxidant and anti-inflammatory. J. Ethnopharmacol. 2021, 281, 114490. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, E.N.; Sampietro, D.A.; Sgariglia, M.A.; Soberón, J.R.; Vattuone, M.A. Antimycotic activity of 5′-prenylisoflavanones of the plant Geoffroea decorticans, against Aspergillus species. Int. J. Food Microbiol. 2009, 132, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Lamarque, A.L.; Fortunato, R.H.; Maestri, D.M.; Guzmahn, C.A. Seed components and taxonomy of some Acacia species. Biochem. Syst. Ecol. 2000, 28, 53–56. [Google Scholar] [CrossRef]
- Costamagna, M.S.; Ordonez, R.M.; Zampini, I.C.; Sayago, J.E.; Isla, M.I. Nutritional and antioxidant properties of Geoffroea decorticans, an Argentinean fruit, and derived products (flour, arrope, decoction and hydroalcoholic beverage). Food Res. Int. 2013, 54, 160–168. [Google Scholar] [CrossRef]
- Maestri, D.M.; Fortunato, R.H.; Greppi, J.A.; Lamarque, A.L. Compositional Studies of Seeds and Fruits from Two Varieties of Geoffroea decorticans. J. Food Compos. Anal. 2001, 14, 585–590. [Google Scholar] [CrossRef]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenéz, D.; Muñoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of Chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 61, 104824. [Google Scholar] [CrossRef]
- Meyers, J.R. Zebrafish: Development of a vertebrate model organism. Curr. Protoc. Essent. Lab. Tech. 2018, 16, e19. [Google Scholar] [CrossRef]
- Kurosawa, T.M. Alternative Research (3Rs) in the World, Asia and Japan. In Alternatives to Animal Testing; Kojima, H., Seidle, T., Spielmann, H., Eds.; Springer: Singapore, 2019; pp. 33–36. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Hunter-Chang, S.; Luo, L.; Li, K.; Liu, K.H.; Robinson, B.S. Oxidative stress mediates end-organ damage in a novel model of acetaminophen-toxicity in Drosophila. Sci. Rep. 2022, 12, 19309. [Google Scholar] [CrossRef]
- Dinh, H.; Semenec, L.; Kumar, S.S.; Curta, F.L.; Cain, A.K. Microbiology’s next top model: Galleria in the molecular age. Pathog Dis. 2021, 79, ftab006. [Google Scholar] [CrossRef]
- Nascimento, A.K.L.; Melo-Silveira, R.F.; Dantas-Santos, N.; Fernandes, J.M.; Zucolotto, S.M.; Rocha, H.A.O.; Scortecci, K.C. Antioxidant and Antiproliferative Activities of Leaf Extracts from Plukenetia volubilis Linneo (Euphorbiaceae). Evid. Based Complement. Altern. Med. 2013, 2013, 950272. [Google Scholar] [CrossRef]
- Athukorala, Y.; Kim, K.N.; Jeon, Y.J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef]
- Sousa, E.O.; Miranda, C.M.B.A.; Nobre, C.B.; Boligon, A.A.; Athayde, M.L.; Costa, J.G.M. Phytochemical analysis and antioxidant activities of Lantana camara and Lantana montevidensis extracts. Ind. Crops Prod. 2015, 70, 7–15. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Megías, E.; Pastor-Cavada, E.; Torres-Fuentes, C.; Girón-Calle, J.; Alaiz, M.; Rocio, J.; Pastor, J.; Vioque, J. Chelating, antioxidant and antiproliferative activity of Vicia sativa polyphenol extracts. Eur. Food Res. Technol. 2009, 230, 353–359. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ke, w.; Zhao, X.; Lu, Z. Foeniculum vulgare seed extract induces apoptosis in lung cancer cells partly through the down-regulation of Bcl-2. Biomed. Pharmacother. 2021, 135, 111213. [Google Scholar] [CrossRef]
- Presa, F.B.; Marques, M.L.M.; Viana, R.L.S.; Nobre, L.T.D.B.; Costa, L.S.; Rocha, H.A.O. The Protective Role of Sulfated Polysaccharides from Green Seaweed Udotea flabellum in Cells Exposed to Oxidative Damage. Mar. Drugs 2018, 16, 135. [Google Scholar] [CrossRef]
- Djouadi, A.; Sales, J.R.; Carvalho, M.O.; Raymundo, A. Development of Healthy Protein-Rich Crackers Using Tenebrio molitor Flour. Foods 2022, 11, 702. [Google Scholar] [CrossRef]
- Silva, T.F.; Cavalcanti-Filho, J.R.N.; Barreto-Fonsêca, M.M.L.; Santos, N.M.D.; Barbosa da Silva, A.C.; Zagmignan, A.; Abreu, A.G.; Sant’Anna da Silva, A.P.; Lima, V.L.d.M.; Silva, N.H.D.; et al. Products Derived from Buchenavia tetraphylla Leaves Have In Vitro Antioxidant Activity and Protect Tenebrio molitor Larvae against Escherichia coli-Induced Injury. Pharmaceuticals 2020, 13, 46. [Google Scholar] [CrossRef]
