Effects of Milk and Dairy on the Risk and Course of Inflammatory Bowel Disease versus Patients’ Dietary Beliefs and Practices: A Systematic Review
Abstract
1. Introduction
2. Material and Methods
3. Results
- Eight studies (n = 8) on the risk of milk and dairy consumption on the occurrence of IBD;
- Twenty-seven studies (n = 27) on the dietary beliefs and practices regarding milk and dairy products (one study covered beliefs and practices regarding milk and dairy consumption and the impact of milk and dairy consumption on the IBD course);
- Three studies (n = 3) on the impact of milk and dairy consumption on the IBD course.
3.1. Interpretative Synthesis of Data: Milk and Dairy Products and the Occurrence of Inflammatory Bowel Disease
| Author | Study Country Quality Level * | Methods | Patients | Findings |
|---|---|---|---|---|
| Maconi et al. [55], 2010 | Case–control Italy D | Questionnaire previously used for studies on the relationship between cancer and diet; consumption of foods and beverages was subdivided into tertiles (low, moderate, and high consumptions) | 41 UC, 42 CD, 160 sex- and age-matched healthy blood donors; patients not changing their dietary habits (26 UC and 25 CD) were analyzed concerning the IBD risk | CD was significantly associated with high consumption of cheese (OR = 3.7, 95% CI: 1.14–12.01); an increased risk of ileal or ileocolonic CD was associated with high cheese consumption (OR = 2.61, 95% CI: 1.26–5.41). Remark: to conclude abiut the effect of milk and dairy consumption on the risk of IBD is impossible. |
| Opstelten et al. [50], 2016 | Prospective cohort study; 12 centers in Denmark, France, Germany, Greece, Italy, the Netherlands, the United Kingdom, and Sweden C | FFQ for the intake of total and specific dairy | 110 CD and 244 UC patients from 401,326 participants of the EPIC cohort | Compared with the lowest quartile, the ORs for the highest quartile of total dairy products were 0.61 (95% CI, 0.32–1.19) for CD and 0.80 (95% CI, 0.50–1.30) for UC. Compared with non-consumers, participants consuming milk had significantly reduced odds of CD (OR 0.30, 95% CI, 0.13–0.65) and non-significantly reduced odds of UC (OR 0.85, 95% CI, 0.49–1.47). |
| Han et al. [53], 2020 | A secondary analysis of National Health Interview Survey 2015 United States C | Survey | In the total population of 103,789 individuals 33,672 adults (44.76% males; 55.24% females) 454 responders (1.35%) were ever told by health professionals that they had IBD. The prevalence of IBD among estimated US adults is 1.28% (95% CI 1.27–1.28) | Drinking milk was associated with a smaller likelihood of IBD (OR 0.70, 95% CI 0.497–0.998). A significant association between IBD and consumption of cheese (OR = 1.006, 95% CI [1.0021–1.0104], p = 0.003), ice cream (OR = 1.011, 95% CI [1.0022–1.0203], p = 0.015), was shown. |
| Van der Sloot et al. [52], 2020 | Case–control study The Netherlands D | The validated GIEQ identifying factors through different stages of life using 844 items | 674 IBD patients of the 1000IBD cohort, frequency-matched based on sex and age with 1348 controls from the population-based Lifelines Cohort Study Mean age at study inclusion 50.4 years | Cow’s milk hypersensitivity (OR 5.87; 95% CI 2.72–12.68) was related to CD. |
| Preda et al. [57], 2020 | An observational, retrospective, case–control study Romania, Belgium D | 76 Romanian and 53 Belgian patients | Lower intake of yogurt by IBD patients (25.6 vs. 43.8%) Remark: small, retrospective study; to conclude the effect of milk and dairy consumption on the risk of IBD is impossible. | |
| Bikbavova et al. [56], 2021 | Retrospective case–control study Russia (Western Siberia) D | World Health Organization countrywide integrated noncommunicable diseases intervention questionnaire | 81 UC (42 men and 39 women, age 18–79 years); 39 controls (healthy volunteers, 14 men, 25 women, aged 22–81 years) | UC patients poorly tolerated milk and fermented milk products before the first disease symptoms more frequent compared to the control group (27.2 ± 4.9% vs. 7.7 ± 4.3%, p = 0.011) Remark: small, retrospective study; to conclude about the effect of milk and dairy consumption on the risk of IBD is impossible |
| Narula et al. [51], 2021 | PURE study 21 countries ** C | Country-specific validated FFQs | The PURE study cohort of 116,087 adults aged 35–70 years. 90 CD and 377 UC 273 women The mean age of IBD patients was 50.5 years. | Intake of dairy was not associated with incident IBD. |
