Unraveling the Potential of γ-Aminobutyric Acid: Insights into Its Biosynthesis and Biotechnological Applications
Abstract
:1. Introduction
2. Source of GAD
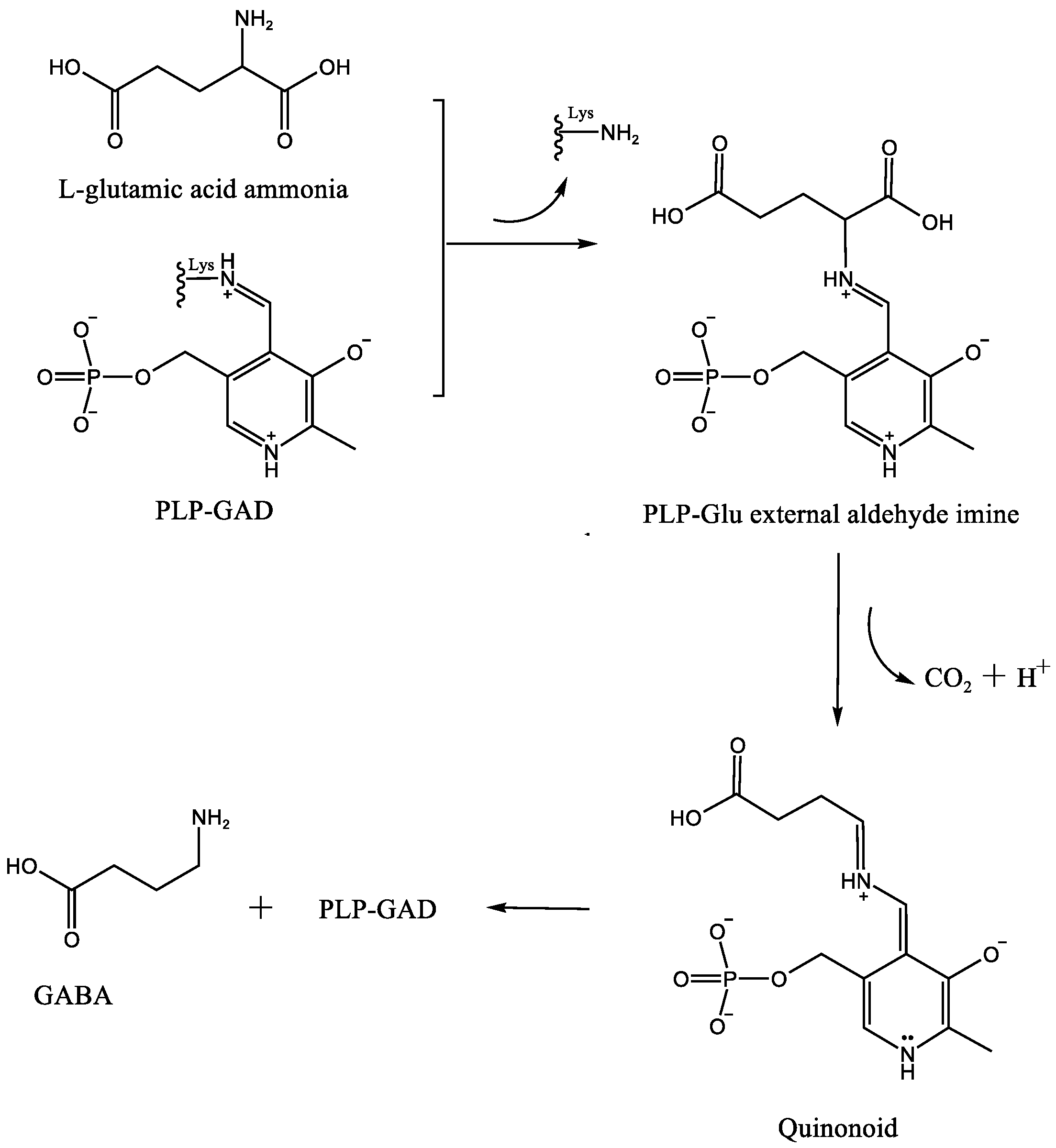
3. The Catalytic Mechanism of GAD
4. The Structural Characteristics of GAD from Different Sources
4.1. Microbial GAD
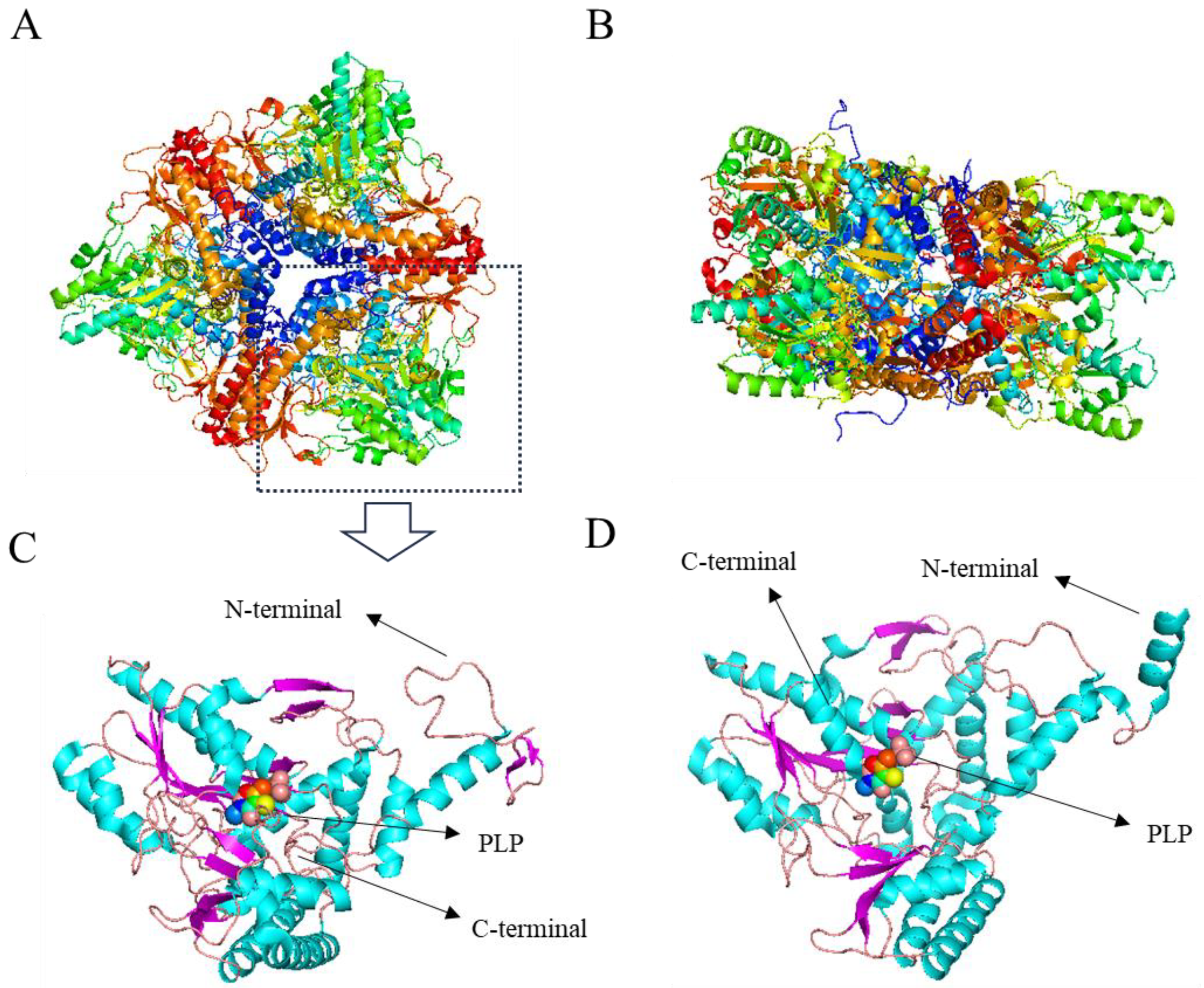
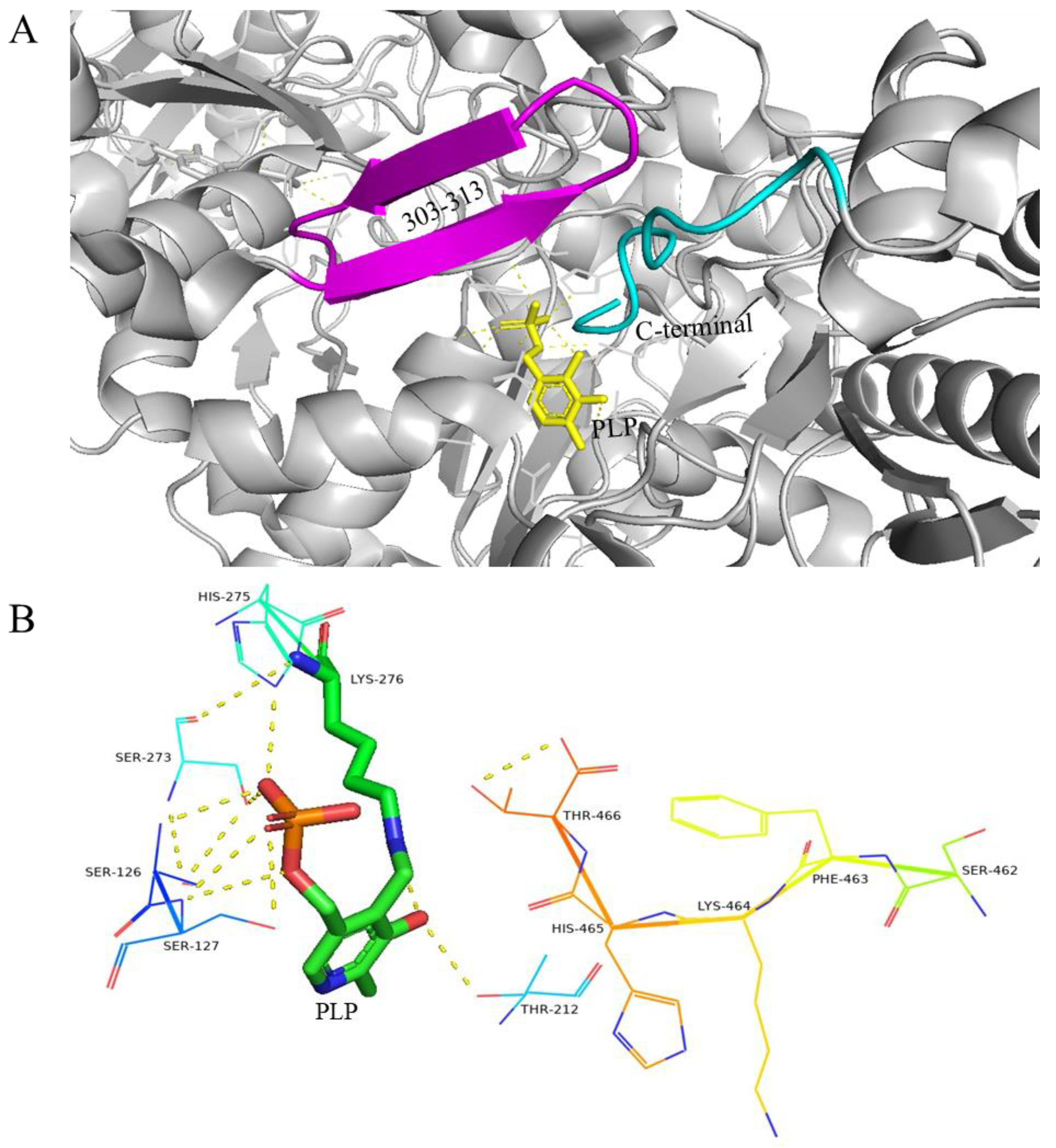
4.1.1. GAD of E. coli
4.1.2. GAD of Lactobacillus Brevis CGMCC 1306
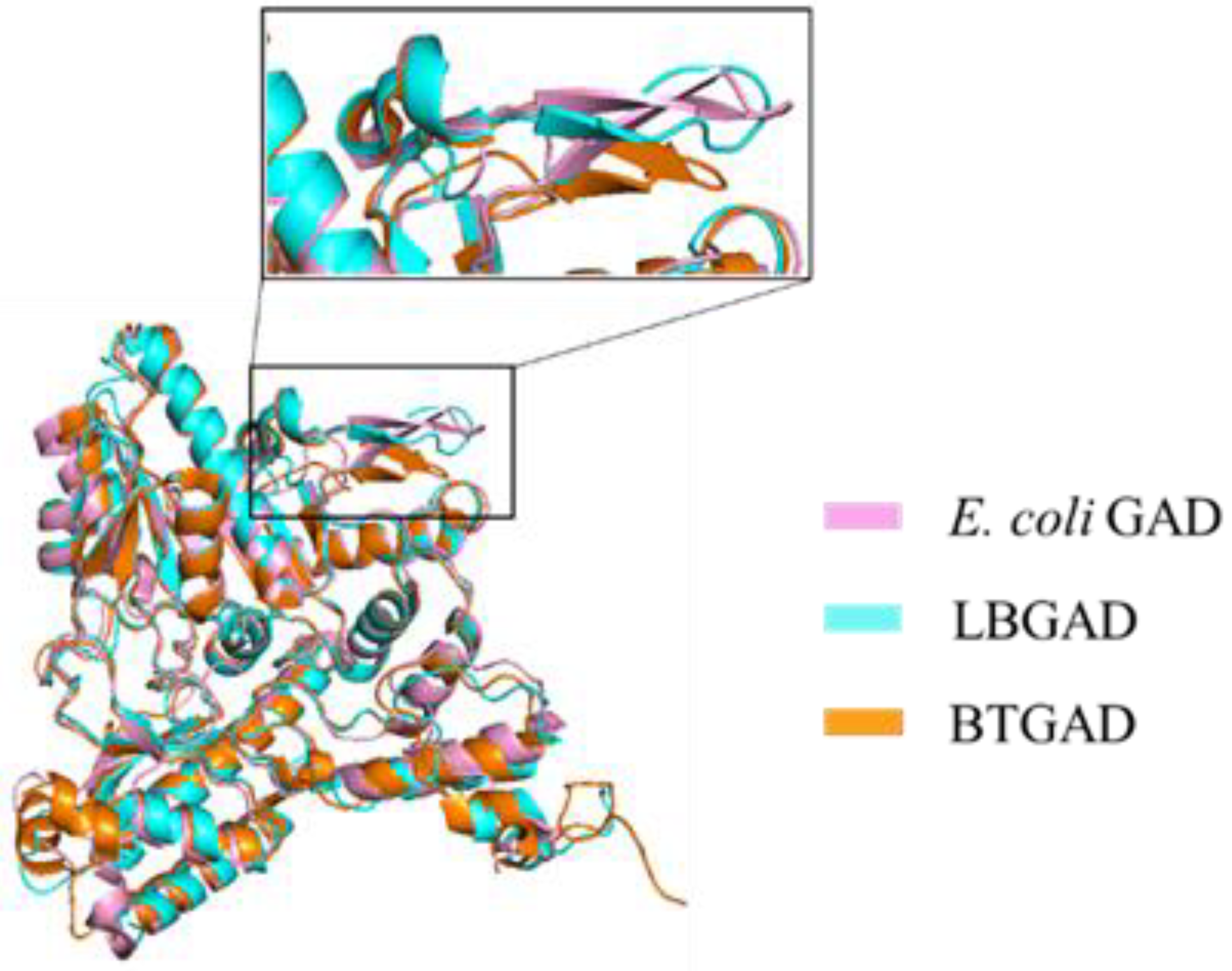
4.1.3. GAD of Bacteroides thetaiotaomicron
4.2. Plant GAD
4.3. Animal GAD
5. Multiple Strategies for High Production of GABA in Microbial Cell Factories
5.1. GABA Pathway
5.2. Directed Evolution of GAD
5.3. Optimization of Fermentation Parameters
5.3.1. Carbon and Nitrogen Sources
5.3.2. pH
5.3.3. Initial Concentration of L-Glutamate
5.3.4. Temperature
5.3.5. Addition of Pyridoxal-5′-Phosphate (PLP) and Trace Elements
6. GABA Regulates Physiological Metabolism
6.1. Animal Feeding
6.2. Endocrine and Gastrointestinal Function
6.3. Dietary Supplement
6.4. Immunoregulation
6.5. Detoxification of Ammonia
6.6. Neurotrophic Factor
6.7. Cardioprotection
7. Prospects and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roberts, E.; Frankel, S. γ-Aminobutyric acid in brain: Its formation from glutamic acid. J. Biol. Chem. 1950, 187, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, Y. Lactic Acid Bacterial Cell Factories for Gamma-Aminobutyric Acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef]
- Hao, R.; Schmit, J.C. Purification and Characterization of Glutamate Decarboxylase from Neurospora Crassa Conidia. J. Biol. Chem. 1991, 266, 5135–5139. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Jalil, S.U.; Chopra, P.; Chhillar, H.; Ferrante, A.; Khan, N.A.; Ansari, M.I. Role of GABA in Plant Growth, Development and Senescence. Plant Gene 2021, 26, 100283. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma Aminobutyric Acid (GABA) and Plant Responses to Stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Takahashi, H.; Sumi, M.; Koshino, F. Effect of γ-Aminobutyric Acid (GABA) on Normotensive or Hypertensive Rats and Men. Jpn. J. Physiol. 1961, 11, 89–95. [Google Scholar] [CrossRef]
- Wassef, A.; Baker, J.; Kochan, L.D. GABA and Schizophrenia: A Review of Basic Science and Clinical Studies. J. Clin. Psychopharmacol. 2003, 23, 601–640. [Google Scholar] [CrossRef]
- Martins-Marques, T. Cardioprotective Role of GABA-B Receptor Activation on Ventricular Arrhythmia Following Myocardial Infarction. Rev. Port. Cardiol. 2023, 42, 137–138. [Google Scholar] [CrossRef]
- Hagan, D.W.; Ferreira, S.M.; Santos, G.J.; Phelps, E.A. The Role of GABA in Islet Function. Front. Endocrinol. 2022, 13, 972115. [Google Scholar] [CrossRef]
- Martin, A.; Mick, G.J.; Choat, H.M.; Lunsford, A.A.; Tse, H.M.; McGwin, G.G.; McCormick, K.L. A Randomized Trial of Oral Gamma Aminobutyric Acid (GABA) or the Combination of GABA with Glutamic Acid Decarboxylase (GAD) on Pancreatic Islet Endocrine Function in Children with Newly Diagnosed Type 1 Diabetes. Nat. Commun. 2022, 13, 7928. [Google Scholar] [CrossRef]
- Wherrett, D.K.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Marks, J.B.; et al. Antigen-Based Therapy with Glutamic Acid Decarboxylase (GAD) Vaccine in Patients with Recent-Onset Type 1 Diabetes: A Randomised Double-Blind Trial. Lancet 2011, 378, 319–327. [Google Scholar] [CrossRef]
- Hata, T.; Rehman, F.; Hori, T.; Nguyen, J.H. GABA, γ-Aminobutyric Acid, Protects against Severe Liver Injury. J. Surg. Res. 2019, 236, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, L.; Li, W.; Zhang, Y.; Zhang, S.; Ge, B.; Yang, H.; Du, G.; Tang, B.; Wang, H.; et al. GABAergic Signaling as a Potential Therapeutic Target in Cancers. Biomed. Pharmacother. 2023, 161, 114410. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Zhong, H.-J.; Wang, S.-Q.; Zhang, R.-X.; Zhuang, Y.-P.; Li, L.; Yi, S.-Z.; Li, Y.; Wu, L.; Ding, Y.; Zhang, J.; et al. Supplementation with High-GABA-Producing Lactobacillus Plantarum L5 Ameliorates Essential Tremor Triggered by Decreased Gut Bacteria-Derived GABA. Transl. Neurodegener. 2023, 12, 58. [Google Scholar] [CrossRef]