- Jorjão, A.L.; De Oliveira, F.E.; Leão, M.V.P.; Jorge, A.O.C.; De Oliveira, L.D. Effect of Lactobacillus rhamnosus on the response of Galleria mellonella against Staphylococcus aureus and Escherichia coli infections. Arch. Microbiol. 2018, 200, 383–389. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Maury, G.L.; Rodríguez, D.M.; Hendrix, S.; Arranz, J.C.E.; Boix, Y.F.; Pacheco, A.O.; Díaz, J.G.; Morris-Quevedo, H.J.; Dubois, A.F.; Aleman, E.I.; et al. Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants-A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R.A. Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islã, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Prazeres, L.D.K.T.; Aragão, T.P.; Brito, S.A.; Almeida, C.L.F.; Silva, A.D.; Paula, M.M.F.; Farias, J.S.; Vieira, L.D.; Damasceno, B.P.G.L.; Rolim, L.A.; et al. Antioxidant and Antiulcerogenic Activity of the Dry Extract of Pods of Libidibia ferrea Mart. ex Tul. (Fabaceae). Oxid. Med. Cell. Longev. 2019, 2019, 1983137. [Google Scholar] [CrossRef]
- Luna, L.C.; Pigni, N.B.; Torras-Claveria, L.; Monferran, M.V.; Maestri, D.; Wunderlin, D.A.; Feresin, G.E.; Bastida, J.; Tapia, A. Ramorinoa girolae Speg (Fabaceae) seeds, an Argentinean traditional indigenous food: Nutrient composition and antioxidant activity. J. Food Compos. Anal. 2013, 31, 120–128. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Haber, F.; Wilstätter, R. Unpaarigheit und radikalketten im reaktion-mechanismus organscher und enzimatischer vorgange. Chem. Eur. 1931, 64, 2844–2856. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Soriano, M.D.P.C.; Ugalde-Arbizu, M.; Alberto, M.R.; Zampini, I.C.; Ilha, M.I.; Simirgiotis, M.J.; Schmeda-Hirschmann, G. The Native Fruit Geoffroea decorticans from Arid Northern Chile: Phenolic Composition, Antioxidant Activities and In Vitro Inhibition of Pro-Inflammatory and Metabolic Syndrome-Associated Enzymes. Molecules 2017, 22, 1565. [Google Scholar] [CrossRef]
- Afanas’ev, I.B.; Ostrachovitch, E.A.; Abramova, N.E.; Korkina, L.G. Different antioxid ant activities of bioflavonoid rutin in normal and ironoverloading rats. Biochem. Pharmacol. 1995, 50, 627–635. [Google Scholar] [CrossRef]
- Amaral, S.; Mira, L.; Nogueira, J.M.F.; da Silva, A.P.; Florêncio, M.H. Plant extracts with anti-inflammatory properties—A new approach for characterization of their bioactive compounds and establishment of structure–antioxidant activity relationships. Bioorg. Med. Chem. 2009, 17, 1876–1883. [Google Scholar] [CrossRef]
- Spiegel, M.; Sroka, Z. Quantum-mechanical characteristics of apigenin: Antiradical, metal chelation and inhibitory properties in physiologically relevant media. Fitoterapia 2023, 164, 105352. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Monteagudo, A.; Ripollés, E.; Berenguer, M.; Espiños, C. Wilson’s Disease: Facing the Challenge of Diagnosing a Rare Disease. Biomedicines 2021, 9, 1100. [Google Scholar] [CrossRef]
- Andrade, B.F.M.T.; Barbosa, L.N.; Probst, I.S.; Júnior, A.F. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Costa, J.P.; Ferreira, P.B.; De Sousa, D.P.; Jordan, J.; Freitas, R.M. Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci. Lett. 2012, 523, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; Almeida, A.A.C.; Oliveira, G.A.L.; Costa, J.P.; Sousa, D.P.; Freitas, R.M.; Almeida, R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Silva, R.O.; Sousa, F.B.; Damasceno, S.R.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant Secondary Metabolites: An Opportunity for Circular Economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Streck, L.; Paz, M.F.C.J.; Sousa, J.M.; Alencar, M.V.; Mata, A.; de Carvalho, R.M.; Santos, J.V.; Silva-Júnior, A.A.D.; Ferreira, P.; et al. Preparation of Phytol-Loaded Nanoemulsion and Screening for Antioxidant Capacity. Int. Arch. Med. 2016, 9, 1–15. Available online: http://repositorio.ufc.br/handle/riufc/18772 (accessed on 1 May 2023).