| Li et al. [54] 2021 | United Kingdom C | A two-sample Mendelian randomization analysis | 20,200 whole-milk consumers and 67,847 skimmed-milk consumers from the UK Biobank | The genome-wide association study identified five lead nucleotide polymorphisms associated with whole-milk preference. Whole-milk preference significantly decreased the risk of IBD (b = 1.735, p = 0.048) and CD (b = 2.549, p = 0.032), but had no significant effect on the risk of UC (b = 1.002, p = 0.44). |
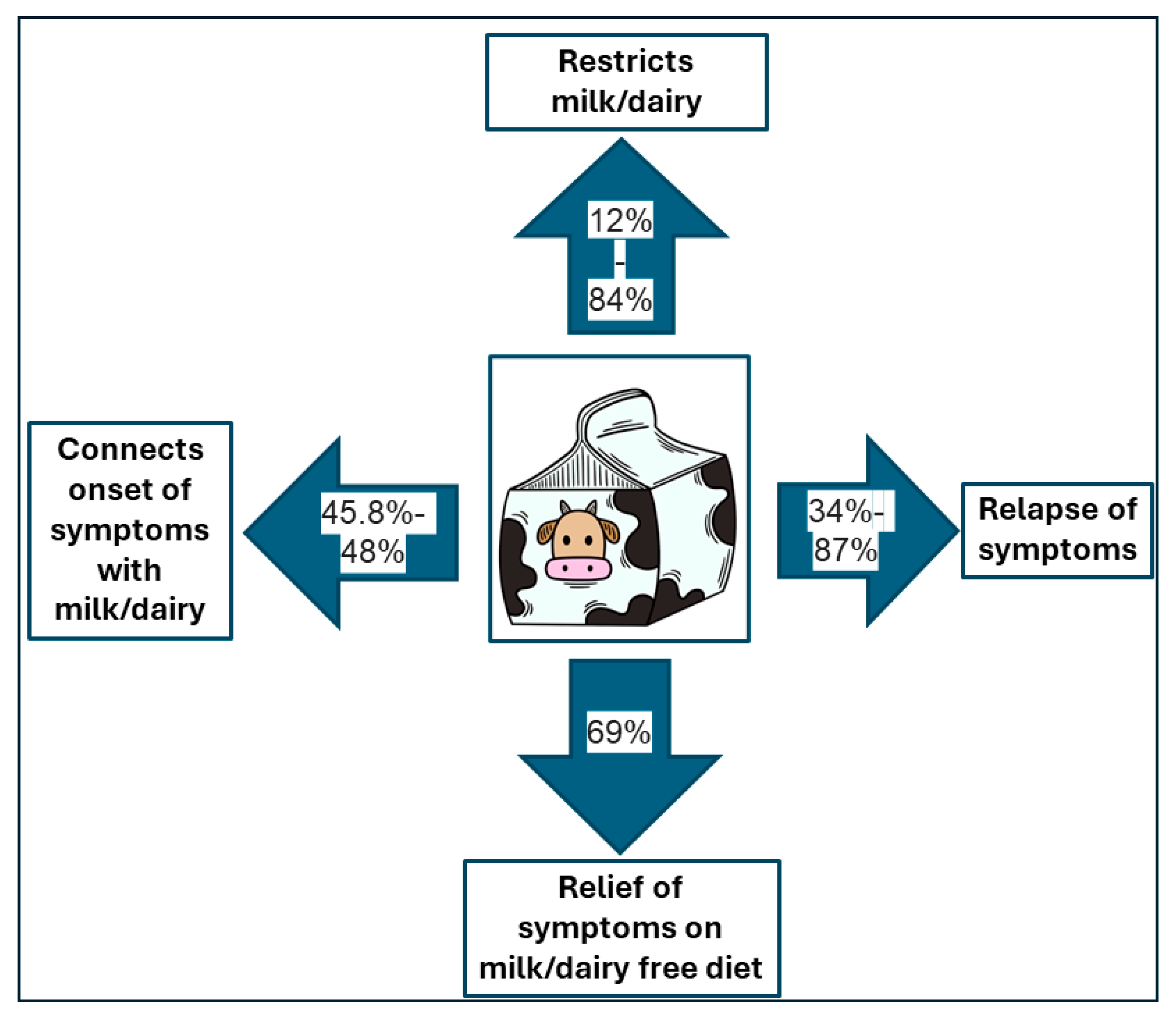
3.2. Interpretative Synthesis of Data: Dietary Beliefs and Practices Related to Milk and Dairy Consumption of Patients with Inflammatory Bowel Disease: The Impact of Milk and Dairy Consumption on the IBD Course
| Author Year | Study Design Country Quality Level * | Methods (a Tool Used to Assess Milk and Dairy Consumption) | Patients | Beliefs | Practice |
|---|---|---|---|---|---|
| Vagianos et al. [81] 2006–2007 | Cohort study Canada C | Surveys semi-annually and annual in-person interviews | 319 IBD patients from the University of Manitoba IBD Cohort Study Diets of those with IBD (n = 256) were compared with a matched, non-IBD Canadian cohort | Potential “gastrointestinal upset” was the reason to avoid milk and milk products in 51% of patients. | 12% always avoid milk and milk products. 29% normally eat milk/milk products but avoid them when the disease is active. |
| Triggs C. et al. [78] 2010 | Case–controlled New Zealand D | A dietary questionnaire (257 food items); self-reported dietary tolerances and intolerances. | 446 CD | Yogurt beneficial:19.1%; adverse 18.1%; Dairy products beneficial: 5.6%; adverse: 20.5%; no difference 46.7% | |
| Cohen A. et al. [63] 2013 | Internet-based cohort study United States C | A semi-quantitative FFQ developed by National Cancer Institute quantified the average daily consumption in the prior month of several food items | 2329 IBD: 1121 CD a 597 UC 405 CD-O 206 UC-P approximately 70% females median age 42–49 years 58–73% of participants reported having minimal disease activity | yogurt improved symptoms: CD 108 vs. 7 ** UC 54 vs. 3 ** CD-O 26 vs. 0 ** UC-P 19 vs. 0 **; milk worsened symptoms: CD 105 ** vs. 6 UC 49 ** vs. 0 CD-O 28 ** vs. 5 UC-P 14 # vs. 2 dairy worsened symptoms: CD 94 ** vs. 3 UC 56 ** vs. 1 CD-O NR UC-P 12 # vs. 0 | Patients ate less or more milk, cheese, and ice cream when they reported that these items worsened or improved their symptoms (p < 0.05). CD patients with disease flare consumed less milk than those in remission (OR 0.78, 95% CI 0.62–0.97). |
| Zallot C. et al. [77] 2013 | Cross-sectional France E | A questionnaire by the Departments of Gastroenterology and Diabetes and Nutrition of the Nancy University Hospital | 244 IBD 60.7% females 72.5% CD | 27.5% perceived milk to be a risk factor for relapse; 27.4% believed a dairy-free diet can improve symptoms during relapse | Patients excluding dairy products also excluded spicy food, carbonated beverages, fat, vegetables, and fruits in 86.6%, 62.7%, 59.7%, 58.2%, and 56.7% of cases, respectively. |
| Limdi et al. [82] 2013–2014 | Single-center, prospective study UK D | Prospective questionnaire | 205 UC 156 CD | 48% of IBD patients reported the link between diet and disease onset, while 57% between diet and disease flare Worse milk and milk products worsened symptoms in 16% of patients. | |
| Bach et al. [76] 2014 | Denmark D | Semi-structured qualitative interview | 25 UC 46% males 46.7 ± 15.6 years | 60% of the patients did not drink milk at all; restrictions based on personal experience. | |
| Lopes et al. [61] 2014 | Cross-sectional study Brazil E | Quantitative FFQ | 44 UC 21 CD 61.5% females Mean age 44.8 ± 13.5 years | 45.5% of patients reported a relation between exacerbation or onset of symptoms and cow’s milk and dairy consumption. | 64.7% of patients restricted milk and dairy products consumption. There was no difference between UC and CD patients. |
| Vidarsdottir et al. [71] 2016 | Cross-sectional Iceland E | 3-day food record | 43 CD 35 UC 35 men and 43 women aged 18–74 years | 87% of participants reported that diet influences gastrointestinal symptoms and 72% had changed their diet accordingly | 60% restricted dairy products due to negative effects on symptoms. |