- Perucca, E.; White, H.S.; Bialer, M. New GABA-Targeting Therapies for the Treatment of Seizures and Epilepsy: II. Treatments in Clinical Development. CNS Drugs 2023, 37, 781–795. [Google Scholar] [CrossRef]
- Felice, D.; Cryan, J.F.; O’Leary, O.F. GABAB Receptors: Anxiety and Mood Disorders. Curr. Top. Behav. Neurosci. 2022, 52, 241–265. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, A.; Madsen, K.K.; Barker-Haliski, M.L.; White, H.S. The GABA Synapse as a Target for Antiepileptic Drugs: A Historical Overview Focused on GABA Transporters. Neurochem. Res. 2014, 39, 1980–1987. [Google Scholar] [CrossRef]
- Erlander, M.G.; Tobin, A.J. The Structural and Functional Heterogeneity of Glutamic Acid Decarboxylase: A Review. Neurochem. Res. 1991, 16, 215–226. [Google Scholar] [CrossRef]
- Zhang, Q.-F.; Hu, S.; Zhao, W.-R.; Huang, J.; Mei, J.-Q.; Mei, L.-H. Parallel Strategy Increases the Thermostability and Activity of Glutamate Decarboxylase. Molecules 2020, 25, 690. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Singhania, R.R.; Pandey, A. Chapter 2—Production, Purification, and Application of Microbial Enzymes. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 13–41. ISBN 978-0-12-803725-6. [Google Scholar]
- Xiong, W.; Liu, B.; Shen, Y.; Jing, K.; Savage, T.R. Protein Engineering Design from Directed Evolution to de Novo Synthesis. Biochem. Eng. J. 2021, 174, 108096. [Google Scholar] [CrossRef]
- Wahab, M.K.H.A.; El-Enshasy, H.A.; Bakar, F.D.A.; Murad, A.M.A.; Illias, R.M. Improvement of Cross-Linking and Stability on Cross-Linked Enzyme Aggregate (CLEA)-Xylanase by Protein Surface Engineering. Process Biochem. 2019, 86, 40–49. [Google Scholar] [CrossRef]
- Ueno, H. Enzymatic and Structural Aspects on Glutamate Decarboxylase. J. Mol. Catal. B Enzym. 2000, 10, 67–79. [Google Scholar] [CrossRef]
- Najjar, V.A.; Fisher, J. Studies on l-glutamic acid decarboxylase from escherichia coli. J. Biol. Chem. 1954, 206, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, D.H.; Kang, H.J.; Shin, M.; Yang, S.-Y.; Yang, J.; Jung, Y.H. Enhanced Production of γ-Aminobutyric Acid (GABA) Using Lactobacillus Plantarum EJ2014 with Simple Medium Composition. LWT 2021, 137, 110443. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, Y.; Wang, X.; Dai, W.; Piao, C.; Yu, H. Optimization of Fermentation for γ-Aminobutyric Acid (GABA) Production by Yeast Kluyveromyces Marxianus C21 in Okara (Soybean Residue). Bioprocess. Biosyst. Eng. 2022, 45, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jeong, S.-J.; Kim, J.H. Characterization of a Glutamate Decarboxylase (GAD) Gene from Lactobacillus Zymae. Biotechnol. Lett. 2014, 36, 1791–1799. [Google Scholar] [CrossRef]
- Yarabbi, H.; Mortazavi, S.A.; Yavarmanesh, M.; Javadmanesh, A. Molecular Cloning, Gene Overexpression and Characterization of Glutamate Decarboxylase from Enterococcus Faecium DO. LWT 2021, 148, 111699. [Google Scholar] [CrossRef]
- Somasundaram, S.; Jeong, J.; Kumaravel, A.; Hong, S.H. Whole-Cell Display of Pyrococcus Horikoshii Glutamate Decarboxylase in Escherichia Coli for High-Titer Extracellular Gamma-Aminobutyric Acid Production. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab039. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-D.; Li, X.; Shi, H.-L.; Jia, Y.-Y.; Dong, Z.-X.; Jiao, Z.-J.; Wang, L.-F.; Xu, J.-H.; Yao, L.-G.; Kan, Y.-C. Efficient Expression of Novel Glutamate Decarboxylases and High Level Production of γ-Aminobutyric Acid Catalyzed by Engineered Escherichia Coli. Int. J. Biol. Macromol. 2020, 160, 372–379. [Google Scholar] [CrossRef]