- Ko, G.A.; Cho, S.K. Phytol suppresses melanogenesis through proteasomal degradation of MITF via the ROS-ERK signaling pathway. Chem. Biol. Interact. 2018, 286, 132–140. [Google Scholar] [CrossRef]
- Shukla, A.; Tripathi, R. Phytol as a hepatoprotective compound in the leaves of Eichhornia crassipes. Appl. Biol. Chem. J. 2022, 3, 79–82. [Google Scholar] [CrossRef]
- Gupta, K.; Taj, T.; Thansiya, B.; Kamath, J.V. Pre-clinical evaluation of hepatoprotective activity of phytol in Wistar albino rats. Int. J. Compr. Adv. Pharmacol. 2019, 4, 17–20. [Google Scholar] [CrossRef]
- Sakthivel, R.; Malar, D.S.; Archunan, G.; Devi, K.P. Phytol ameliorated benzo (a) pyrene induced lung carcinogenesis in Swiss albino mice via inhibition of oxidative stress and apoptosis. Environ. Toxicol. 2019, 34, 355–363. [Google Scholar] [CrossRef]
- Miret, J.A.; Munné-Bosch, S. Redox signaling and stress tolerance in plants: A focus on vitamin E. Ann. N. Y. Acad. Sci. 2015, 1340, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Fahrenholtz, S.R.; Doleiden, F.H.; Trozzolo, A.M.; Lamola, A.A. On the quenching of singlet oxygen by alpha-tocopherol. Photochem. Photobiol. 1974, 20, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Della-Penna, D. Tocopherol works in photosynthetic organisms. Curr. Opin. Plant Biol. 2007, 10, 260–265. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Kalmykova, A. Electrochemical Characterization of the Antioxidant Properties of Medicinal Plants and Products: A Review. Molecules 2023, 28, 2308. [Google Scholar] [CrossRef]
- Cittadini, M.C.; García-Estévez, I.; Escribano-Bailón, A.T.; Bodoira, R.M.; Barrionuevo, D.; Maestri, D. Nutritional and nutraceutical compounds of fruits from native trees (Ziziphus mistol and Geoffroea decorticans) of the dry chaco forest. J. Food Compos. Anal. 2021, 97, 103775. [Google Scholar] [CrossRef]
- McCluskey, S.; Hall, M.; Stanton, C.; Devery, R. α-tocopherol inhibits oxidative stress induced by cholestanetriol and 25-hydroxycholesterol in porcine ovarian granulosa cells. Mol. Cell. Biochem. 1999, 194, 217–225. [Google Scholar] [CrossRef]
- Amin, K.A.; Hashem, K.S. Deltamethrin-induced oxidative stress and biochemical changes in tissues and blood of catfish (Clarias gariepinus): Antioxidant defense and role of alpha-tocopherol. BMC Vet. Res. 2012, 8, 45. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Satish, K.C.S.; Rani, M.U.; Srikanth, M.K.; Boobalan, G.; Reddy, A.G. An evaluation of the protective role of α-tocopherol on free radical induced hepatotoxicity and nephrotoxicity due to chromium in rats. Indian J. Pharmacol. 2013, 45, 490–495. [Google Scholar] [CrossRef]
- Ciocoiu, M.; Badescu, M.; Paduraru, I. Protecting antioxidative effects of vitamins E and C in experimental physical stress. J. Physiol. Biochem. 2007, 63, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Min, J.W.; Kong, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on phytochemical profile and health promoting effects of mung bean (Vigna radiata). Food Sci. Hum. Wellness 2018, 7, 11–33. [Google Scholar] [CrossRef]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.N.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin Inhibits Inflammatory Pain in Mice by Targeting TRPV1, Oxidative Stress, and Cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M.; Al-Sayed, E.; Moghannem, S.; Azam, F.; El-Shazly, M.; Singab, A.N. Breaking down the barriers to a natural antiviral agent: Antiviral activity and molecular docking of Erythrina speciosa extract, fractions, and the major compound. Chem. Biodivers. 2020, 17, 1900511. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Yıldırım, S.; Kucukler, S.; Caglayan, C.; Darendelioğlu, E.; Dortbudak, M.B. Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: A multi-biomarker approach. Food Chem. Toxicol. 2020, 138, 111190. [Google Scholar] [CrossRef] [PubMed]
