Impact of Vitamin D Status and Nutrition on the Occurrence of Long Bone Fractures Due to Falls in Elderly Subjects in the Vojvodina Region of Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations and Study Participants
- 65 or over years of age
- Fracture of a long bone to a ground-level fall
- Voluntary consent of a patient to participate in the study
- 65 or over years of age
- No fracture of a long bone to a ground-level fall
- Voluntary consent of a patient to participate in the study
- Patients under the age of 65
- Refusal of the patient to participate in the study
- Inability to give written consent
- Foreign citizens
- Communication problems
- Death in the course of the study
- Vitamin D and calcium substitution therapy started less than 6 months ago
- Exposure to the UV-B light 1 month before the start of the test (going to the sea, mountain, and solarium).
- Medical conditions that can influence the overall/bone health or vitamin D status (kidney diseases, malignant diseases, hematologic disorders, idiopathic hypercalciuria, diabetes mellitus type 1, parathyroid gland diseases, adrenal gland diseases, acromegaly, rheumatoid diseases, chronic gastrointestinal and liver disorders—inflammatory bowel diseases, celiac disease, gastrointestinal resection and bariatric surgery, cystic fibrosis, neurological disorders, long-term immobilization, etc.) [33].
- Long-term uses of medications connected with vitamin D, calcium, and bone metabolism (bisphosphonates, calcitonin, corticosteroids, antiepileptic drugs, SSRIs, thyroxin, TSH, gonadotropin-releasing hormone antagonists, progestins, tamoxifen, loop diuretics, aluminum-containing antacids, chemotherapy, and heparin) [34].
- Missing any presented data
2.2. Required Sample Size Calculation
2.3. Socioeconomic, Medical, Lifestyle, Anthropometric, and Blood Pressure Data
2.4. Dietary Intakes
2.5. Determination of Vitamin D Status
2.6. Statistics
3. Results
3.1. General Data
3.2. The Dietary Intake of Energy and Macronutrients
3.3. The Intake of Different Food Groups
3.4. The Intake of Vitamin D and Calcium through Food and Supplements
3.5. Serum Vitamin D Status
3.6. Logistic Regression Models for Predicting the Risk of Fractures
3.7. ROC Curve Analysis for Serum Vitamin D Cutoff Values for the Increased Risk for Fractures
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.-M.; Bisignano, C.; James, S.L.; Abady, G.G.; Abedi, A.; Abu-Gharbieh, E.; Alhassan, R.K.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. GBD 2019 Fracture Collaborators. Global, Regional, and National Burden of Bone Fractures in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Ristic, B.; Stojadinovic, I.; Ilic, M. Epidemiology of Hip Fractures Due to Falls. Medicina 2023, 59, 1528. [Google Scholar] [CrossRef] [PubMed]
- Singaram, S.; Naidoo, M. The Physical, Psychological and Social Impact of Long Bone Fractures on Adults: A Review. Afr. J. Prim. Health Care Fam. Med. 2019, 11, a1908. [Google Scholar] [CrossRef]
- Brennan, M.; O’Shea, P.M.; O’Keeffe, S.T.; Mulkerrin, E.C. Spontaneous Insufficiency Fractures. J. Nutr. Health Aging 2019, 23, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Li, J.; Hao, S.; Liu, M.; Jin, H.; Zheng, L.; Xia, M.; Jin, B.; Zhu, C.; Alfreds, S.T.; et al. Identification of Elders at Higher Risk for Fall with Statewide Electronic Health Records and a Machine Learning Algorithm. Int. J. Med. Inform. 2020, 137, 104105. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.B.; Lee, S.K.; An, Y.S.; Kim, W.; Choy, W.S. The Clinical Necessity of a Distal Forearm DEXA Scan for Predicting Distal Radius Fracture in Elderly Females: A Retrospective Case-Control Study. BMC Musculoskelet. Disord. 2023, 24, 177. [Google Scholar] [CrossRef] [PubMed]
- Tulipan, J.; Jones, C.M.; Ilyas, A.M. The Effect of Osteoporosis on Healing of Distal Radius Fragility Fractures. Orthop. Clin. N. Am. 2015, 46, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Senohradski, K.; Markovic-Denic, L.; Lesic, A.; Bumbasirevic, V.; Bumbasirevic, M. Trends in the Incidence of Hip Fractures. Osteoporos. Int. 2013, 24, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Seppala, L.J.; Wermelink, A.M.A.T.; de Vries, M.; Ploegmakers, K.J.; van de Glind, E.M.M.; Daams, J.G.; van der Velde, N.; Blain, H.; Bousquet, J.; Bucht, G.; et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-Analysis: II. Psychotropics. J. Am. Med. Dir. Assoc. 2018, 19, 371.e11–371.e17. [Google Scholar] [CrossRef]
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Khurrum, M.; Chehab, M.; Ditillo, M.; Richards, J.; Douglas, M.; Bible, L.; Spece, L.; Joseph, B. Trends in Geriatric Ground-Level Falls: Report from the National Trauma Data Bank. J. Surg. Res. 2021, 266, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Court-Brown, C.M.; Duckworth, A.D.; Clement, N.D.; McQueen, M.M. Fractures in Older Adults. A View of the Future? Injury 2018, 49, 2161–2166. [Google Scholar] [CrossRef]
- Haleem, S.; Choudri, M.J.; Kainth, G.S.; Parker, M.J. Mortality Following Hip Fracture: Trends and Geographical Variations over the Last SIXTY Years. Injury 2023, 54, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Merron, G.; Coughlan, T. 1 Year Mortality after Hip Fracture in an Irish Urban Trauma Centre. BMC Musculoskelet. Disord. 2023, 24, 487. [Google Scholar] [CrossRef]
- Dimet-Wiley, A.; Golovko, G.; Watowich, S.J. One-Year Postfracture Mortality Rate in Older Adults with Hip Fractures Relative to Other Lower Extremity Fractures: Retrospective Cohort Study. JMIR Aging 2022, 5, e32683. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Y.; Ji, J.; Chang, J.; Yu, S.; Yu, B. The Relationship between Serum Vitamin D and Fracture Risk in the Elderly: A Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 81. [Google Scholar] [CrossRef]
- Zheng, R.; Byberg, L.; Larsson, S.C.; Höijer, J.; Baron, J.A.; Michaëlsson, K. Prior Loss of Body Mass Index, Low Body Mass Index, and Central Obesity Independently Contribute to Higher Rates of Fractures in Elderly Women and Men. J. Bone Miner. Res. 2021, 36, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Bifolco, G.; Caffarelli, C.; Mazziotti, G.; Migliaccio, S.; Napoli, N.; Ruggiero, C.; Cipriani, C. Nutrition, Vitamin D, and Calcium in Elderly Patients before and after a Hip Fracture and Their Impact on the Musculoskeletal System: A Narrative Review. Nutrients 2024, 16, 1773. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Xiao, X.; Zhang, X.; He, L.; Zhang, Q. Association of Dietary Carbohydrate and Fiber Ratio with Postmenopausal Bone Mineral Density and Prevalence of Osteoporosis: A Cross-Sectional Study. PLoS ONE 2024, 19, e0297332. [Google Scholar] [CrossRef]
- Luokkala, T.; Laitinen, M.K.; Hevonkorpi, T.P.; Raittio, L.; Mattila, V.M.; Launonen, A.P. Distal Radius Fractures in the Elderly Population. EFORT Open Rev. 2020, 5, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Karpouzos, A.; Diamantis, E.; Farmaki, P.; Savvanis, S.; Troupis, T. Nutritional Aspects of Bone Health and Fracture Healing. J. Osteoporos. 2017, 2017, 4218472. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Maleitzke, T.; Eder, C.; Ahmad, S.; Stöckle, U.; Braun, K.F. Management of Proximal Femur Fractures in the Elderly: Current Concepts and Treatment Options. Eur. J. Med. Res. 2021, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Reginster, J.-Y.; Cabrerizo, S.; Bruyère, O.; Kanis, J.; Martinez, J.; Zulet, M. Nutritional Status and Nutritional Treatment Are Related to Outcomes and Mortality in Older Adults with Hip Fracture. Nutrients 2018, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Cass, A.R.; Charlton, K.E. Prevalence of Hospital-acquired Malnutrition and Modifiable Determinants of Nutritional Deterioration during Inpatient Admissions: A Systematic Review of the Evidence. J. Hum. Nutr. Diet. 2022, 35, 1043–1058. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.A.; Morrison, M.M. For You Were Hungry and I Gave You Food: The Prevalence and Treatment of Malnutrition in Patients with Acute Hip Fracture. Nutr. Clin. Pract. 2022, 37, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Wilson, J.M.; Ahn, J.; Shapiro, M.; Schenker, M.L. Malnutrition and the Orthopaedic Trauma Patient: A Systematic Review of the Literature. J. Orthop. Trauma 2018, 32, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Polzonetti, V.; Pucciarelli, S.; Vincenzetti, S.; Polidori, P. Dietary Intake of Vitamin D from Dairy Products Reduces the Risk of Osteoporosis. Nutrients 2020, 12, 1743. [Google Scholar] [CrossRef]