- Liu, S.; Wen, B.; Du, G.; Wang, Y.; Ma, X.; Yu, H.; Zhang, J.; Fan, S.; Zhou, H.; Xin, F. Coordinated Regulation of Bacteroides Thetaiotaomicron Glutamate Decarboxylase Activity by Multiple Elements under Different pH. Food Chem. 2023, 403, 134436. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Jung, D.-H.; Cho, E.-S.; Seo, M.-J. Expression, Purification, and Characterization of Glutamate Decarboxylase from Human Gut-Originated Lactococcus Garvieae MJF010. World J. Microbiol. Biotechnol. 2022, 38, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, G.; Ge, F.; Dang, T.; Ren, Y.; Zeng, B.; Li, W. Characterization and Mutagenesis of a Novel Mycobacterium Smegmatis-Derived Glutamate Decarboxylase Active at Neutral pH. World J. Microbiol. Biotechnol. 2022, 38, 75. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, K.; Harris, E.B.; Kirchheimer, W.F. Glutamic Acid Decarboxylase in Mycobacterium Leprae. Arch. Microbiol. 1983, 134, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Snedden, W.A.; Arazi, T.; Fromm, H.; Shelp, B.J. Calcium/Calmodulin Activation of Soybean Glutamate Decarboxylase. Plant Physiol. 1995, 108, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yao, H.; Chen, F.; Wang, X. Purification and Characterization of Glutamate Decarboxylase from Rice Germ. Food Chem. 2007, 101, 1670–1676. [Google Scholar] [CrossRef]
- Satyanarayan, V.; Nair, P.M. Purification and Characterization of Glutamate Decarboxylase from Solanum Tuberosum. Eur. J. Biochem. 1985, 150, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Trobacher, C.P.; Zarei, A.; Liu, J.; Clark, S.M.; Bozzo, G.G.; Shelp, B.J. Calmodulin-Dependent and Calmodulin-Independent Glutamate Decarboxylases in Apple Fruit. BMC Plant Biol. 2013, 13, 144. [Google Scholar] [CrossRef]
- Yang, R.; Yin, Y.; Guo, Q.; Gu, Z. Purification, Properties and cDNA Cloning of Glutamate Decarboxylase in Germinated Faba Bean (Vicia faba L.). Food Chem. 2013, 138, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- On the Nature of Glutamic Acid Decarboxylase in Wheat Embryos 12|Plant Physiology|Oxford Academic. Available online: https://academic.oup.com/plphys/article/35/1/68/6089595?login=false (accessed on 8 August 2024).
- Mei, X.; Xu, X.; Yang, Z. Characterization of Two Tea Glutamate Decarboxylase Isoforms Involved in GABA Production. Food Chem. 2020, 305, 125440. [Google Scholar] [CrossRef]
- Kanwal, S.; Incharoensakdi, A. Characterization of Glutamate Decarboxylase from Synechocystis Sp. PCC6803 and Its Role in Nitrogen Metabolism. Plant Physiol. Biochem. 2016, 99, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hamel, E.; Krause, D.N.; Roberts, E. Characterization of Glutamic Acid Decarboxylase Activity in Cerebral Blood Vessels. J. Neurochem. 1982, 39, 842–849. [Google Scholar] [CrossRef]
- Wald, U.; Selzer, M.E.; Krieger, N.R. Glutamic Acid Decarboxylase in Sea Lamprey (Petromyzon marinus): Characterization, Localization, and Developmental Changes. J. Neurochem. 1981, 36, 363–368. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Matsuda, T.; Roberts, E. Purification and Characterization of Glutamate Decarboxylase from Mouse Brain. J. Biol. Chem. 1973, 248, 3029–3034. [Google Scholar] [CrossRef]
- A Glutamic Acid Decarboxylase (CgGAD) Highly Expressed in Hemocytes of Pacific Oyster Crassostrea Gigas. Dev. Comp. Immunol. 2016, 63, 56–65. [CrossRef]
- Grossfeld, R.M.; Yancey, S.W.; Baxter, C.F. Assay and Properties of Glutamic Acid Decarboxylase in Homogenates of Crayfish Nervous Tissue. Comp. Biochem. Physiol. Part B Comp. Biochem. 1984, 78, 287–298. [Google Scholar] [CrossRef]
- Chude, O.; Roberts, E.; Wu, J. Partial purification of drosophila glutamate decarboxylase. J. Neurochem. 1979, 32, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Meza, G. Some Characteristics of Glutamic Acid Decarboxylase of Chick Ampullary Cristae. J. Neurochem. 1984, 43, 634–639. [Google Scholar] [CrossRef]
- Steffen-Munsberg, F.; Vickers, C.; Kohls, H.; Land, H.; Mallin, H.; Nobili, A.; Skalden, L.; van den Bergh, T.; Joosten, H.-J.; Berglund, P.; et al. Bioinformatic Analysis of a PLP-Dependent Enzyme Superfamily Suitable for Biocatalytic Applications. Biotechnol. Adv. 2015, 33, 566–604. [Google Scholar] [CrossRef]