- Noor, K.K.; Ijaz, M.U.; Ehsan, N.; Tahir, A.; Yeni, D.K.; Zihad, S.N.K.; Uddin, S.J.; Ashraf, A.; Simal-Gandara, J. Hepatoprotective role of vitexin against cadmium-induced liver damage in male rats: A biochemical, inflammatory, apoptotic and histopathological investigation. Biomed. Pharmacother. 2022, 150, 112934. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Wang, H.; Chen, Y. Vitexin ameliorated diabetic nephropathy via suppressing GPX4-mediated ferroptosis. Eur. J. Pharmacol. 2023, 951, 175787. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Ahmed, H.; Ashraf, A.; Aziz, S.; Al-Ghanim, K.; Akhtar, M.; Riaz, M.N.; Mahboob, S. Vitexin attenuates cisplatin-induced renal toxicity by reducing oxidative stress and inflammation. J. King Saud Univ.-Sci. 2021, 33, 101657. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin—A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Arjumand, W.; Seth, A.; Sultana, S. Rutin attenuates cisplatin induced renal inflammation and apoptosis by reducing NFκB, TNF-α and caspase-3 expression in wistar rats. Food Chem. Toxicol. 2011, 49, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Naraki, K.; Roohbakhsh, A.; Hayes, A.W.; Karimi, G. The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Sci. Nutr. 2023, 11, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.R.; Mohseni-Moghaddam, P.; Hosseini, M.; Gholami, L.; Baluchnejadmojarad, T.; Roghani, M. Rutin, a quercetin glycoside, alleviates acute endotoxemic kidney injury in C57BL/6 mice via suppression of inflammation and up-regulation of antioxidants and SIRT1. Eur. J. Pharmacol. 2018, 833, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Shukla, P.; Verma, A.; Nirala, S.K.; Bhadauria, M. Protective role of rutin against combined exposure to lipopolysaccharide and D-galactosamine-induced dysfunctions in liver, kidney, and brain: Hematological, biochemical, and histological evidences. J. Food Biochem. 2021, 45, e13605. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Lee, J.J.; Saiful Yazan, L.; Kassim, N.K.; Che Abdullah, C.A.; Esa, N.; Lim, P.C.; Tan, D.C. Cytotoxic Activity of Christia vespertilionis Root and Leaf Extracts and Fractions against Breast Cancer Cell Lines. Molecules 2020, 25, 2610. [Google Scholar] [CrossRef]
- Costamagna, M.S.; Zampini, I.C.; Alberto, M.R.; Cuello, S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef]
- Tam, C.W.J.; Ko, C.-H.; Lau, K.-M.; To, M.-H.; Kwok, H.-F.; Chan, Y.-W.; Siu, W.-S.; Etienne-Selloum, N.; Lau, C.-P.; Chan, W.-Y.; et al. A Chinese 2-herb formula (NF3) promotes hindlimb ischemia-induced neovascularization and wound healing of diabetic rats. J. Diabetes Its Complicat. 2014, 28, 436–447. [Google Scholar]
- Tewtrakul, S.; Tungcharoen, P.; Sudsai, T.; Karalai, C.; Ponglimanont, C.; Yodsaoue, O. Antiinflammatory and Wound Healing Effects of Caesalpinia sappan L. Phytother. Res. 2015, 29, 850–856. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 30. [Google Scholar] [CrossRef]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast Proliferation and Migration in Wound Healing by Phytochemicals: Evidence for a Novel Synergic Outcome. Int. J. Med. Sci. 2020, 17, 1030–1042. [Google Scholar] [CrossRef]