- Van Schoor, N.; Lips, P. Worldwide Vitamin D Status. In Vitamin D; Elsevier: Amsterdam, The Netherlands, 2018; pp. 15–40. [Google Scholar]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Jovičić, S.; Ignatović, S.; Kangrga, R.; Beletić, A.; Mirković, D.; Majkić-Singh, N. Comparison of Three Different Methods for 25(OH)-Vitamin D Determination and Vitamin D Status in General Population—Serbian Experience. J. Med. Biochem. 2012, 31, 347–357. [Google Scholar] [CrossRef]
- Ganesan, K.; Jandu, J.S.; Anastasopoulou, C.; Ahsun, S.; Roane, D. Secondary Osteoporosis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Audran, M. Iatrogenic Demineralizing Osteopathies. Presse Méd. 1994, 23, 271–273. [Google Scholar]
- Ždrale, I.; Ćorić, A. Impact of Nutrition on the Occurrence of Long Bone Fractures in Elderly Patients. In Proceedings of the 62nd Congress of the Students of Biomedical Sciences, with an International Contribution, Kopaonik, Serbia, 24–28 April 2023; p. 916. [Google Scholar]
- Hulley, S.B.; Cummings, S.R.; Browner, W.S.; Grady, D.G.; Newman, T.B. Designing Clinical Research: An Epidemiologic Approach, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A Simulation Study of the Number of Events per Variable in Logistic Regression Analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Guidance on the EU Menu Methodology. EFSA J. 2014, 12, 3944. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and Managing the Global Epidemic; WHO Technical Report Series, No. 894; WHO: Geneva, Switzerland, 1999. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Gurinović, M.; Zeković, M.; Milešević, J.; Nikolić, M.; Glibetić, M. Nutritional Assessment. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Gurinović, M.; Milešević, J.; Kadvan, A.; Nikolić, M.; Zeković, M.; Djekić-Ivanković, M.; Dupouy, E.; Finglas, P.; Glibetić, M. Development, Features and Application of DIET ASSESS & PLAN (DAP) Software in Supporting Public Health Nutrition Research in Central Eastern European Countries (CEEC). Food Chem. 2018, 238, 186–194. [Google Scholar] [CrossRef]
- Milešević, J.; Samaniego, L.; Kiely, M.; Glibetić, M.; Roe, M.; Finglas, P. Specialized Food Composition Dataset for Vitamin D Content in Foods Based on European Standards: Application to Dietary Intake Assessment. Food Chem. 2018, 240, 544–549. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. December 2020. Available online: https://www.DietaryGuidelines.gov (accessed on 8 August 2024).
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for vitamin D. EFSA J. 2016, 14, e4547. [Google Scholar] [CrossRef]
- Avci, E.; Demir, S.; Aslan, D.; Nar, R.; Şenol, H. Assessment of Abbott Architect 25-OH Vitamin D Assay in Different Levels of Vitamin D. J. Med. Biochem. 2020, 39, 100–107. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930, Correction in J. Clin. Endocrinol. Metab. 2024, 2024, dgae373. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Fuleihan, G.E.; Josse, R.G.; Lips, P.; Morales-Torres, J.; Yoshimura, N. IOF position statement: Vitamin D recommendations for older adults. Osteoporos. Int. 2010, 21, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. J. Am. Geriatr. Soc. 2014, 62, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Nahm, F.S. Receiver Operating Characteristic Curve: Overview and Practical Use for Clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Rizzoli, R.; Biver, E.; Bonjour, J.P.; Coxam, V.; Goltzman, D.; Kanis, J.A.; Lappe, J.; Rejnmark, L.; Sahni, S.; Weaver, C.; et al. Benefits and safety of dietary protein for bone health-an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos. Int. 2018, 29, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Baerlocher, K.; Bauer, J.M.; Elmadfa, I.; Heseker, H.; Leschik-Bonnet, E.; Stangl, G.; Volkert, D.; Stehle, P.; on behalf of the German Nutrition Society (DGE). Revised Reference Values for the Intake of Protein. Ann. Nutr. Metab. 2019, 74, 242–250. [Google Scholar] [CrossRef]
- Chotiyarnwong, P.; McCloskey, E.V.; Harvey, N.C.; Lorentzon, M.; Prieto-Alhambra, D.; Abrahamsen, B.; Adachi, J.D.; Borgström, F.; Bruyere, O.; Carey, J.J.; et al. Is It Time to Consider Population Screening for Fracture Risk in Postmenopausal Women? A Position Paper from the International Osteoporosis Foundation Epidemiology/Quality of Life Working Group. Arch. Osteoporos. 2022, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Chandanwale, R.; Pundkar, A.; Kanani, K.; Bukhari, R.; Khan, S. Increased Risk of Osteoporosis in Postmenopausal Women: Review. Pravara Med. Rev. 2022, 14, 37–42. [Google Scholar]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Osteoporosis in the World: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Sørensen, N.N.; Andreasen, C.M.; Jensen, P.R.; Hauge, E.M.; Bollerslev, J.; Delaissé, J.-M.; Kassem, M.; Jafari, A.; Diaz-delCastillo, M.; Andersen, T.L. Disturbed Bone Marrow Adiposity in Patients with Cushing’s Syndrome and Glucocorticoid- and Postmenopausal- Induced Osteoporosis. Front. Endocrinol. 2023, 14, 1232574. [Google Scholar] [CrossRef]
- Charde, S.H.; Joshi, A.; Raut, J. A Comprehensive Review on Postmenopausal Osteoporosis in Women. Cureus 2023, 15, e48582. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.J.; Beeram, I.; Florance, J.; Momenzadeh, K.; Mohamadi, A.; Rodriguez, E.K.; von Keudell, A.; Nazarian, A. Modifiable Lifestyle Factors Associated with Fragility Hip Fracture: A Systematic Review and Meta-Analysis. J. Bone Miner. Metab. 2021, 39, 893–902. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, Physical Activity, and Dietary Supplementation to Prevent Bone Mineral Density Loss: A Food Pyramid. Nutrients 2021, 14, 74. [Google Scholar] [CrossRef]
- Cauley, J.A. Osteoporosis: Fracture Epidemiology Update 2016. Curr. Opin. Rheumatol. 2017, 29, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Marinelli Busilacchi, E.; Morsia, E.; Poloni, A. Bone Marrow Adipose Tissue. Cells 2024, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Sebo, Z.L.; Rendina-Ruedy, E.; Ables, G.P.; Lindskog, D.M.; Rodeheffer, M.S.; Fazeli, P.K.; Horowitz, M.C. Bone Marrow Adiposity: Basic and Clinical Implications. Endocr. Rev. 2019, 40, 1187–1206. [Google Scholar] [CrossRef]
- Dzubanova, M.; Benova, A.; Ferencakova, M.; Coupeau, R.; Tencerova, M. Nutrition and Bone Marrow Adiposity in Relation to Bone Health. Physiol. Res. 2024, 73, 1–32. [Google Scholar]
- Je, M.; Kang, K.; Yoo, J.-I.; Kim, Y. The Influences of Macronutrients on Bone Mineral Density, Bone Turnover Markers, and Fracture Risk in Elderly People: A Review of Human Studies. Nutrients 2023, 15, 4386. [Google Scholar] [CrossRef]
- Kerstetter, J.E.; O’Brien, K.O.; Caseria, D.M.; Wall, D.E.; Insogna, K.L. The Impact of Dietary Protein on Calcium Absorption and Kinetic Measures of Bone Turnover in Women. J. Clin. Endocrinol. Metab. 2005, 90, 26–31. [Google Scholar] [CrossRef]
- Al-Aama, T. Falls in the Elderly: Spectrum and Prevention. Can. Fam. Physician 2011, 57, 771–776. [Google Scholar] [PubMed]
- Institute of Medicine (US) Division of Health Promotion and Disease Prevention; Berg, R.L.; Cassells, J.S. (Eds.) Falls in Older Persons: Risk Factors and Prevention. In The Second Fifty Years: Promoting Health and Preventing Disability; National Academies Press: Washington, DC, USA, 1992; p. 15. Available online: https://www.ncbi.nlm.nih.gov/books/NBK235613/ (accessed on 20 July 2024).