- Saxena, V.K.; Vedamurthy, G.V.; Singh, R. A Novel Concept of Pyridoxal 5’-Phosphate Permeability in E.Coli for Modulating the Heterologous Expression of PLP Dependent Proteins. Process Biochem. 2022, 113, 37–46. [Google Scholar] [CrossRef]
- Du, Y.-L.; Ryan, K.S. Pyridoxal Phosphate-Dependent Reactions in the Biosynthesis of Natural Products. Nat. Prod. Rep. 2019, 36, 430–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, R.; Sun, H.; Li, B. Quantum Chemistry Studies of the Catalysis Mechanism Differences between the Two Isoforms of Glutamic Acid Decarboxylase. J. Mol. Model. 2013, 19, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Biase, D.D.; Tramonti, A.; John, R.A.; Bossa, F. Isolation, Overexpression, and Biochemical Characterization of the Two Isoforms of Glutamic Acid Decarboxylase fromEscherichia Coli. Protein Expr. Purif. 1996, 8, 430–438. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Tramonti, A.; Bossa, F.; Visca, P. The Response to Stationary-Phase Stress Conditions in Escherichia coli: Role and Regulation of the Glutamic Acid Decarboxylase System. Mol. Microbiol. 1999, 32, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Capitani, G.; Biase, D.D.; Aurizi, C.; Gut, H.; Bossa, F.; Grütter, M.G. Crystal Structure and Functional Analysis of Escherichia Coli Glutamate Decarboxylase. EMBO J. 2003, 22, 4027–4037. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Brown, W.C.; Harms, E.; Smith, J.L. Crystal Structures Capture Three States in the Catalytic Cycle of a Pyridoxal Phosphate (PLP) Synthase. J. Biol. Chem. 2015, 290, 5226–5239. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fang, H.; Gai, Z.-C.; Mei, J.-Q.; Li, J.-N.; Hu, S.; Lv, C.-J.; Zhao, W.-R.; Mei, L.-H. Lactobacillus Brevis CGMCC 1306 Glutamate Decarboxylase: Crystal Structure and Functional Analysis. Biochem. Biophys. Res. Commun. 2018, 503, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Grzechowiak, M.; Sliwiak, J.; Jaskolski, M.; Ruszkowski, M. Structural and Functional Studies of Arabidopsis Thaliana Glutamate Dehydrogenase Isoform 2 Demonstrate Enzyme Dynamics and Identify Its Calcium Binding Site. Plant Physiol. Biochem. 2023, 201, 107895. [Google Scholar] [CrossRef]
- Arazi, T.; Baum, G.; Snedden, W.A.; Shelp, B.J.; Fromm, H. Molecular and Biochemical Analysis of Calmodulin Interactions with the Calmodulin-Binding Domain of Plant Glutamate Decarboxylase. Plant Physiol. 1995, 108, 551–561. [Google Scholar] [CrossRef]
- Gut, H.; Dominici, P.; Pilati, S.; Astegno, A.; Petoukhov, M.V.; Svergun, D.I.; Grütter, M.G.; Capitani, G. A Common Structural Basis for pH- and Calmodulin-Mediated Regulation in Plant Glutamate Decarboxylase. J. Mol. Biol. 2009, 392, 334–351. [Google Scholar] [CrossRef]
- Astegno, A.; Capitani, G.; Dominici, P. Functional Roles of the Hexamer Organization of Plant Glutamate Decarboxylase. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2015, 1854, 1229–1237. [Google Scholar] [CrossRef]
- Zik, M.; Arazi, T.; Snedden, W.A.; Fromm, H. Two Isoforms of Glutamate Decarboxylase in Arabidopsis Are Regulated by Calcium/Calmodulin and Differ in Organ Distribution. Plant Mol. Biol. 1998, 37, 967–975. [Google Scholar] [CrossRef]
- Tokumitsu, H.; Sakagami, H. Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction. Int. J. Mol. Sci. 2022, 23, 11025. [Google Scholar] [CrossRef]
- Teresinski, H.J.; Hau, B.; Symonds, K.; Kilburn, R.; Munro, K.A.; Doner, N.M.; Mullen, R.; Li, V.H.; Snedden, W.A. Arabidopsis Calmodulin-like Proteins CML13 and CML14 Interact with Proteins That Have IQ Domains. Plant Cell Environ. 2023, 46, 2470–2491. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Rainaldi, M.; Vogel, H.J. Structural Studies of Soybean Calmodulin Isoform 4 Bound to the Calmodulin-Binding Domain of Tobacco Mitogen-Activated Protein Kinase Phosphatase-1 Provide Insights into a Sequential Target Binding Mode*. J. Biol. Chem. 2009, 284, 28292–28305. [Google Scholar] [CrossRef]