| Casanova et al. [73] 2017 | Multicentre, observational, prospective study conducted at 30 centers Spain E | A questionnaire by the IBD Unit and the Endocrinology and Nutrition Department | 1271 patients 60% CD 51% females median age of 45 years | 23% of patients avoided dairy to prevent relapse of the disease, 36% avoided dairy during relapse. | |
| Lim et al. [58] 2018 | Cross-sectional Korea E | Survey | 61 CD 43 UC 2 groups: exclusion diet (n = 49) and non-exclusion diet (n = 55) | In the exclusion diet group, milk and dairy were the most frequently restricted foods. | |
| Larussa et al. [74] 2019 | Single-center Italy E | Self-prescribed dietary restrictions in patients with IBD | 67 UC 23 CD | 27% of patients avoided milk alone. 84% of patients avoided at least one dairy product. | |
| Opstelten J. et al. [75] 2019 | Cohort study The Netherlands C | Self-completed semiquantitative FFQ | 165 patients with longstanding IBD vs. 1469 Dutch population-based study | In IBD patients consumption of dairy products (except cheese) was −36.3 g/d lower than in control group. | |
| Głabska et al. [69] 2019 | Matched case–control study Poland D | Three-day dietary record | 44 male UC patients in remission | Consumption of milk and dairy beverages did not differ between UC patients and controls. | |
| Marsh et al. [80] 2019 | Prospective Cross-sectional Australia E | Structured interview, nutritional assessment and medical record review | 117 IBD | 40% of patients in flare and 33% in remission avoided lactose. | |
| Crooks et al. [66] 2019–2020 | Single-center, prospective study UK E | Prospective questionnaire | 208 patients with inactive UC | 31% of patients reported a relationship between diet and UC onset. | 21% avoided milk products. |
| Crooks et al. [83] 2019–2020 | A prospective, cross-sectional, multi-center study UK E | 29 questions questionnaire | 91 UC 45 CD All ≥ 60 years | 68% of patients followed dietary restrictions to prevent a flare of IBD; milk products were avoided in 23%. | |
| Han et al. [53] 2020 | A secondary analysis of the National Health Interview Survey 2015 US E | Survey | 454 responders from a total population of 103,789 were ever told by health professionals that they have IBD | Consumption of milk in the past 30 days was similar among patients with IBD (71.29%; 95% CI 65.06–76.81) and without IBD (75.83%; 95% CI 75.10–76.55) | |
| Krela Kazmierczak et al. [67] 2020 | Cross-sectional study Poland E | Questionnaire including consumption of milk and dairy products (qualitatively), especially before and after diagnosis of IBD. | 208 adult IBD individuals (mean age 37.7 ± 14 years; 101 women) CD 103 UC 105 | Milk consumption decreased after diagnosis (87% vs. 26.9%; p < 0.0001), but only in men. Percentage of people consuming dairy did not significantly decrease after diagnosis (100% vs. 90.4%; p = 0.0407). | |
| Kamp et al. [84] 2020 | Cross-sectional Study United States E | Dietary screening questionnaire, self-directed diet modifications, dietary beliefs questionnaire | 147 adult IBD patients aged 18–35 with IBD 64% CD 90% females | 69% of participants reported that diet modification could reduce IBD symptoms | 66% of patients modified dairy consumption as a result of IBD. |
| Peters et al. [64], 2021 | Case–control study The Netherlands D | FFQ by the nutritional department of Wageningen University | Groningen 1000IBD cohort and the Lifelines DEEP Cohort 493 IBD 207 UC 286 CD 61% females healthy population controls 1297 | Compared to controls, patients with active disease consumed less cheese (27.2 ± 28.8 vs. 30.2 ± 26.8, p = 0.030), dairy (229 ± 185 vs. 285 ± 192, p < 0.001); UC patients ate less dairy (250 ± 171 vs. 285 ± 192, p = 0.014); CD patients consumed less cheese (26.2 ± 7.1 vs. 30.2 ± 26.8, p = 0.006) Patients in remission ate less dairy (227 ± 186 vs. 285 ± 192, p < 0.001). | |
| Crooks et al. [66] 2021 | Cross-sectional, multicenter study UK E | 30-item questionnaire | 255 British South Asians with IBD 154 UC 93 CD IBDU 8 | 42% of patients reported milk as a dietary trigger for IBD relapse. | 49% of patients avoided milk products. |
| Sienkiewicz M. et al. [68] 2021 | Cross-sectional Poland E | 186-item FFQ | 73 IBD 44% UC 103 healthy volunteers | IBD patients ate milk and dairy products less frequently (1.06 vs. 1.81, p < 0.001) than the control group. | |