- Salehi, B.; Albayrak, S.; Antolak, H.; Krégiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Tsouh Fokou, P.V.; Yousef, Z.; Amiruddin Zakaria, Z.; et al. Aloe Genus Plants: From Farm to Food Applications and Phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yan, Z.; Miao, Y.; Há, W.; Li, Z.; Yang, L.; Mi, D. Copper in cancer: From limiting nutrient to therapeutic target. Front. Oncol. 2023, 13, 1209156. [Google Scholar] [CrossRef]
- Yang, F.; Pei, R.; Zhang, Z.; Liao, J.; Yu, W.; Qiao, N.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol. Vitr. 2019, 54, 310–316. [Google Scholar] [CrossRef]
- Liu, W.; Guo, H.; Jian, Z.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Copper Induces Oxidative Stress and Apoptosis in the Mouse Liver. Oxidative Med. Cell. Longev. 2020, 2020, 20. [Google Scholar] [CrossRef]
- Zhong, G.; He, Y.; Wan, F.; Wu, S.; Jiang, X.; Tang, Z.; Hu, L. Effects of Long-Term Exposure to Copper on the Keap1/Nrf2 Signaling Pathway and Msr-Related Redox Status in the Kidneys of Rats. Biol. Trace Elem. Res. 2021, 199, 4205–4217. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Ma, X.; Yang, F.; Liao, J.; Qiao, N.; Yu, W.; Han, Q.; Li, Y.; Pan, J.; Hu, L.; et al. Exposure to copper induces endoplasmic reticulum (ER) stress-mediated apoptosis in chicken (Gallus gallus) myocardium. Vet. Res. Commun. 2023, 47, 2027–2040. [Google Scholar] [CrossRef] [PubMed]
- Pal, A. Copper toxicity induced hepatocerebral and neurodegenerative diseases: An urgent need for prognostic biomarkers. NeuroToxicology 2014, 40, 97–101. [Google Scholar] [CrossRef]
- Magazenkova, D.N.; Skomorokhova, E.A.; Farroukh, M.A.; Zharkova, M.S.; Jassem, Z.M.; Rekina, V.E.; Shamova, O.V.; Puchkova, L.V.; Ilyechova, E.Y. Influence of Silver Nanoparticles on the Growth of Ascitic and Solid Ehrlich Adenocarcinoma: Focus on Copper Metabolism. Pharmaceutics 2023, 15, 1099. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidant defence mechanisms in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Ho, T.T.; Murthy, H.N.; Park, S.Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Ke-yi, N.K.V.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Brai, A.; Poggialini, F.; Vagaggini, C.; Pasqualini, C.; Simoni, S.; Francardi, V.; Dreassi, E. Tenebrio molitor as a Simple and Cheap Preclinical Pharmacokinetic and Toxicity Model. Int. J. Mol. Sci. 2023, 24, 2296. [Google Scholar] [CrossRef]
- Mylonakis, E.; Casadevall, A.; Ausubel, F.M. Exploiting Amoeboid and Non-Vertebrate Animal Model Systems to Study the Virulence of Human Pathogenic Fungi. PLoS Pathog. 2007, 3, e101. [Google Scholar] [CrossRef]
- Li, D.; Deng, L.; Hu, G.H.; Zhao, L.X.; Hu, D.D.; Jiang, Y.Y.; Wang, Y. Using Galleria mellonella Candida albicans Infection Model to Evaluate Antifungal Agents. Biol. Pharm. Bull. 2013, 36, 1482–1487. [Google Scholar] [CrossRef]
- Canteri, S.P.; Custódio, C.C.; Wilson, D.; Sergio, A.R. An Invertebrate Host to Study Fungal Infections, Mycotoxins and Antifungal Drugs: Tenebrio molitor. J. Fungi 2018, 4, 125. [Google Scholar] [CrossRef]