- Pigott, T.; McPeak, A.; de Chastelain, A.; DeMayo, M.M.; Rasic, N.; Rayner, L.; Noel, M.; Miller, J.V.; Harris, A.D. Changes in Brain GABA and Glutamate and Improvements in Physical Functioning Following Intensive Pain Rehabilitation in Youth with Chronic Pain. J. Pain. 2023, 24, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.R.; Mabasa, L.; Crane, C.; Park, C.S.; Venâncio, V.P.; Bianchi, M.L.P.; Antunes, L.M.G. Maternal Vitamin B 6 Deficient or Supplemented Diets on Expression of Genes Related to GABAergic, Serotonergic, or Glutamatergic Pathways in Hippocampus of Rat Dams and Their Offspring. Mol. Nutr. Food Res. 2016, 60, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, T.; Oriuchi, N.; Yanagawa, Y. GAD65/GAD67 Double Knockout Mice Exhibit Intermediate Severity in Both Cleft Palate and Omphalocele Compared with GAD67 Knockout and VGAT Knockout Mice. Neuroscience 2015, 288, 86–93. [Google Scholar] [CrossRef]
- Reetz, A.; Solimena, M.; Matteoli, M.; Folli, F.; Takei, K.; De Camilli, P. GABA and Pancreatic Beta-Cells: Colocalization of Glutamic Acid Decarboxylase (GAD) and GABA with Synaptic-like Microvesicles Suggests Their Role in GABA Storage and Secretion. EMBO J. 1991, 10, 1275–1284. [Google Scholar] [CrossRef]
- Szpręgiel, I.; Wrońska, D.; Kmiecik, M.; Pałka, S.; Kania, B.F. Glutamic Acid Decarboxylase Concentration Changes in Response to Stress and Altered Availability of Glutamic Acid in Rabbit (Oryctolagus cuniculus) Brain Limbic Structures. Animals 2021, 11, 455. [Google Scholar] [CrossRef]
- Fenalti, G.; Law, R.H.P.; Buckle, A.M.; Langendorf, C.; Tuck, K.; Rosado, C.J.; Faux, N.G.; Mahmood, K.; Hampe, C.S.; Banga, J.P.; et al. GABA Production by Glutamic Acid Decarboxylase Is Regulated by a Dynamic Catalytic Loop. Nat. Struct. Mol. Biol. 2007, 14, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Langendorf, C.G.; Tuck, K.L.; Key, T.L.G.; Fenalti, G.; Pike, R.N.; Rosado, C.J.; Wong, A.S.M.; Buckle, A.M.; Law, R.H.P.; Whisstock, J.C. Structural Characterization of the Mechanism through Which Human Glutamic Acid Decarboxylase Auto-Activates. Biosci. Rep. 2013, 33, e00013. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Lei, D.; Lu, Y.; Huang, Q.; Wu, Y.; Yang, S.; Wu, Y. Parvalbumin in the Metabolic Pathway of Glutamate and γ-Aminobutyric Acid: Influence on Expression of GAD65 and GAD67. Arch. Biochem. Biophys. 2023, 734, 109499. [Google Scholar] [CrossRef]
- Lazarus, M.S.; Krishnan, K.; Huang, Z.J. GAD67 Deficiency in Parvalbumin Interneurons Produces Deficits in Inhibitory Transmission and Network Disinhibition in Mouse Prefrontal Cortex. Cereb. Cortex 2015, 25, 1290–1296. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, R.; Zhao, J.; Li, C.; Guo, T.; Yang, S.; Han, T.; Kong, J. Fast-Acidification Promotes GABA Synthesis in Response to Acid Stress in Streptococcus Thermophilus. LWT 2022, 164, 113671. [Google Scholar] [CrossRef]
- Sarasa, S.B.; Mahendran, R.; Muthusamy, G.; Thankappan, B.; Selta, D.R.F.; Angayarkanni, J. A Brief Review on the Non-Protein Amino Acid, Gamma-Amino Butyric Acid (GABA): Its Production and Role in Microbes. Curr. Microbiol. 2020, 77, 534–544. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, R.; Guo, Q.; Gu, Z. NaCl Stress and Supplemental CaCl2 Regulating GABA Metabolism Pathways in Germinating Soybean. Eur. Food Res. Technol. 2014, 238, 781–788. [Google Scholar] [CrossRef]
- Sun, Y.; Mehmood, A.; Battino, M.; Xiao, J.; Chen, X. Enrichment of Gamma-Aminobutyric Acid in Foods: From Conventional Methods to Innovative Technologies. Food Res. Int. 2022, 162, 111801. [Google Scholar] [CrossRef]
- Li, E.; Luo, X.; Liao, S.; Shen, W.; Li, Q.; Liu, F.; Zou, Y. Accumulation of Γ-aminobutyric Acid during Cold Storage in Mulberry Leaves. Int. J. Food Sci. Tech. 2018, 53, 2664–2672. [Google Scholar] [CrossRef]
- Pannerchelvan, S.; Rios-Solis, L.; Wong, F.W.F.; Zaidan, U.H.; Wasoh, H.; Mohamed, M.S.; Tan, J.S.; Mohamad, R.; Halim, M. Strategies for Improvement of Gamma-Aminobutyric Acid (GABA) Biosynthesis via Lactic Acid Bacteria (LAB) Fermentation. Food Funct. 2023, 14, 3929–3948. [Google Scholar] [CrossRef]