| Guida et al. [65] 2021 | Prospective Italy E | Questionnaire on eating habits And food intolerances | 167 IBD: 81 UC 86 CD 57.5% males the mean age 48.6 ± 16 years | Milk and dairy products were reported as symptom triggers by 57 patients (34.1%). | 67.9% of participants avoided milk and dairy as they suspected, that they may be responsible for the occurrence of symptoms. |
| Vagianos et al. [79] 2022 | Matched case–control longitudinal study Canada D | Biweekly online surveys for 1 year | 61% of patients reported at baseline that food may induce flares, and 25% of those with active disease believed that food may have caused their current flare | Consumption of assessed foods in the previous 2 weeks between patients with active and inactive disease did not differ. | |
| Cheng-Tzu Hsieh et al. 2022 [60] | Cross-sectional Taiwan E | Questionnaire developed by researchers from the UK, Japan, and Taiwan | 50 UC 60% males 46.9 years | 45.8% of patients reported, that diet triggered UC onset, and 48.0% of patients believed diet has ever caused flares. | 59.2% of patients restricted consumption of milk and dairy. |
| Godala et al. [70] 2023 | Prospective, questionnaire-based study Poland E | Questionnaire | 82 IBD: 48 CD 34 UC 48.8% males 38.1 ± 11.6 years | 47.8% believed, that milk and dairy should be avoided | 28.9% of participants followed lactose-free diet, but 65.8% on lactose-free diet consumed cheese and yogurts. |
| Rana et al. [59] 2023 | Cross-sectional study India E | Questionnaire | 84 UC 38 years (24–49.3) 55.9% males | Amongst the predictors of flare consumption of dairy did not differ between patients and controls (88.2% and 75.8%). |
3.3. The Impact of Milk and Dairy Consumption on the IBD Course
| Author Year | Study Design Country Quality Level * | Methods | Patients | Results |
|---|---|---|---|---|
| Tasson et al. [85] 2017 | Cross-sectional Italy E | 146-item FFQ on the intake of several foods over 1 year correlated with disease activity measured with fecal calprotectin | 103 IBD 54 CD 49 UC 50 active 53 remission age 45.85 ± 14.2 years | No statistically significant associations were found between disease flare and the consumption of dairy. |
| Komperød et al. [86] 2018 | Prospective Norway D | The intervention consisted of two weeks of a habitual diet and two weeks of an elimination diet. | 16 CD in remission | Cow’s milk was the most commonly reported dietary trigger of GI symptoms (13 of 16 patients) The elimination diet was effective in CD patients in remission. |
| Vagianos et al. [79] 2022 | Matched case–control longitudinal study Canada D | Biweekly online surveys for 1 year Self-reported flares | 95 IBD flare 64 (67%) CD 31 (33%) UC 95 non-flare controls | Increased consumption of milk products in the past 2 weeks did not differ between flares (n = 25; 36%) and controls (n = 31; 33%, p = 0.257). |
4. Summary
5. Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kellermayer, R.; Zilbauer, M. The Gut Microbiome and the Triple Environmental Hit Concept of Inflammatory Bowel Disease Pathogenesis. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 589–595. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s Disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Lamb, C.A.; Titterton, C.; Banerjee, R.; Gomberg, A.; Rubin, D.T.; Hart, A.L. Inflammatory Bowel Disease Has No Borders: Engaging Patients as Partners to Deliver Global, Equitable and Holistic Health Care. Lancet 2024, S0140673624009838. [Google Scholar] [CrossRef]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The Epidemiology of Inflammatory Bowel Disease: East Meets West. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Cui, G.; Liu, H.; Xu, G.; Laugsand, J.-B.; Pang, Z. Exploring Links Between Industrialization, Urbanization, and Chinese Inflammatory Bowel Disease. Front. Med. 2021, 8, 757025. [Google Scholar] [CrossRef]
- Aniwan, S.; Santiago, P.; Loftus, E.V.; Park, S.H. The Epidemiology of Inflammatory Bowel Disease in Asia and Asian Immigrants to Western Countries. UEG J. 2022, 10, 1063–1076. [Google Scholar] [CrossRef]