- Meng, X.; Zhu, K.; Devine, A.; Kerr, D.A.; Binns, C.W.; Prince, R.L. A 5-Year Cohort Study of the Effects of High Protein Intake on Lean Mass and BMC in Elderly Postmenopausal Women. J. Bone Miner. Res. 2009, 24, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Greenwood, D.C.; Cade, J.E. Foods, nutrients and hip fracture risk: A prospective study of middle-aged women. Clin. Nutr. 2022, 41, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chen, S.; Wang, X.; Jin, J.; Li, X.; Li, Z. Association between muscle strength and mass and bone mineral density in the US general population: Data from NHANES 1999–2002. J. Orthop. Surg. Res. 2023, 18, 397. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein Intake and Exercise for Optimal Muscle Function with Aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Gacs, G.; Barltrop, D. Significance of Ca-Soap Formation for Calcium Absorption in the Rat. Gut 1977, 18, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Yu, X. Fat, Sugar, and Bone Health: A Complex Relationship. Nutrients 2017, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dellatore, P.; Douard, V.; Qin, L.; Watford, M.; Ferraris, R.P.; Lin, T.; Shapses, S.A. High Fat Diet Enriched with Saturated, but Not Monounsaturated Fatty Acids Adversely Affects Femur, and Both Diets Increase Calcium Absorption in Older Female Mice. Nutr. Res. 2016, 36, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, O.; Djafarian, K.; Ghorabi, S.; Khodadost, M.; Nasiri, M.; Shab-Bidar, S. Dietary Intake of Fish, n-3 Polyunsaturated Fatty Acids and Risk of Hip Fracture: A Systematic Review and Meta-Analysis on Observational Studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1320–1333. [Google Scholar] [CrossRef]
- Benova, A.; Ferencakova, M.; Bardova, K.; Funda, J.; Prochazka, J.; Spoutil, F.; Cajka, T.; Dzubanova, M.; Balcaen, T.; Kerckhofs, G.; et al. Omega-3 PUFAs Prevent Bone Impairment and Bone Marrow Adiposity in Mouse Model of Obesity. Commun. Biol. 2023, 6, 1043. [Google Scholar] [CrossRef]
- Orchard, T.S.; Pan, X.; Cheek, F.; Ing, S.W.; Jackson, R.D. A Systematic Review of Omega-3 Fatty Acids and Osteoporosis. Br. J. Nutr. 2012, 107, S253–S260. [Google Scholar] [CrossRef] [PubMed]
- Longo, A.B.; Ward, W.E. PUFAs, Bone Mineral Density, and Fragility Fracture: Findings from Human Studies. Adv. Nutr. 2016, 7, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.S.; Ing, S.W.; Lu, B.; Belury, M.A.; Johnson, K.; Wactawski-Wende, J.; Jackson, R.D. The Association of Red Blood Cell N-3 and n-6 Fatty Acids with Bone Mineral Density and Hip Fracture Risk in the Women’s Health Initiative. J. Bone Miner. Res. 2013, 28, 505–515. [Google Scholar] [CrossRef]
- Mangano, K.M.; Sahni, S.; Kerstetter, J.E.; Kenny, A.M.; Hannan, M.T. Polyunsaturated fatty acids and their relation with bone and muscle health in adults. Curr. Osteoporos. Rep. 2013, 11, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xie, C.; Yang, J.; Tian, C.; Zhang, M.; Lu, Z.; Meng, X.; Cai, J.; Guo, X.; Gao, T. The Effects of n-3 PUFA Supplementation on Bone Metabolism Markers and Body Bone Mineral Density in Adults: A Systematic Review and Meta-Analysis of RCTs. Nutrients 2023, 15, 2806. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, K.; Wei, F.; Ballesteros, A.; Meckmongkol, T.; Calder, A.; Gilbertson, T.; Orlovskaya, N.; Coathup, M.J. Do Polyunsaturated Fatty Acids Protect against Bone Loss in Our Aging and Osteoporotic Population? Bone 2021, 143, 115736. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, S.; Juonala, M.; Fogelholm, M.; Pahkala, K.; Laaksonen, M.; Kähönen, M.; Sievänen, H.; Viikari, J.; Raitakari, O. Dietary Saturated Fat and Bone Health in Young Adults: The Young Finns Cohort. Calcif. Tissue Int. 2022, 111, 419–429. [Google Scholar] [CrossRef]
- Fang, Z.-B.; Wang, G.-X.; Cai, G.-Z.; Zhang, P.-X.; Liu, D.-L.; Chu, S.-F.; Li, H.-L.; Zhao, H.-X. Association between Fatty Acids Intake and Bone Mineral Density in Adults Aged 20–59: NHANES 2011–2018. Front. Nutr. 2023, 10, 1033195. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.; Djafarian, K.; Mofrad, M.D.; Shab-Bidar, S. Dietary Fat, Saturated Fatty Acid, and Monounsaturated Fatty Acid Intakes and Risk of Bone Fracture: A Systematic Review and Meta-Analysis of Observational Studies. Osteoporos. Int. 2018, 29, 1949–1961. [Google Scholar] [CrossRef]
- Martínez-Ramírez, M.J.; Palma, S.; Martínez-González, M.A.; Delgado-Martínez, A.D.; de la Fuente, C.; Delgado-Rodríguez, M. Dietary Fat Intake and the Risk of Osteoporotic Fractures in the Elderly. Eur. J. Clin. Nutr. 2007, 61, 1114–1120. [Google Scholar] [CrossRef]
- Corwin, R.L.; Hartman, T.J.; Maczuga, S.A.; Graubard, B.I. Dietary saturated fat intake is inversely associated with bone density in humans: Analysis of NHANES III. J. Nutr. 2006, 136, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Yoo, H.J.; Park, S.J.; Kwak, M.K.; Lee, S.H.; Kim, S.J.; Hamrick, M.W.; Isales, C.M.; Ahn, S.H.; Koh, J.-M. Association of Blood N-3 Fatty Acid with Bone Mass and Bone Marrow TRAP-5b in the Elderly with and without Hip Fracture. Osteoporos. Int. 2019, 30, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Coetzer, H.; Claassen, N.; van Papendorp, D.H.; Kruger, M.C. Calcium Transport by Isolated Brush Border and Basolateral Membrane Vesicles: Role of Essential Fatty Acid Supplementation. Prostaglandins Leukot. Essent. Fatty Acids 1994, 50, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-M.; Kim, G.W.; Yim, H.W.; Paek, Y.J.; Lee, K.-S. Association between Dietary Fat Intake and Bone Mineral Density in Korean Adults: Data from Korea National Health and Nutrition Examination Survey IV (2008∼2009). Osteoporos. Int. 2015, 26, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Beom, S.H.; Lee, S.H.; Koh, J.-M.; Kim, B.-J.; Kim, T.H. Differential Association of Dietary Fat Intake with DXA-Based Estimates of Bone Strength According to Sex in the KNHANES IV Population. Arch. Osteoporos. 2020, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, M.; Ma, H.; Li, X.; Heianza, Y.; Qi, L. Dietary Fiber, Genetic Variations of Gut Microbiota-Derived Short-Chain Fatty Acids, and Bone Health in UK Biobank. J. Clin. Endocrinol. Metab. 2021, 106, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, Y.; Lu, N.; Felson, D.T.; Kiel, D.P.; Sahni, S. Association Between Dietary Fiber Intake and Bone Loss in the Framingham Offspring Study. J. Bone Miner. Res. 2018, 33, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Suh, H.S. Associations between Dietary Fiber Intake and Bone Mineral Density in Adult Korean Population: Analysis of National Health and Nutrition Examination Survey in 2011. J. Bone Metab. 2019, 26, 151. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, J.; Zhang, X.; Geng, B. Dietary Fiber Intake and Femoral Bone Mineral Density in Middle-Aged and Older US Adults: A Cross-Sectional Study of National Health and Nutrition Examination Survey 2013–2014. Front. Nutr. 2022, 9, 851820. [Google Scholar] [CrossRef]