- Soma, Y.; Fujiwara, Y.; Nakagawa, T.; Tsuruno, K.; Hanai, T. Reconstruction of a Metabolic Regulatory Network in Escherichia Coli for Purposeful Switching from Cell Growth Mode to Production Mode in Direct GABA Fermentation from Glucose. Metab. Eng. 2017, 43, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhao, J.; Wang, Y.; Gao, J.; Du, M.; Zhang, Y.; Xu, N.; Du, H.; Ju, J.; Liu, Q.; et al. Engineering of Corynebacterium Glutamicum for High-Level γ-Aminobutyric Acid Production from Glycerol by Dynamic Metabolic Control. Metab. Eng. 2022, 69, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.-F.; Lyu, C.-J.; Cao, J.-R.; Mei, J.-Q.; Hu, S.; Zhao, W.-R.; Xu, T.-Y.; Wang, Y.-T.; Wang, D.-L.; Huang, J.; et al. High Level Production of γ-Aminobutyric Acid in Engineered Escherichia Coli by Refactoring the Glutamate Decarboxylase. Process Biochem. 2022, 118, 243–251. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Chen, J.; Zhang, Y.; Feng, Z. Expanding the pH Range of Glutamate Decarboxylase from L. Pltarum LC84 by Site-Directed Mutagenesis. Front. Bioeng. Biotechnol. 2023, 11, 1160818. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-Q.; Li, M.-W.; Qiu, Y.; Chen, Q.; Jiang, S.-J.; Shang, Y.-J.; Zhao, L.-M. Increasing Thermal Stability of Glutamate Decarboxylase from Escherichia. Coli by Site-Directed Saturation Mutagenesis and Its Application in GABA Production. J. Biotechnol. 2018, 278, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Gao, L.; Ge, R.; Cui, X.; Zhou, J.; Li, Z. Gamma-Aminobutyric Acid (GABA) Promotes Characteristics of Levilactobacillus Sp. LB-2. LWT 2023, 184, 115014. [Google Scholar] [CrossRef]
- Park, S.; Sohn, Y.J.; Park, S.J.; Choi, J. Effect of DR1558, a Deinococcus Radiodurans Response Regulator, on the Production of GABA in the Recombinant Escherichia Coli under Low pH Conditions. Microb. Cell Fact. 2020, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Edalatian Dovom, M.R.; Habibi Najafi, M.B.; Rahnama Vosough, P.; Norouzi, N.; Ebadi Nezhad, S.J.; Mayo, B. Screening of Lactic Acid Bacteria Strains Isolated from Iranian Traditional Dairy Products for GABA Production and Optimization by Response Surface Methodology. Sci. Rep. 2023, 13, 440. [Google Scholar] [CrossRef]
- Asun, A.C.; Lin, S.-T.; Ng, H.S.; Lan, J.C.-W. Production of Gamma-Aminobutyric Acid (GABA) by Bacillus Subtilis BBEL02 Fermentation Using Nitrogen-Rich Industrial Wastes as Crude Feedstocks. Biochem. Eng. J. 2022, 187, 108654. [Google Scholar] [CrossRef]
- Villegas, J.M.; Brown, L.; Savoy de Giori, G.; Hebert, E.M. Optimization of Batch Culture Conditions for GABA Production by Lactobacillus Brevis CRL 1942, Isolated from Quinoa Sourdough. LWT Food Sci. Technol. 2016, 67, 22–26. [Google Scholar] [CrossRef]
- Laroute, V.; Mazzoli, R.; Loubière, P.; Pessione, E.; Cocaign-Bousquet, M. Environmental Conditions Affecting GABA Production in Lactococcus Lactis NCDO 2118. Microorganisms 2021, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Ng, I.-S. Investigation of enzymatic quality and quantity using pyridoxal 5′-phosphate (PLP) regeneration system as a decoy in escherichia coli. Int. J. Biol. Macromol. 2023, 235, 123814. [Google Scholar] [CrossRef]
- Yang, X.; Huo, X.; Tang, Y.; Zhao, M.; Tao, Y.; Huang, J.; Ke, C. Integrating Enzyme Evolution and Metabolic Engineering to Improve the Productivity of Γ-Aminobutyric Acid by Whole-Cell Biosynthesis in Escherichia Coli. J. Agric. Food. Chem. 2023, 71, 4656–4664. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Gan, M.; Jin, Y.; Gao, X.; Chen, Q.; Guan, J.; Wang, Z. Application of Simultaneous Saccharification and Fermentation (SSF) from Viscosity Reducing of Raw Sweet Potato for Bioethanol Production at Laboratory, Pilot and Industrial Scales. Bioresour. Technol. 2011, 102, 4573–4579. [Google Scholar] [CrossRef]
- Han, J.; Zhao, X.; Zhao, X.; Wang, Q.; Li, P.; Gu, Q. Microbial-Derived γ-Aminobutyric Acid: Synthesis, Purification, Physiological Function, and Applications. J. Agric. Food. Chem. 2023, 71, 14931–14946. [Google Scholar] [CrossRef]