- Banerjee, R.; Pal, P.; Prakash, N.; Mudigonda, S.; Joseph, S.; Patel, R.; Khalil, M.; Komawar, A.; Korikana, S.; Mekala, D.; et al. DOP60 Environmental Risk Factors for Inflammatory Bowel Disease: A Large Prospective Case-Control Study in India. J. Crohn’s Colitis 2023, 17, i132–i135. [Google Scholar] [CrossRef]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.-F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology 2018, 154, 1037–1046.e2. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Sigall-Boneh, R.; Levine, A.; Lomer, M.; Wierdsma, N.; Allan, P.; Fiorino, G.; Gatti, S.; Jonkers, D.; Kierkus, J.; Katsanos, K.H.; et al. Research Gaps in Diet and Nutrition in Inflammatory Bowel Disease. A Topical Review by D-ECCO Working Group [Dietitians of ECCO]. J. Crohn’s Colitis 2017, 11, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Ghosh, S. Diet and Nutrition in IBD—Progress and Gaps. Nutrients 2019, 11, 1740. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Chan, S.S.M.; Lochhead, P.; Ananthakrishnan, A.N.; Hart, A.R.; Chan, A.T. The Role of Diet in the Aetiopathogenesis of Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 525–535. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; MacDonald, J.K.; Gordon, M.; Mullin, G.E. Dietary Interventions for Induction and Maintenance of Remission in Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2019, 2, CD012839. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C. Milk Nutritional Composition and Its Role in Human Health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-J.; Tan, J.-S.; Gao, X.-J.; Yang, J.-G.; Yang, Y.-J. Effect of Cheese Intake on Cardiovascular Diseases and Cardiovascular Biomarkers. Nutrients 2022, 14, 2936. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.M.; Stephensen, C.B.; Kratz, M.; Bolling, B.W. Exploring the Links between Diet and Inflammation: Dairy Foods as Case Studies. Adv. Nutr. 2021, 12, 1S–13S. [Google Scholar] [CrossRef]
- Nieman, K.M.; Anderson, B.D.; Cifelli, C.J. The Effects of Dairy Product and Dairy Protein Intake on Inflammation: A Systematic Review of the Literature. J. Am. Coll. Nutr. 2021, 40, 571–582. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B.; Gil, A.; Rangel-Huerta, O.D. Milk and Dairy Product Consumption and Inflammatory Biomarkers: An Updated Systematic Review of Randomized Clinical Trials. Adv. Nutr. 2019, 10, S239–S250. [Google Scholar] [CrossRef]
- Díaz-López, A.; Bulló, M.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Fitó, M.; Gómez-Gracia, E.; Fiol, M.; García de la Corte, F.J.; Ros, E.; et al. Dairy Product Consumption and Risk of Type 2 Diabetes in an Elderly Spanish Mediterranean Population at High Cardiovascular Risk. Eur. J. Nutr. 2016, 55, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk Consumption and Multiple Health Outcomes: Umbrella Review of Systematic Reviews and Meta-Analyses in Humans. Nutr. Metab. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Thomson, P.; Medina, D.A.; Garrido, D. Human Milk Oligosaccharides and Infant Gut Bifidobacteria: Molecular Strategies for Their Utilization. Food Microbiol. 2018, 75, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, L.-Q.; Liu, F.; Wu, J.-Y. Human Milk Oligosaccharides and Infant Gut Microbiota: Molecular Structures, Utilization Strategies and Immune Function. Carbohydr. Polym. 2022, 276, 118738. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Du, M.; Mao, X. Bovine α-Lactalbumin Hydrolysates Ameliorate Obesity-Associated Endotoxemia and Inflammation in High-Fat Diet-Fed Mice through Modulation of Gut Microbiota. Food Funct. 2019, 10, 3368–3378. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Shen, Y.; Su, E.; Du, L.; Xie, J.; Wei, D. Anti-Hyperuricemic, Nephroprotective, and Gut Microbiota Regulative Effects of Separated Hydrolysate of α-Lactalbumin on Potassium Oxonate- and Hypoxanthine-Induced Hyperuricemic Mice. Mol. Nutr. Food Res. 2023, 67, 2200162. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, X.; Zheng, Y.; Li, L.; Wang, F.; Wei, H.; Peng, J. Paneth Cells Protect Intestinal Stem Cell Niche to Alleviate Deoxynivalenol-Induced Intestinal Injury. Ecotoxicol. Environ. Saf. 2023, 264, 115457. [Google Scholar] [CrossRef]
- Maga, E.A.; Desai, P.T.; Weimer, B.C.; Dao, N.; Kültz, D.; Murray, J.D. Consumption of Lysozyme-Rich Milk Can Alter Microbial Fecal Populations. Appl. Env. Microbiol. 2012, 78, 6153–6160. [Google Scholar] [CrossRef]
- Manuyakorn, W.; Tanpowpong, P. Cow Milk Protein Allergy and Other Common Food Allergies and Intolerances. Paediatr. Int. Child. Health 2019, 39, 32–40. [Google Scholar] [CrossRef]
- Szilagyi, A.; Galiatsatos, P.; Xue, X. Systematic Review and Meta-Analysis of Lactose Digestion, Its Impact on Intolerance and Nutritional Effects of Dairy Food Restriction in Inflammatory Bowel Diseases. Nutr. J. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, Regional, and Global Estimates for Lactose Malabsorption in Adults: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef]