- Travinsky-Shmul, T.; Beresh, O.; Zaretsky, J.; Griess-Fishheimer, S.; Rozner, R.; Kalev-Altman, R.; Penn, S.; Shahar, R.; Monsonego-Ornan, E. Ultra-Processed Food Impairs Bone Quality, Increases Marrow Adiposity and Alters Gut Microbiome in Mice. Foods 2021, 10, 3107. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The Gut-Bone Axis: How Bacterial Metabolites Bridge the Distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Nutritional Influence on Bone: Role of Gut Microbiota. Aging Clin. Exp. Res. 2019, 31, 743–751. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, Gut Microbiome, and Bone Health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.; Cao, M.-M.; Li, Y.-J.; Zhang, R.-L.; Wu, M.-T.; Yu, Q.; Rui, Y.-F. Fecal Microbiota Transplantation as a Promising Treatment Option for Osteoporosis. J. Bone Miner. Metab. 2022, 40, 874–889. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, Goitrogens, Phytates and Oxalates, Friends or Foe? J. Funct. Foods 2022, 89, 104938. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef] [PubMed]
- Warensjö Lemming, E.; Byberg, L.; Höijer, J.; Larsson, S.C.; Wolk, A.; Michaëlsson, K. Combinations of Dietary Calcium Intake and Mediterranean-Style Diet on Risk of Hip Fracture: A Longitudinal Cohort Study of 82,000 Women and Men. Clin. Nutr. 2021, 40, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, Y.; Onoda, T.; Tanno, K.; Kuribayashi, T.; Sakata, K.; Orimo, H. Association of Hip Fracture Incidence and Intake of Calcium, Magnesium, Vitamin D, and Vitamin K. Eur. J. Epidemiol. 2008, 23, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lumbers, M.; New, S.A.; Gibson, S.; Murphy, M.C. Nutritional Status in Elderly Female Hip Fracture Patients: Comparison with an Age-Matched Home Living Group Attending Day Centres. Br. J. Nutr. 2001, 85, 733–740. [Google Scholar] [CrossRef]
- Zhao, J.-G.; Zeng, X.-T.; Wang, J.; Liu, L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults. JAMA 2017, 318, 2466. [Google Scholar] [CrossRef]
- Bolland, M.J.; Leung, W.; Tai, V.; Bastin, S.; Gamble, G.D.; Grey, A.; Reid, I.R. Calcium Intake and Risk of Fracture: Systematic Review. BMJ 2015, 2015, h4580. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Shi, B.-M.; Qin, L.-Q. Systematic Review and Meta-Analysis of the Association between Dairy Consumption and the Risk of Hip Fracture: Critical Interpretation of the Currently Available Evidence. Osteoporos. Int. 2020, 31, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Dadra, A.; Aggarwal, S.; Kumar, P.; Kumar, V.; Dibar, D.P.; Bhadada, S.K. High. prevalence of vitamin D deficiency and osteoporosis in patients with fragility fractures of hip: A pilot study. J. Clin. Orthop. Trauma 2019, 10, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Cavalier, E.; Reginster, J.Y. Vitamin D and osteosarcopenia: An update from epidemiological studies. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; da Silva, D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, Physiological Role, Biokinetics, Deficiency, Therapeutic Use, Toxicity, and Overview of Analytical Methods for Detection of Vitamin D and Its Metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef]
- O’Neill, C.; Kazantzidis, A.; Ryan, M.; Barber, N.; Sempos, C.; Durazo-Arvizu, R.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Chevalley, T.; Brandi, M.L.; Cashman, K.D.; Cavalier, E.; Harvey, N.C.; Maggi, S.; Cooper, C.; Al-Daghri, N.; Bock, O.; Bruyère, O.; et al. Role of Vitamin D Supplementation in the Management of Musculoskeletal Diseases: Update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) Working Group. Aging Clin. Exp. Res. 2022, 34, 2603–2623. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, K.; Cumming, R.G.; Naganathan, V.; Blyth, F.M.; Le Couteur, D.G.; Handelsman, D.J.; Waite, L.M.; Seibel, M.J. U-Shaped Association between Serum 25-Hydroxyvitamin D and Fracture Risk in Older Men: Results from the Prospective Population-Based CHAMP Study. J. Bone Miner. Res. 2014, 29, 2024–2031. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Harvey, N.C.; Biver, E.; Kaufman, J.M.; Bauer, J.; Branco, J.; Brandi, M.L.; Bruyère, O.; Coxam, V.; Cruz-Jentoft, A.; Czerwinski, E.; et al. The role of calcium supplementation in healthy musculoskeletal ageing: An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos. Int. 2017, 28, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Xue, W.-Q.; Wu, B.-H.; He, M.-G.; Xie, H.-L.; Ouyang, W.-F.; Tu, S.; Chen, Y.-M. Higher Fish Intake Is Associated with a Lower Risk of Hip Fractures in Chinese Men and Women: A Matched Case-Control Study. PLoS ONE 2013, 8, e56849. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Ho, S.C.; Lam, S.S. Higher Sea Fish Intake Is Associated with Greater Bone Mass and Lower Osteoporosis Risk in Postmenopausal Chinese Women. Osteoporos. Int. 2010, 21, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Garcia, J.; Moran, J.; Roncero-Martin, R.; Rey-Sanchez, P.; Rodriguez-Velasco, F.; Pedrera-Zamorano, J. Dietary Habits, Nutrients and Bone Mass in Spanish Premenopausal Women: The Contribution of Fish to Better Bone Health. Nutrients 2012, 5, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Farina, E.K.; Kiel, D.P.; Roubenoff, R.; Schaefer, E.J.; Cupples, L.A.; Tucker, K.L. Protective Effects of Fish Intake and Interactive Effects of Long-Chain Polyunsaturated Fatty Acid Intakes on Hip Bone Mineral Density in Older Adults: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2011, 93, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Pujia, R.; Ferro, Y.; Maurotti, S.; Mare, R.; Arturi, F.; Montalcini, T.; Pujia, A.; Mazza, E. Relationship between Osteoporosis, Multiple Fractures, and Egg Intake in Healthy Elderly. J. Midlife Health 2021, 12, 287. [Google Scholar] [CrossRef] [PubMed]