- Cordeiro, M.L.S.; Aquino-Martins, V.G.Q.; da Silva, A.P.; Naliato, G.F.S.; Silveira, E.R.; Theodoro, R.C.; da Santos, D.Y.A.C.; Rocha, H.A.O.; Scortecci, K.C. Exploring the Antioxidant Potential of Talisia esculenta Using In Vitro and In Vivo Approaches. Nutrients 2023, 15, 3855. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, A.; Jehan, C.; Rigaud, T.; Moret, Y. Immune Defenses of a Beneficial Pest: The Mealworm Beetle, Tenebrio molitor. Front. Physiol. 2019, 10, 138. [Google Scholar] [CrossRef]
- Sanchez, W.; Palluel, O.; Meunier, L.; Coquery, M.; Porcher, J.M.; Selim, A. Copper-induced oxidative stress in three-spined stickleback: Relationship with hepatic metal levels. Environ. Toxicol. Pharmacol. 2005, 19, 177–183. [Google Scholar] [CrossRef]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. Assessing anticancer potential of blueberry flavonoids, quercetin, kaempferol, and gentisic acid, through oxidative stress and apoptosis parameters on HCT-116 cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Söderhall, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]


| Compounds | G. decorticans Extracts | |
|---|---|---|
| EE | EA | |
| Total Phenolic Compounds (mg EAG/g Extract) | 238 ± 5 a | 211 ± 3 b |
| Total Flavonoids (mg EQ/g Extract) | 13.7 ± 2 a | 35.4 ± 3 b |
| Compounds | G. decorticans Extracts | |
|---|---|---|
| EE | EA | |
| Phytol | 10.94 ± 0.07 ng·mL−1 a | 2.34 ± 0.31 ng·mL−1 b |
| Vitexin | 18.17 ± 0.08 ng·mL−1 a | 1.56 ± 0.05 ng·mL−1 b |
| Rutin | 28.13 ± 0.23 ng·mL−1 a | 25.07 ± 0.16 ng·mL−1 b |
| α-Tocopherol | 5.51 ± 0.36 ng·mL−1 a | 4.88 ± 0.09 ng·mL−1 b |
| Amonafide | nd | nd |
| Caffeic acid | nd | nd |
| Isovitexin | nd | nd |
| Epifriedelanol | nd | nd |
| Leucocyanidin | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.P.; Cordeiro, M.L.d.S.; Aquino-Martins, V.G.d.Q.; de Moura Melo, L.F.; Paiva, W.d.S.; Naliato, G.F.d.S.; Theodoro, R.C.; Meneses, C.H.S.G.; Rocha, H.A.O.; Scortecci, K.C. Prospecting of the Antioxidant Activity from Extracts Obtained from Chañar (Geoffroea decorticans) Seeds Evaluated In Vitro and In Vivo Using the Tenebrio molitor Model. Nutrients 2024, 16, 2813. https://doi.org/10.3390/nu16172813
Silva AP, Cordeiro MLdS, Aquino-Martins VGdQ, de Moura Melo LF, Paiva WdS, Naliato GFdS, Theodoro RC, Meneses CHSG, Rocha HAO, Scortecci KC. Prospecting of the Antioxidant Activity from Extracts Obtained from Chañar (Geoffroea decorticans) Seeds Evaluated In Vitro and In Vivo Using the Tenebrio molitor Model. Nutrients. 2024; 16(17):2813. https://doi.org/10.3390/nu16172813
Chicago/Turabian StyleSilva, Ariana Pereira, Maria Lucia da Silva Cordeiro, Verônica Giuliani de Queiroz Aquino-Martins, Luciana Fentanes de Moura Melo, Weslley de Souza Paiva, Georggia Fatima da Silva Naliato, Raquel Cordeiro Theodoro, Carlos Henrique Salvino Gadelha Meneses, Hugo Alexandre Oliveira Rocha, and Katia Castanho Scortecci. 2024. "Prospecting of the Antioxidant Activity from Extracts Obtained from Chañar (Geoffroea decorticans) Seeds Evaluated In Vitro and In Vivo Using the Tenebrio molitor Model" Nutrients 16, no. 17: 2813. https://doi.org/10.3390/nu16172813
APA StyleSilva, A. P., Cordeiro, M. L. d. S., Aquino-Martins, V. G. d. Q., de Moura Melo, L. F., Paiva, W. d. S., Naliato, G. F. d. S., Theodoro, R. C., Meneses, C. H. S. G., Rocha, H. A. O., & Scortecci, K. C. (2024). Prospecting of the Antioxidant Activity from Extracts Obtained from Chañar (Geoffroea decorticans) Seeds Evaluated In Vitro and In Vivo Using the Tenebrio molitor Model. Nutrients, 16(17), 2813. https://doi.org/10.3390/nu16172813