- Zingone, F.; Bertin, L.; Maniero, D.; Palo, M.; Lorenzon, G.; Barberio, B.; Ciacci, C.; Savarino, E.V. Myths and Facts about Food Intolerance: A Narrative Review. Nutrients 2023, 15, 4969. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and Health Attributes of Milk and Milk Imitations. Eur. J. Nutr. 2020, 59, 19–34. [Google Scholar] [CrossRef]
- Facioni, M.S.; Raspini, B.; Pivari, F.; Dogliotti, E.; Cena, H. Nutritional Management of Lactose Intolerance: The Importance of Diet and Food Labelling. J. Transl. Med. 2020, 18, 260. [Google Scholar] [CrossRef]
- Angima, G.; Qu, Y.; Park, S.H.; Dallas, D.C. Prebiotic Strategies to Manage Lactose Intolerance Symptoms. Nutrients 2024, 16, 1002. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Larijani, B.; Esmaillzadeh, A. Consumption of Milk and Dairy Products and Risk of Osteoporosis and Hip Fracture: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 1722–1737. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Du, X.; Shi, B.-M.; Qin, L.-Q. Systematic Review and Meta-Analysis of the Association between Dairy Consumption and the Risk of Hip Fracture: Critical Interpretation of the Currently Available Evidence. Osteoporos. Int. 2020, 31, 1411–1425. [Google Scholar] [CrossRef]
- Hidayat, K.; Chen, J.-S.; Wang, T.-C.; Liu, Y.-J.; Shi, Y.-J.; Su, H.-W.; Liu, B.; Qin, L.-Q. The Effects of Milk Supplementation on Bone Health Indices in Adults: A Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 1186–1199. [Google Scholar] [CrossRef]
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507. [Google Scholar] [CrossRef]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, Function, Denaturation and Digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Practical Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.E.; Wilson, B.; Wall, C.L. British Dietetic Association Consensus Guidelines on the Nutritional Assessment and Dietary Management of Patients with Inflammatory Bowel Disease. J. Hum. Nutr. Diet. 2022, 36, 336–377. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients With Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 752–759. [Google Scholar] [CrossRef]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-Based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef]
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s Disease Exclusion Diet for Induction and Maintenance of Remission in Adults with Mild-to-Moderate Crohn’s Disease (CDED-AD): An Open-Label, Pilot, Randomised Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Straus, S.E.; Glasziou, P.; Richardson, W.S.; Haynes, R.B. Evidence-Based Medicine; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Opstelten, J.L.; Leenders, M.; Dik, V.K.; Chan, S.S.M.; Van Schaik, F.D.M.; Khaw, K.T.; Luben, R.; Hallmans, G.; Karling, P.; Lindgren, S.; et al. Dairy Products, Dietary Calcium, and Risk of Inflammatory Bowel Disease: Results From a European Prospective Cohort Investigation. Inflamm. Bowel Dis. 2016, 22, 1403–1411. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of Ultra-Processed Food Intake with Risk of Inflammatory Bowel Disease: Prospective Cohort Study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Van Der Sloot, K.W.J.; Weersma, R.K.; Alizadeh, B.Z.; Dijkstra, G. Identification of Environmental Risk Factors Associated With the Development of Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Han, M.K.; Anderson, R.; Viennois, E.; Merlin, D. Examination of Food Consumption in United States Adults and the Prevalence of Inflammatory Bowel Disease Using National Health Interview Survey 2015. PLoS ONE 2020, 15, e0232157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, H.; Tian, A.; Guo, Y.; Zhao, X.; Zhang, M.; Chen, L.; Wen, J.; Yang, J.; Qi, B.; et al. Whole-Milk Consumption Decreases the Risk of Inflammatory Bowel Disease: A Two-Sample Mendelian Randomization Analysis. J. Bio-X Res. 2021, 04, 114–119. [Google Scholar] [CrossRef]
- Maconi, G.; Ardizzone, S.; Cucino, C.; Bezzio, C.; Russo, A.G.; Porro, G.B. Pre-Illness Changes in Dietary Habits and Diet as a Risk Factor for Inflammatory Bowel Disease: A Case-Control Study. World J. Gastroenterol. 2010, 16, 4297–4304. [Google Scholar] [CrossRef]
- Bikbavova, G.R.; Livzan, M.A.; Turchaninov, D.V.; Sovalkin, V.I.; Akhmedov, V.A. Ulcerative Colitis: Nutritional Habits as the Disease Risk Factor. Russ. Open Med. J. 2021, 10, 108. [Google Scholar] [CrossRef]