- Coheley, L.M.; Kindler, J.M.; Laing, E.M.; Oshri, A.; Hill Gallant, K.M.; Warden, S.J.; Peacock, M.; Weaver, C.M.; Lewis, R.D. Whole Egg Consumption and Cortical Bone in Healthy Children. Osteoporos. Int. 2018, 29, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Larijani, B.; Esmaillzadeh, A. Consumption of Milk and Dairy Products and Risk of Osteoporosis and Hip Fracture: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 1722–1737. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Esmaillzadeh, A. Letter to Editor: Careful Literature Search and Exact Data Extraction Are Milestones of a Meta-Analysis: The Case of Dairy Consumption and Hip Fracture. BMC Public Health 2018, 18, 1253. [Google Scholar] [CrossRef]
- Rizzoli, R. Dairy Products and Bone Health. Aging Clin. Exp. Res. 2021, 34, 9–24. [Google Scholar] [CrossRef]
- Laird, E.; Molloy, A.M.; McNulty, H.; Ward, M.; McCarroll, K.; Hoey, L.; Hughes, C.F.; Cunningham, C.; Strain, J.J.; Casey, M.C. Greater Yogurt Consumption Is Associated with Increased Bone Mineral Density and Physical Function in Older Adults. Osteoporos. Int. 2017, 28, 2409–2419. [Google Scholar] [CrossRef]
- Na, X.; Xi, Y.; Qian, S.; Zhang, J.; Yang, Y.; Zhao, A. Association between Dairy Product Intake and Risk of Fracture among Adults: A Cohort Study from China Health and Nutrition Survey. Nutrients 2022, 14, 1632. [Google Scholar] [CrossRef]
- Al-Algawy, A.A.H.; Baiee, H.A.; Hasan, S.; Jassim, I.; Razaq, M.; Kamel, F.; Ali, A.; Khudhair, E. Risk Factors Associated with Hip Fractures among Adult People in Babylon City, Iraq. Open Access Maced. J. Med. Sci. 2019, 7, 3608–3614. [Google Scholar] [CrossRef]
- Mishra, S.; Baruah, K.; Malik, V.S.; Ding, E.L. Dairy Intake and Risk of Hip Fracture in Prospective Cohort Studies: Non-Linear Algorithmic Dose-Response Analysis in 486,950 Adults. J. Nutr. Sci. 2023, 12, e96. [Google Scholar] [CrossRef]
- Matía-Martín, P.; Torrego-Ellacuría, M.; Larrad-Sainz, A.; Fernández-Pérez, C.; Cuesta-Triana, F.; Rubio-Herrera, M.Á. Effects of Milk and Dairy Products on the Prevention of Osteoporosis and Osteoporotic Fractures in Europeans and Non-Hispanic Whites from North America: A Systematic Review and Updated Meta-Analysis. Adv. Nutr. 2019, 10, S120–S143. [Google Scholar] [CrossRef]
- Bian, S.; Hu, J.; Zhang, K.; Wang, Y.; Yu, M.; Ma, J. Dairy Product Consumption and Risk of Hip Fracture: A Systematic Review and Meta-Analysis. BMC Public Health 2018, 18, 165. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Mozaffarian, D.; Cauley, J.A.; Mukamal, K.J.; Robbins, J.; Siscovick, D.S. Fish Consumption, Bone Mineral Density, and Risk of Hip Fracture among Older Adults: The Cardiovascular Health Study. J. Bone Miner. Res. 2010, 25, 1972–1979. [Google Scholar] [CrossRef]
- Feskanich, D.; Bischoff-Ferrari, H.A.; Frazier, A.L.; Willett, W.C. Milk Consumption During Teenage Years and Risk of Hip Fractures in Older Adults. JAMA Pediatr. 2014, 168, 54. [Google Scholar] [CrossRef]
- Shang, N.; Wu, J. Egg White Ovotransferrin Attenuates RANKL-Induced Osteoclastogenesis and Bone Resorption. Nutrients 2019, 11, 2254. [Google Scholar] [CrossRef]
- Shang, N.; Bhullar, K.S.; Wu, J. Ovotransferrin Exhibits Osteogenic Activity Partially via Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1) Activation in MC3T3-E1 Cells. J. Agric. Food Chem. 2020, 68, 9427–9435. [Google Scholar] [CrossRef]
- Shang, N.; Wu, J. Egg White Ovotransferrin Shows Osteogenic Activity in Osteoblast Cells. J. Agric. Food Chem. 2018, 66, 2775–2782. [Google Scholar] [CrossRef]
- Kemi, V.E.; Kärkkäinen, M.U.M.; Rita, H.J.; Laaksonen, M.M.L.; Outila, T.A.; Lamberg-Allardt, C.J.E. Low Calcium:Phosphorus Ratio in Habitual Diets Affects Serum Parathyroid Hormone Concentration and Calcium Metabolism in Healthy Women with Adequate Calcium Intake. Br. J. Nutr. 2010, 103, 561–568. [Google Scholar] [CrossRef]
- Kemi, V.E.; Kärkkäinen, M.U.M.; Lamberg-Allardt, C.J.E. High Phosphorus Intakes Acutely and Negatively Affect Ca and Bone Metabolism in a Dose-Dependent Manner in Healthy Young Females. Br. J. Nutr. 2006, 96, 545–552. [Google Scholar] [CrossRef]
- Calvo, M.S.; Tucker, K.L. Is Phosphorus Intake That Exceeds Dietary Requirements a Risk Factor in Bone Health? Ann. N. Y. Acad. Sci. 2013, 1301, 29–35. [Google Scholar] [CrossRef]
- Godos, J.; Giampieri, F.; Chisari, E.; Micek, A.; Paladino, N.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; La Vignera, S.; Musumeci, G.; et al. Alcohol Consumption, Bone Mineral Density, and Risk of Osteoporotic Fractures: A Dose–Response Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1515. [Google Scholar] [CrossRef]
- Ratajczak, A.E.; Szymczak-Tomczak, A.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Impact of Cigarette Smoking on the Risk of Osteoporosis in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 1515. [Google Scholar] [CrossRef]
- Yoon, V.; Maalouf, N.M.; Sakhaee, K. The Effects of Smoking on Bone Metabolism. Osteoporos. Int. 2012, 23, 2081–2092. [Google Scholar] [CrossRef]
- Babić Leko, M.; Pleić, N.; Gunjača, I.; Zemunik, T. Environmental Factors That Affect Parathyroid Hormone and Calcitonin Levels. Int. J. Mol. Sci. 2021, 23, 44. [Google Scholar] [CrossRef]
- Troy, K.L.; Mancuso, M.E.; Butler, T.A.; Johnson, J.E. Exercise Early and Often: Effects of Physical Activity and Exercise on Women’s Bone Health. Int. J. Environ. Res. Public Health 2018, 15, 878. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Cao, M.M.; Li, Y.J.; Chen, X.X.; Yu, Q.; Rui, Y.F. A narrative review of the moderating effects and repercussion of exercise intervention on osteoporosis: Ingenious involvement of gut microbiota and its metabolites. J. Transl. Med. 2022, 20, 490. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Z.B. Exercise: A Possibly Effective Way to Improve Vitamin D Nutritional Status. Nutrients 2020, 14, 2652. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Claeson, M.; Khan, A.; Neale, R.E. The effect of physical activity on vitamin D: A systematic review and meta-analysis of intervention studies in humans. Public Health Pract. 2024, 7, 100495. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. Web–Based Injury Statistics Query and Reporting System (WISQARS). Available online: https://www.cdc.gov/injury/wisqars/fatal/trends.html (accessed on 20 July 2024).
- Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. Mortality 1999–2021 on CDC WONDER Online Database: Older Adult Falls Data. Available online: https://www.cdc.gov/falls/data-research/index.html#cdc_data_surveillance_section_3-deaths-from-older-adult-falls (accessed on 20 July 2024).