- Vosoughi, A.; Zendehdel, M.; Zarei, H.; Hassanpour, S. Central Effects of the Serotoninergic, GABAergic, and Cholecystokinin Systems on Neuropeptide VF (NPVF)-Induced Hypophagia and Feeding Behavior in Neonatal Broiler Chicken. Neurosci. Lett. 2024, 818, 137557. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Jain, M.R.; Horvath, T.L.; Diano, S.; Kalra, P.S.; Kalra, S.P. Interactions between Neuropeptide Y and γ -Aminobutyric Acid in Stimulation of Feeding: A Morphological and Pharmacological Analysis*. Endocrinology 1999, 140, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Saiyidi, N.M.; Nie, B.; Han, B.; Yang, K. Effects of γ-Aminobutyric Acid on the Growth Performance and Blood Indicators in Sheep with 6-8 Months Old. China Feed 2015, 20, 29–32. [Google Scholar]
- Chen, Q.; Liang, J. The Effect of γ-aminobutyric on Growth Performance and Serum Levels of Protein and Enzyme Activity in Weaned Piglets. Chin. Agric. Sci. Bull. 2012, 28, 47–50. [Google Scholar]
- Fan, Z.Y.; Deng, J.P.; Liu, G.H.; Cai, H.Y.; He, J.H.; Wu, M.X.; Zhen, F.Y. Effects of γ-Aminobutyric Acid on the Performance and Internal Hormone Levels in Growing Pigs. Chin. J. Anim. Nutr. 2007, 04, 350–356. [Google Scholar]
- Tanaka, C. γ-aminobutyric acid in peripheral tissues. Life Sci. 1985, 37, 2221–2235. [Google Scholar] [CrossRef] [PubMed]
- Xiang-Gui, X.; Zai-Fu, Y.; Shen-He, H.A.; Xiao-Hong, Y. Promotive Effects of Gaba on Acid Secretion from Isolated Mouse Stomach In Vitro. Acta Zool. Sin. 2001, 47, 170–175. [Google Scholar]
- Nakajima, K.; Tooyama, I.; Kuriyama, K.; Kimura, H. Immunohistochemical Demonstration of GABAB Receptors in the Rat Gastrointestinal Tract. Neurochem. Res. 1996, 21, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Auteri, M.; Zizzo, M.; Serio, R. The GABAergic System and the Gastrointestinal Physiopathology. Curr. Pharm. Des. 2015, 21, 4996–5016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jiang, X.; Ge, Y.; Xu, Q.; Li, Z.; Tang, H.; Cao, D.; Zhang, D. The Effects of GABA-Rich Adzuki Beans on Glycolipid Metabolism, as Well as Intestinal Flora, in Type 2 Diabetic Mice. Front. Nutr. 2022, 9, 849529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Wang, H.; Huang, Y.; Qi, M.; Liao, S.; Bin, P.; Yin, Y. Effects of GABA Supplementation on Intestinal SIgA Secretion and Gut Microbiota in the Healthy and ETEC-Infected Weanling Piglets. Mediat. Inflamm. 2020, 2020, 7368483. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.C.; Draguhn, A. GABA Metabolism and Transport: Effects on Synaptic Efficacy. Neural Plast. 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.-M.E.; Shehata, A.M.; Khidr, R.E.; Paswan, V.K.; Ibrahim, N.S.; El-Ghoul, A.A.; Aldhumri, S.A.; Gabr, S.A.; Mesalam, N.M.; Elbaz, A.M.; et al. Nutritional Manipulation to Combat Heat Stress in Poultry—A Comprehensive Review. J. Therm. Biol. 2021, 98, 102915. [Google Scholar] [CrossRef] [PubMed]
- Al Wakeel, R.A.; Shukry, M.; Abdel Azeez, A.; Mahmoud, S.; Saad, M.F. Alleviation by Gamma Amino Butyric Acid Supplementation of Chronic Heat Stress-Induced Degenerative Changes in Jejunum in Commercial Broiler Chickens. Ann. Ny. Acad. Sci. 2017, 20, 562–572. [Google Scholar] [CrossRef]
- Park, K.-T.; Oh, M.; Joo, Y.; Han, J.-K. Effects of Gamma Aminobutyric Acid on Performance, Blood Cell of Broiler Subjected to Multi-Stress Environments. Anim. Biosci. 2023, 36, 248–255. [Google Scholar] [CrossRef]
- Ullah, A.; Jahan, S.; Razak, S.; Pirzada, M.; Ullah, H.; Almajwal, A.; Rauf, N.; Afsar, T. Protective Effects of GABA against Metabolic and Reproductive Disturbances in Letrozole Induced Polycystic Ovarian Syndrome in Rats. J. Ovarian Res. 2017, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Yin, J.; Yang, W.; Shi, B.; Shan, A. Effects of Dietary γ-Aminobutyric Acid Supplementation on Antioxidant Status, Blood Hormones and Meat Quality in Growing-Finishing Pigs Undergoing Transport Stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 590–596. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, R.; Long, D.; Xie, Y.; Jiang, S. Effects of γ-aminobutyric Acid on Growth Performance and Meat Quality of Finishing Pigs under the Hot and Hu-mid Conditions. Chin. J. Anim. Sci. 2016, 52, 50–54. [Google Scholar]