- Preda, C.; Manuc, T.; Chifulescu, A.E.; Istratescu, D.; Louis, E.; Baicus, C.; Sandra, I.; Diculescu, M.; Reenaers, C.; Van Kemseke, C.; et al. Diet as an Environmental Trigger in Inflammatory Bowel Disease: A Retrospective Comparative Study in Two European Cohorts. Rev. Esp. Enfermedades Dig. Organo Of. Soc. Esp. Patol. Dig. 2020, 112, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Kim, S.-K.; Hong, S.-J. Food Elimination Diet and Nutritional Deficiency in Patients with Inflammatory Bowel Disease. Clin. Nutr. Res. 2018, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.S.; Mahajan, G.; Patil, A.N.; Singh, A.K.; Jearth, V.; Sekar, A.; Singh, H.; Saroch, A.; Dutta, U.; Sharma, V. Factors Contributing to Flares of Ulcerative Colitis in North India- a Case-Control Study. BMC Gastroenterol. 2023, 23, 336. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-T.; Weng, M.-T.; Tung, C.-C.; Chen, N.-C.; Chen, H.-C.; Chien, K.-L.; Wei, S.-C. Dietary Beliefs and Information Resources of Ulcerative Colitis Patients in Clinical Remission: A Cross-Sectional Survey in Taiwan. Clin. Nutr. ESPEN 2022, 51, 430–436. [Google Scholar] [CrossRef]
- Lopes, M.B.; Rocha, R.; Lyra, A.C.; Oliveira, V.R.; Coqueiro, F.G.; Almeida, N.S.; Valois, S.S.; Santana, G.O. Restriction of Dairy Products; a Reality in Inflammatory Bowel Disease Patients. Nutr. Hosp. 2014, 29, 575–581. [Google Scholar] [CrossRef]
- Crooks, B.; Misra, R.; Arebi, N.; Kok, K.; Brookes, M.J.; McLaughlin, J.; Limdi, J.K. The Dietary Practices and Beliefs of British South Asian People Living with Inflammatory Bowel Disease: A Multicenter Study from the United Kingdom. Intest. Res. 2022, 20, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary Patterns and Self-Reported Associations of Diet with Symptoms of Inflammatory Bowel Disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Tigchelaar-Feenstra, E.F.; Imhann, F.; Dekens, J.A.M.; Swertz, M.A.; Franke, L.H.; Wijmenga, C.; Weersma, R.K.; Alizadeh, B.Z.; Dijkstra, G.; et al. Habitual Dietary Intake of IBD Patients Differs from Population Controls: A Case-Control Study. Eur. J. Nutr. 2021, 60, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Guida, L.; Di Giorgio, F.M.; Busacca, A.; Carrozza, L.; Ciminnisi, S.; Almasio, P.L.; Di Marco, V.; Cappello, M. Perception of the Role of Food and Dietary Modifications in Patients with Inflammatory Bowel Disease: Impact on Lifestyle. Nutrients 2021, 13, 759. [Google Scholar] [CrossRef] [PubMed]
- Crooks, B.; McLaughlin, J.; Matsuoka, K.; Kobayashi, T.; Yamazaki, H.; Limdi, J.K. The Dietary Practices and Beliefs of People Living with Inactive Ulcerative Colitis. Eur. J. Gastroenterol. Hepatol. 2021, 33, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Krela-Kaźmierczak, I.; Michalak, M.; Szymczak-Tomczak, A.; Czarnywojtek, A.; Wawrzyniak, A.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Dobrowolska, A.; Eder, P. Milk and Dairy Product Consumption in Patients with Inflammatory Bowel Disease: Helpful or Harmful to Bone Mineral Density? Nutrition 2020, 79–80, 110830. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Szymańska, P.; Maciejewska, O.; Niewiadomska, J.; Wiśniewska-Jarosińska, M.; Fichna, J. Assessment of Dietary Habits in Inflammatory Bowel Disease Patients: A Cross-Sectional Study from Poland. Nutr. Bull. 2021, 46, 432–442. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Lech, G. Analysis of the Nutrients and Food Products Intake of Polish Males with Ulcerative Colitis in Remission. Nutrients 2019, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Godala, M.; Gaszyńska, E.; Durko, Ł.; Małecka-Wojciesko, E. Dietary Behaviors and Beliefs in Patients with Inflammatory Bowel Disease. JCM 2023, 12, 3455. [Google Scholar] [CrossRef]
- Vidarsdottir, J.B.; Johannsdottir, S.E.; Thorsdottir, I.; Bjornsson, E.; Ramel, A. A Cross-Sectional Study on Nutrient Intake and -Status in Inflammatory Bowel Disease Patients. Nutr. J. 2015, 15, 61. [Google Scholar] [CrossRef]
- Walton, M.; Alaunyte, I. Do Patients Living with Ulcerative Colitis Adhere to Healthy Eating Guidelines? A Cross-Sectional Study. Br. J. Nutr. 2014, 112, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients With Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Suraci, E.; Marasco, R.; Imeneo, M.; Abenavoli, L.; Luzza, F. Self-Prescribed Dietary Restrictions Are Common in Inflammatory Bowel Disease Patients and Are Associated with Low Bone Mineralization. Medicina 2019, 55, 507. [Google Scholar] [CrossRef] [PubMed]