- Hsieh, F.Y. Sample Size Tables for Logistic Regression. Stat. Med. 1989, 8, 795–802. [Google Scholar] [CrossRef]
- Djekic-Ivankovic, M.; Weiler, H.A.; Nikolic, M.; Kadvan, A.; Gurinovic, M.; Mandic, L.M.; Glibetic, M. Validity of an FFQ Assessing the Vitamin D Intake of Young Serbian Women Living in a Region without Food Fortification: The Method of Triads Model. Public Health Nutr. 2016, 19, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, N.L.; Ball, A.L.; Neils-Strunjas, J.; Smith, R.; Weiler, E.; Krikorian, R. Using Memory Strategies to Improve 24-Hour Dietary Recalls among Older Adults. J. Allied Health 2003, 32, 196–201. [Google Scholar] [PubMed]
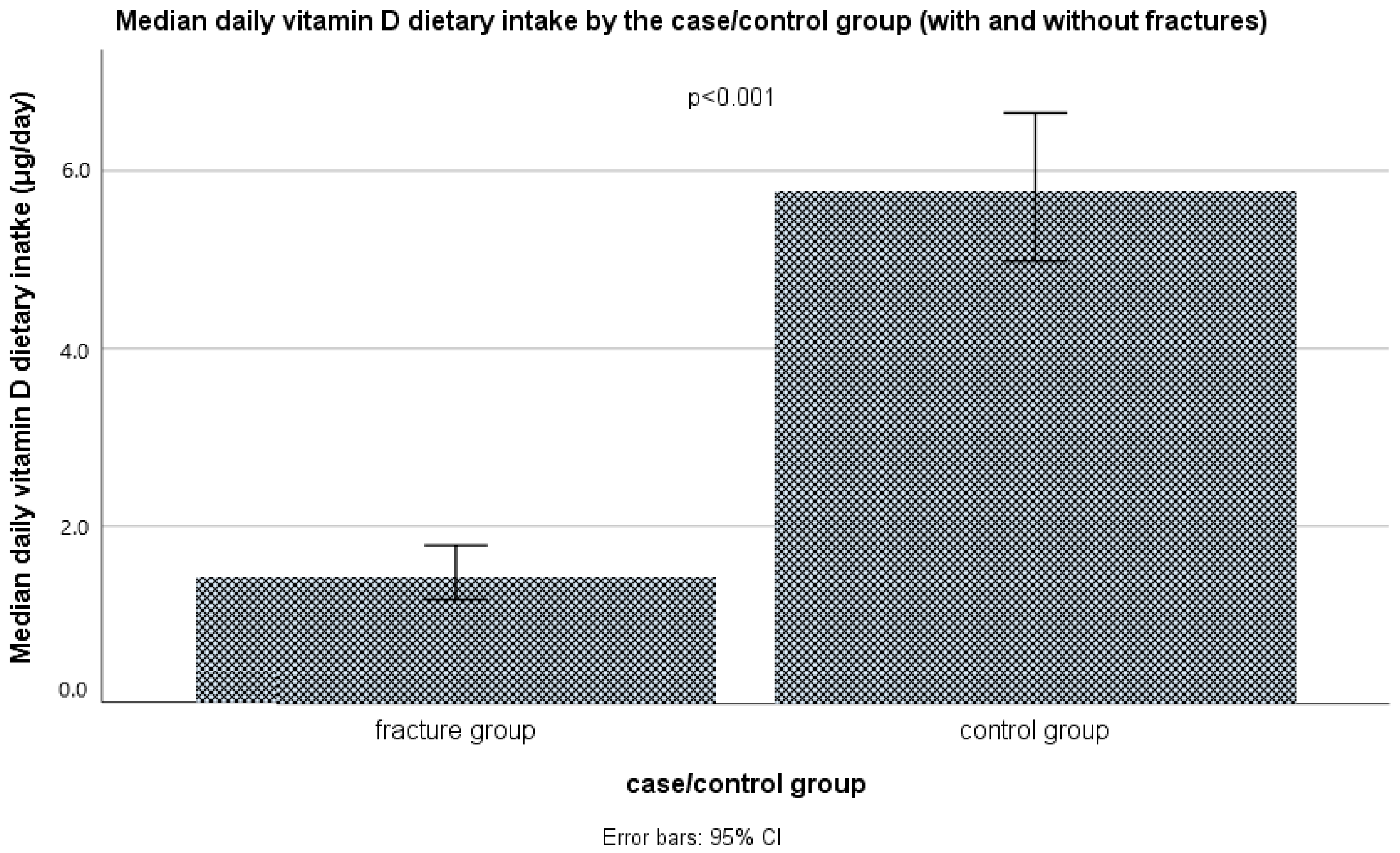
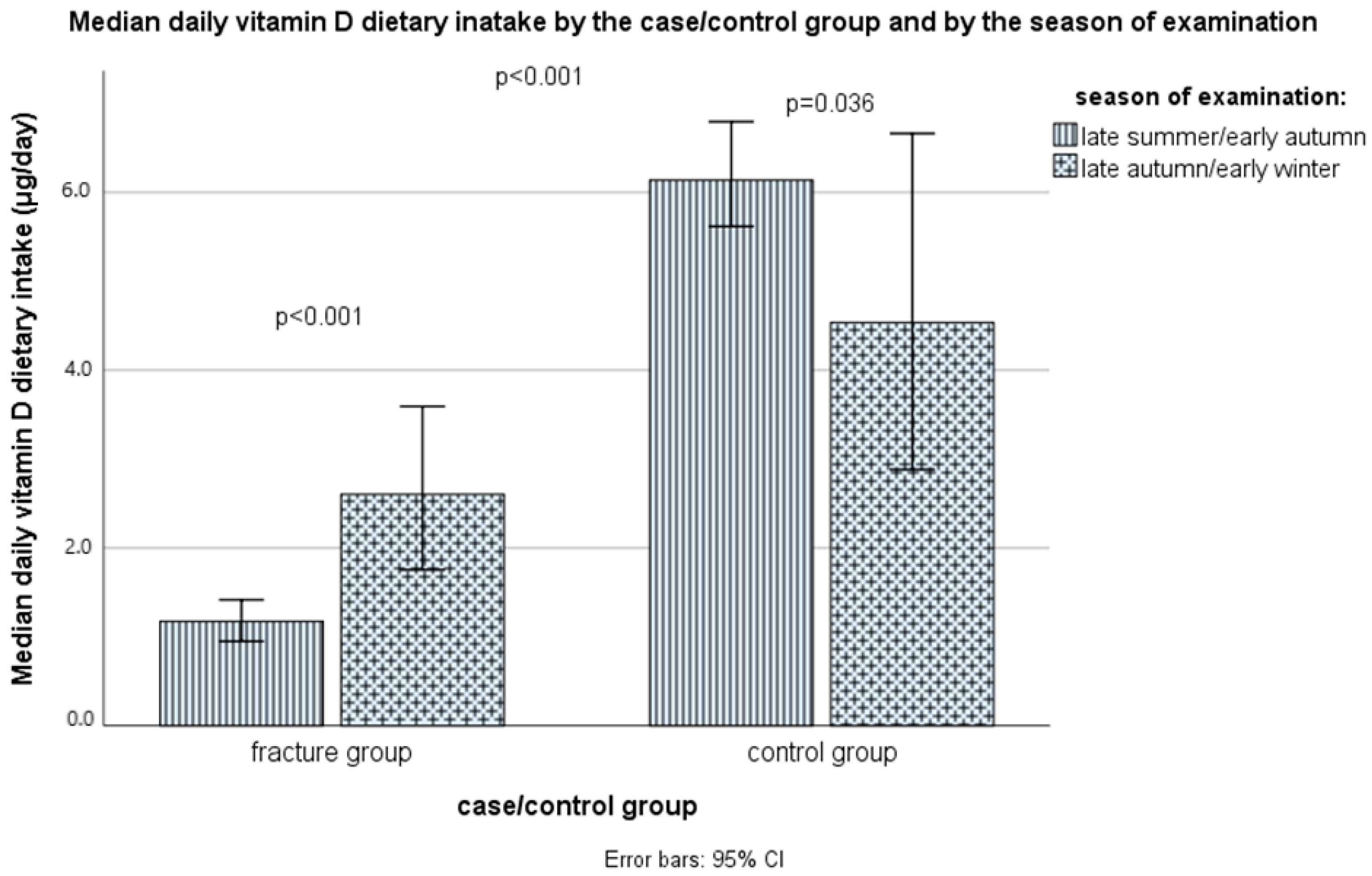



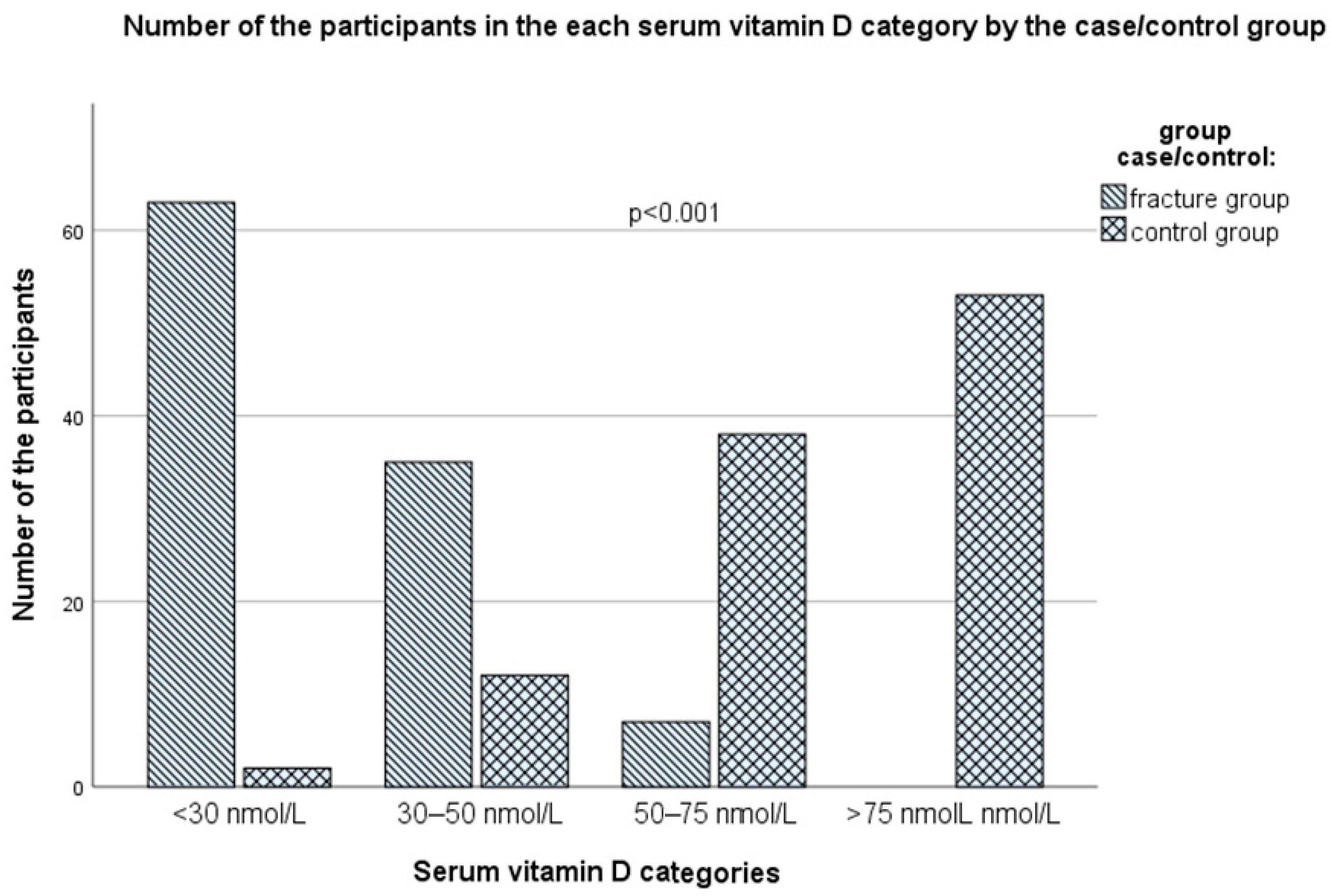
| With Fractures (n = 105) | Controls (n = 105) | ||||
|---|---|---|---|---|---|
| Median/n | (IQR)/(%) | Median/n | (IQR)/(%) | p | |
| Sex: | 1.000 | ||||
| • Men n (%) | 15 | (23.8%) | 15 | (23.8%) | |
| • Women n (%) | 80 | (76.2%) | 80 | (76.2%) | |
| Age (years) | 73.0 | (69.0–78.0) | 71.0 | (69.0–75.0) | 0.044 |
| Education: | 0.440 | ||||
| • Primary education (ISCED 1) or lower n (%) | 15 | (14.3%) | 17 | (16.2%) | |
| • Lower secondary education (ISCED 2) n (%) | 27 | (25.7%) | 16 | (15.2%) | |
| • Upper secondary education (ISCED 3) n (%) | 35 | (33.3%) | 36 | (34.3%) | |
| • Post-secondary but non-tertiary education (ISCED 4) n (%) | 13 | (12.4%) | 17 | (16.2%) | |
| • First stage of tertiary education (ISCED 5) n (%) | 13 | (12.4%) | 14 | (13.3%) | |
| • Second stage of tertiary education (ISCED 6) n (%) | 2 | (1.9%) | 5 | (4.8%) | |
| Settlement type: | 0.774 | ||||
| • Rural | 37 | (35.2%) | 39 | (37.1%) | |
| • Urban | 68 | (64.8%) | 66 | (62.9%) | |
| Marital status: | 0.324 | ||||
| • Married | 77 | (73.3%) | 83 | (79.0%) | |
| • Widowed, single, divorced | 29 | (27.7%) | 22 | (21.0%) | |
| Household person number | 2.0 | (2.0–4.0) | 2.0 | (2.0–4.0) | 0.766 |
| Smoking status: | <0.001 | ||||
| • Never smoker | 12 | (11.4%) | 43 | (41.0%) | |
| • Former smoker | 45 | (42.9%) | 38 | (36.2%) | |
| • Current smoker: | 48 | (45.7%) | 24 | (22.9%) | |
| ∘ Less than 10 cigarettes per day | 11 | (10.5%) | 16 | (15.2%) | <0.001 |
| ∘ 10–20 cigarettes per day | 32 | (30.5%) | 8 | (7.6%) | |
| ∘ Over 20 cigarettes per day | 5 | (4.8%) | 3 | (2.9%) | |
| Physical activity level (scale, scoring 1–5) | 3.0 | (2.0–3.0) | 4.0 | (4.0–5.0) | <0.001 |
| • 1. Very inactive n (%) | 10 | (9.5%) | 3 | (2.9%) | <0.001 |
| • 2. Moderately inactive n (%) | 26 | (24.8%) | 2 | (1.9%) | |
| • 3. Nor active nor inactive n (%) | 45 | (42.9%) | 18 | (17.1%) | |
| • 4. Moderately active n (%) | 20 | (19.0%) | 49 | (46.7%) | |
| • 5. Highly active n (%) | 4 | (3.8%) | 33 | (31.4%) | |
| BMI (kg/m2) | 25.7 | (23.4–27.7) | 26.7 | (24.1–28.9) | 0.057 |
| • Underweight n (%) | 1 | (1.0%) | 0 | (0.0%) | 0.278 |
| • Normal-weight n (%) | 44 | (41.9%) | 33 | (31.4%) | |
| • Overweight n (%) | 43 | (41.0%) | 54 | (51.4%) | |
| • Obese n (%) | 17 | (16.2%) | 18 | (17.1%) | |
| Systolic arterial pressure | 130 | (120–140) | 120 | (120–130) | <0.001 |
| Diastolic arterial pressure | 80 | (80–90) | 80 | (80–90) | 0.438 |
| Bone fractures before (any type of fractures): | <0.001 | ||||
| • No n (%) | 35 | (33.3%) | 88 | (83.8%) | |