- Koop, H.; Arnold, R. Control of Rat Gastric Somatostatin Release by γ-Aminobutyric Acid (GABA). Horm. Metab. Res. 1986, 18, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Harty, R.F.; Franklin, P.A. GABA Affects the Release of Gastrin and Somatostatin from Rat Antral Mucosa. Nature 1983, 303, 623–624. [Google Scholar] [CrossRef]
- Ferone, D.; Van Hagen, P.M.; Semino, C.; Dalm, V.A.; Barreca, A.; Colao, A.; Lamberts, S.W.J.; Minuto, F.; Hofland, L.J. Somatostatin Receptor Distribution and Function in Immune System. Digest. Liver Dis. 2004, 36, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Wiens, S.C.; Trudeau, V.L. Thyroid Hormone and γ-Aminobutyric Acid (GABA) Interactions in Neuroendocrine Systems. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xiao, L.; Xiao, Y.; Chen, C. The Modulatory Role of Growth Hormone in Inflammation and Macrophage Activation. Endocrinology 2022, 163, bqac088. [Google Scholar] [CrossRef]
- Paul, V. Inhibition of Acute Hyperammonemia-Induced Convulsions by Systemically Administered Gamma Aminobutyric Acid in Rats. Pharmacol. Biochem. Behav. 2003, 74, 523–528. [Google Scholar] [CrossRef]
- Fiszman, M.L.; Schousboe, A. Role of Calcium and Kinases on the Neurotrophic Effect Induced by Γ-aminobutyric Acid. J. Neurosci. Res. 2004, 76, 435–441. [Google Scholar] [CrossRef]
- Mumtaz, F.; Khan, M.I.; Zubair, M.; Dehpour, A.R. Neurobiology and Consequences of Social Isolation Stress in Animal Model—A Comprehensive Review. Biomed. Pharmacother. 2018, 105, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Liwinski, T.; Lang, U.E.; Brühl, A.B.; Schneider, E. Exploring the Therapeutic Potential of Gamma-Aminobutyric Acid in Stress and Depressive Disorders through the Gut–Brain Axis. Biomedicines 2023, 11, 3128. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Yuan, H.; Chen, S.; Yu, Y.; Zhang, X.; Gao, Z.; Du, H.; Li, W.; Wang, Y.; et al. Prophylactic Supplementation with Lactobacillus Reuteri or Its Metabolite GABA Protects against Acute Ischemic Cardiac Injury. Adv. Sci. 2024, 11, 2307233. [Google Scholar] [CrossRef]
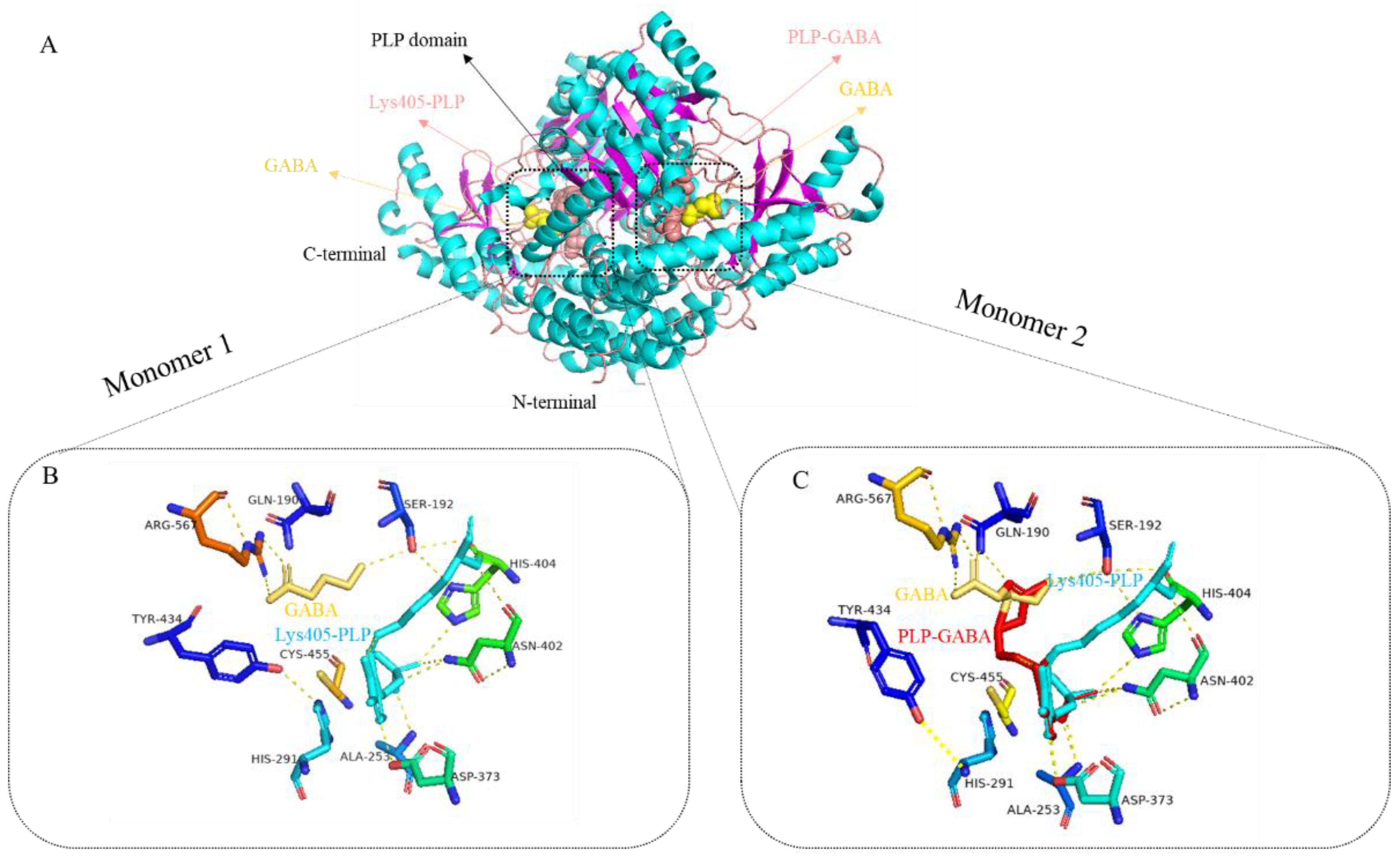
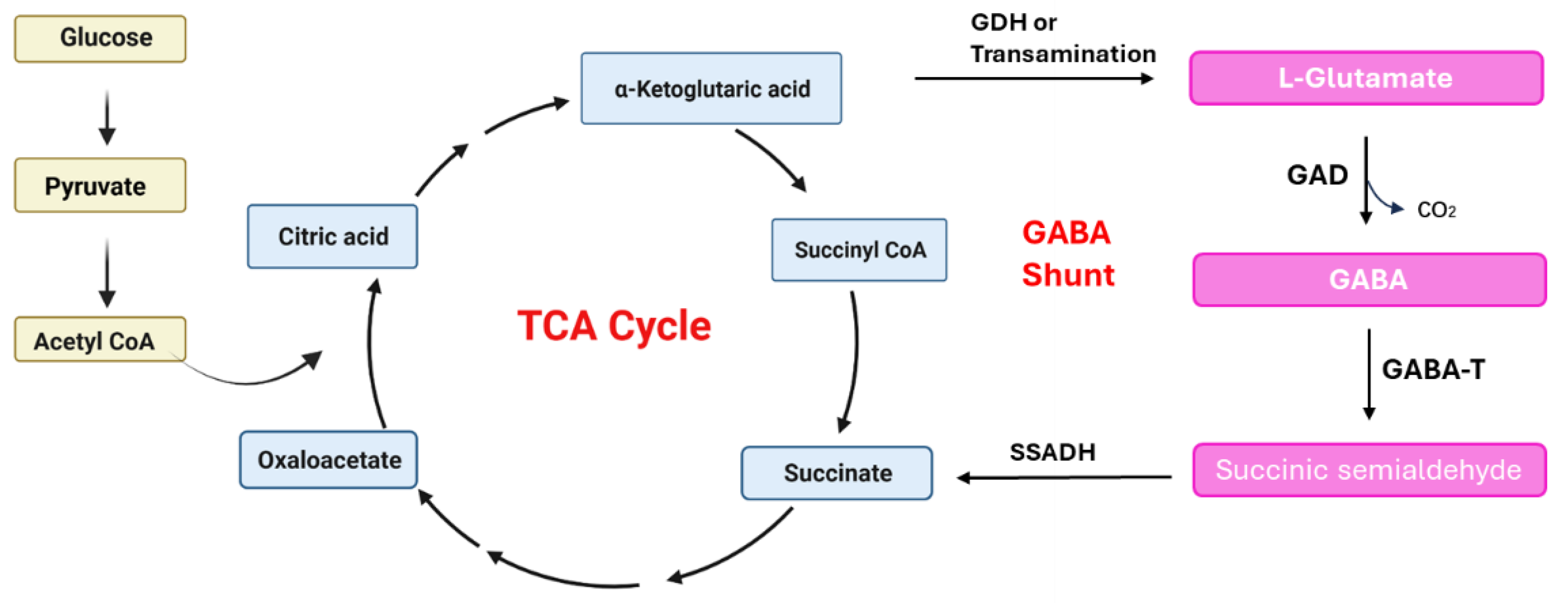

| Microorganism | Optimum Temperature (°C) | Optimum pH | References |
|---|---|---|---|
| E. coli | 37 °C | 4.8~5.0 | [25] |
| L. plantarum EJ2014 | 30 °C | 4.0~5.0 | [26] |
| K. marxianus C21 | 35 °C | 4.0 | [27] |
| Lb. zymae GU240 | 41 °C | 4.5 | [28] |
| E.s faecium | 45 °C | 6.6 | [29] |
| Pyrococcus horikoshii | 60 °C | - | [30] |
| E. sulfureus | 55 °C | 4 | [31] |
| L. lactis | 55 °C | 5.5 | [31] |
| L. senmaizukei | 40 °C | 5.5 | [31] |
| L. brevis | 50 °C | 4.5 | [31] |
| B. thetaiotaomicron VPI-5482 | 60 °C | 3.6 | [32] |
| L. garvieae MJF010 | 35 °C | 5 | [33] |
| M. smegmatis | - | 5.4 | [34] |
| Mycobacterium leprae | 37 °C | 4.5 | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Wang, Z.; Gao, L.; Chen, X. Unraveling the Potential of γ-Aminobutyric Acid: Insights into Its Biosynthesis and Biotechnological Applications. Nutrients 2024, 16, 2760. https://doi.org/10.3390/nu16162760
Zhu L, Wang Z, Gao L, Chen X. Unraveling the Potential of γ-Aminobutyric Acid: Insights into Its Biosynthesis and Biotechnological Applications. Nutrients. 2024; 16(16):2760. https://doi.org/10.3390/nu16162760
Chicago/Turabian StyleZhu, Lei, Zhefeng Wang, Le Gao, and Xiaoyi Chen. 2024. "Unraveling the Potential of γ-Aminobutyric Acid: Insights into Its Biosynthesis and Biotechnological Applications" Nutrients 16, no. 16: 2760. https://doi.org/10.3390/nu16162760
APA StyleZhu, L., Wang, Z., Gao, L., & Chen, X. (2024). Unraveling the Potential of γ-Aminobutyric Acid: Insights into Its Biosynthesis and Biotechnological Applications. Nutrients, 16(16), 2760. https://doi.org/10.3390/nu16162760