| • Yes n (%) | 70 | (66.7%) | 17 | (16.2%) | |
| Season of examination/blood sampling: | 0.318 | ||||
| • Late summer/early autumn n (%) | 71 | (61.0%) | 64 | (67.6%) | |
| • Late autumn/early winter n (%) | 34 | (39.0%) | 41 | (32.4%) | |
| With Fractures (n = 105) | Controls (n = 105) | ||||
|---|---|---|---|---|---|
| Median | (IQR) | Median | (IQR) | p | |
| Energy intake (Kcal/day) | 1298.0 | (1176.6–1531.7) | 1736.5 | (1491.9–1998.0) | <0.001 |
| Protein intake (g/day) | 38.6 | (32.1–55.5) | 70.3 | (55.8–86.0) | <0.001 |
| Fat intake (g/day) | 55.6 | (41.8–71.7) | 82.1 | (69.6–102.1) | <0.001 |
| Carbohydrate intake (g/day) | 157.8 | (134.6–187.0) | 159.9 | (136.5–200.5) | 0.284 |
| Alcohol intake (g/day) | 0.001 | (0.000–0.005) | 0.000 | (0.000–0.003) | 0.049 |
| Fiber intake (g/day) | 11.7 | (9.4–14.8) | 13.5 | (10.5–18.0) | 0.003 |
| Energy intake from protein (%Kcal/day) | 12.3 | (10.1–17.0) | 16.4 | (14.5–18.9) | <0.001 |
| Energy intake from fat (%Kcal/day) | 38.3 | (31.9–43.8) | 44.4 | (40.0–48.6) | <0.001 |
| Energy intake from carbohydrate (%Kcal/day) | 48.4 | (40.4–54.9) | 38.9 | (34.1–43.4) | <0.001 |
| Energy intake from alcohol (%Kcal/day) | 0.000 | (0.000–0.003) | 0.000 | (0.000–0.002) | 0.035 |
| With Fractures (n = 105) | Controls (n = 105) | ||||
|---|---|---|---|---|---|
| Median | (IQR) | Median | (IQR) | p | |
| Energy intake from milk and milk products (Kcal/day) | 87.9 | (42.1–166.1) | 222.7 | (111.7–331.0) | <0.001 |
| Energy intake from eggs and egg products (Kcal/day) | 11.5 | (0.0–51.7) | 64.5 | (14.5–131.4) | <0.001 |
| Energy intake from meat and meat products (Kcal/day) | 122.3 | (68.9–224.7) | 160.9 | (75.8–292.7) | 0.181 |
| Energy intake from fish, seafood, and related products (Kcal/day) | 0.0 | (0.0–0.0) | 93.1 | (0.0–179.0) | <0.001 |
| Energy intake from edible fats, oil, and similar products (Kcal/day) | 168.6 | (107.3–236.2) | 249.5 | (167.2–359.9) | <0.001 |
| Energy intake from grains and grain products (Kcal/day) | 482.2 | (367.0–600.5) | 535.0 | (383.8–680.9) | 0.073 |
| Energy intake from nuts, seeds, and related products (Kcal/day) | 21.7 | (1.2–51.3) | 20.4 | (3.1–52.4) | 0.580 |
| Energy intake from vegetables and vegetable products (Kcal/day) | 71.2 | (34.0–119.8) | 69.6 | (44.5–123.5) | 0.512 |
| Energy intake from fruit and fruit products (Kcal/day) | 44.0 | (0.0–98.2) | 56.2 | (3.3–116.7) | 0.067 |
| Energy intake from sugar and sugar products (Kcal/day) | 62.9 | (9.3–154.9) | 22.9 | (0.0–69.5) | <0.001 |
| Energy intake from alcoholic and non-alcoholic beverages (Kcal/day) | 35.8 | (1.3–86.7) | 32.2 | (1.7–62.2) | 0.151 |
| Energy intake from miscellaneous food products (Kcal/day) | 0.6 | (0.0–1.4) | 1.4 | (0.3–2.6) | <0.001 |
| Energy intake from special nutritional supplements (Kcal/day) | 0.0 | (0.0–0.0) | 0.0 | (0.0–0.0) | 0.317 |
| Model No | Predictors | B | Exponent (B) (Odds Ratio) | 95%CI for Exponent (B) | Predictor Significance p | Model Nagelkerke R2 | Model Significance p |
|---|---|---|---|---|---|---|---|
| Model 1 | Constant | 5.951 | 383.986 | <0.001 | 0.755 | <0.001 | |
| Serum 25(OH)D levels (nmol/L) | −0.123 | 0.884 | (0.854–0.915) | <0.001 | |||
| Model 2 | Constant | 16.047 | 9,309,443.280 | 0.004 | 0.817 | <0.001 | |
| Serum 25(OH)D levels (nmol/L) | −0.131 | 0.878 | (0.841–0.916) | <0.001 | |||
| Physical activity level (1–5) | −0.735 | 0.480 | (0.284–0.811) | 0.006 | |||
| Season (late autumn/early winter) | 1.012 | 2.752 | (0.882–8.584) | 0.081 | |||
| BMI (kg/m2) | −0.105 | 0.900 | (0.766–1.057) | 0.200 | |||
| Smoking status (1–5) | 0.244 | 1.276 | (0.864–1.886) | 0.221 | |||
| Age (years) | −0.069 | 0.933 | (0.832–1.046) | 0.234 | |||
| Sex (male) | −0.395 | 0.674 | (0.169–2.680) | 0.575 | |||
| Model 3 | Constant | 9.975 | 21,485.246 | <0.001 | 0.863 | <0.001 | |
| Serum 25(OH)D levels (nmol/L) | −0.146 | 0.864 | (0.822–0.909) | <0.001 | |||
| Protein intake (g/day) | −0.062 | 0.939 | (0.907–0.973) | <0.001 | |||
| Season (late autumn/early winter) | 2.554 | 12.858 | (2.933–56.373) | 0.001 | |||
| Vitamin D intake (µg/day) | −0.111 | 0.895 | (0.796–1.006) | 0.064 | |||
| Model 4 | Constant | 14.339 | 1,688,174.056 | <0.001 | 0.874 | <0.001 | |
| Serum 25(OH)D levels (nmol/L) | −0.155 | 0.857 | (0.808–0.909) | <0.001 | |||
| Season (late autumn/early winter) | 2.240 | 9.393 | (2.196–40.174) | 0.003 | |||
| Fiber intake (g/day) | −0.173 | 0.841 | (0.739–0.958) | 0.009 | |||
| Fat intake (g/day) | −0.034 | 0.966 | (0.941–0.992) | 0.012 | |||
| Physical activity level (1–5) | −0.733 | 0.481 | (0.252–0.916) | 0.026 | |||
| Vitamin D intake (µg/day) | −0.105 | 0.900 | (0.800–1.012) | 0.079 | |||
| Model 5 | Constant | 14.708 | 2,440,269.079 | <0.001 | 0.919 | <0.001 | |
| Serum 25(OH)D levels (nmol/L) | −0.231 | 0.794 | (0.713–0.885) | <0.001 | |||
| Fish and seafood dietary intakes (Kcal/day) | −0.028 | 0.972 | (0.958–0.987) | <0.001 | |||
| Season (late autumn/early winter) | 3.817 | 45.472 | (3.799–544.322) | 0.003 | |||
| Egg and egg product dietary intakes (Kcal/day) | −0.018 | 0.982 | (0.969–0.996) | 0.011 | |||
| Sugar and sweets dietary intakes (Kcal/day) | 0.013 | 1.013 | (1.002–1.025) | 0.022 | |||
| Physical activity level (1–5) | −0.842 | 0.431 | (0.199–0.933) | 0.033 | |||
| Milk and dairy product dietary intakes (Kcal/day) | −0.008 | 0.992 | (0.985–0.999) | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdenović, N.; Šarac, I.; Ćorić, A.; Karan, S.; Nikolić, S.; Ždrale, I.; Milešević, J. Impact of Vitamin D Status and Nutrition on the Occurrence of Long Bone Fractures Due to Falls in Elderly Subjects in the Vojvodina Region of Serbia. Nutrients 2024, 16, 2702. https://doi.org/10.3390/nu16162702
Gvozdenović N, Šarac I, Ćorić A, Karan S, Nikolić S, Ždrale I, Milešević J. Impact of Vitamin D Status and Nutrition on the Occurrence of Long Bone Fractures Due to Falls in Elderly Subjects in the Vojvodina Region of Serbia. Nutrients. 2024; 16(16):2702. https://doi.org/10.3390/nu16162702
Chicago/Turabian StyleGvozdenović, Nemanja, Ivana Šarac, Andrijana Ćorić, Saša Karan, Stanislava Nikolić, Isidora Ždrale, and Jelena Milešević. 2024. "Impact of Vitamin D Status and Nutrition on the Occurrence of Long Bone Fractures Due to Falls in Elderly Subjects in the Vojvodina Region of Serbia" Nutrients 16, no. 16: 2702. https://doi.org/10.3390/nu16162702
APA StyleGvozdenović, N., Šarac, I., Ćorić, A., Karan, S., Nikolić, S., Ždrale, I., & Milešević, J. (2024). Impact of Vitamin D Status and Nutrition on the Occurrence of Long Bone Fractures Due to Falls in Elderly Subjects in the Vojvodina Region of Serbia. Nutrients, 16(16), 2702. https://doi.org/10.3390/nu16162702