- Opstelten, J.L.; de Vries, J.H.M.; Wools, A.; Siersema, P.D.; Oldenburg, B.; Witteman, B.J.M. Dietary Intake of Patients with Inflammatory Bowel Disease: A Comparison with Individuals from a General Population and Associations with Relapse. Clin. Nutr. 2019, 38, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Bach, U.; Jensen, H.N.; Rasmussen, H.H.; Fallingborg, J.; Holst, M.; Bach, U.; Jensen, H.N.; Rasmussen, H.H.; Fallingborg, J.; Holst, M. Dietary Habits in Patients with Ulcerative Colitis—Cause of Nutrient Deficiency? Food Nutr. Sci. 2014, 5, 1945–1950. [Google Scholar] [CrossRef][Green Version]
- Zallot, C.; Quilliot, D.; Chevaux, J.B.; Peyrin-Biroulet, C.; Guéant-Rodriguez, R.M.; Freling, E.; Collet-Fenetrier, B.; Williet, N.; Ziegler, O.; Bigard, M.A.; et al. Dietary Beliefs and Behavior among Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 2013, 19, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Triggs, C.M.; Munday, K.; Hu, R.; Fraser, A.G.; Gearry, R.B.; Barclay, M.L.; Ferguson, L.R. Dietary Factors in Chronic Inflammation: Food Tolerances and Intolerances of a New Zealand Caucasian Crohn’s Disease Population. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 690, 123–138. [Google Scholar] [CrossRef]
- Vagianos, K.; Shafer, L.A.; Witges, K.; Graff, L.A.; Targownik, L.E.; Bernstein, C.N. Self-reported Flares among People Living with Inflammatory Bowel Disease Are Associated with Stress and Worry but Not Associated with Recent Diet Changes: The Manitoba Living with IBD Study. J. Parenter. Enter. Nutr. 2022, 46, 1686–1698. [Google Scholar] [CrossRef]
- Marsh, A.; Kinneally, J.; Robertson, T.; Lord, A.; Young, A.; Radford –Smith, G. Food Avoidance in Outpatients with Inflammatory Bowel Disease—Who, What and Why. Clin. Nutr. ESPEN 2019, 31, 10–16. [Google Scholar] [CrossRef]
- Vagianos, K.; Clara, I.; Carr, R.; Graff, L.A.; Walker, J.R.; Targownik, L.E.; Lix, L.M.; Rogala, L.; Miller, N.; Bernstein, C.N. What Are Adults With Inflammatory Bowel Disease (IBD) Eating? A Closer Look at the Dietary Habits of a Population-Based Canadian IBD Cohort. JPEN. J. Parenter. Enter. Nutr. 2016, 40, 405–411. [Google Scholar] [CrossRef]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary Practices and Beliefs in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef]
- Crooks, B.; Misra, R.; Arebi, N.; Kok, K.; Brookes, M.J.; McLaughlin, J.; Limdi, J.K. The Dietary Practices and Beliefs of People Living with Older-Onset Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2021, 33, e442–e448. [Google Scholar] [CrossRef]
- Kamp, K.J.; Pennings, B.; Javelli, D.; Wyatt, G.; Given, B. Dietary Patterns, Beliefs and Behaviours among Individuals with Inflammatory Bowel Disease: A Cross-sectional Study. J. Hum. Nutr. Diet. 2021, 34, 257–264. [Google Scholar] [CrossRef]
- Tasson, L.; Canova, C.; Vettorato, M.G.; Savarino, E.; Zanotti, R. Influence of Diet on the Course of Inflammatory Bowel Disease. Dig. Dis. Sci. 2017, 62, 2087–2094. [Google Scholar] [CrossRef]
- Komperød, M.J.; Sommer, C.; Mellin-Olsen, T.; Iversen, P.O.; Røseth, A.G.; Valeur, J. Persistent Symptoms in Patients with Crohn’s Disease in Remission: An Exploratory Study on the Role of Diet. Scand. J. Gastroenterol. 2018, 53, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Orellana, D.; McNulty, K.; Jaffe, N.; Yin, X.; Sauk, J.S.; Limketkai, B.N. Dairy Consumption Patterns and Clinical Disease in Patients with Inflammatory Bowel Disease. Gastroenterology 2024, 164, S-877. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.; Neubauer, K. Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults. Diagnostics 2020, 10, 367. [Google Scholar] [CrossRef]
- He, P.; Yu, L.; Tian, F.; Zhang, H.; Chen, W.; Zhai, Q. Dietary Patterns and Gut Microbiota: The Crucial Actors in Inflammatory Bowel Disease. Adv. Nutr. 2022, 13, 1628–1651. [Google Scholar] [CrossRef] [PubMed]
- Dolovich, C.; Shafer, L.A.; Vagianos, K.; Witges, K.; Targownik, L.E.; Bernstein, C.N. The Complex Relationship between Diet, Symptoms, and Intestinal Inflammation in Persons with Inflammatory Bowel Disease: The Manitoba Living With IBD Study. J. Parenter. Enter. Nutr. 2022, 46, 867–877. [Google Scholar] [CrossRef]
- González, S.; Fernández-Navarro, T.; Arboleya, S.; de los Reyes-Gavilán, C.G.; Salazar, N.; Gueimonde, M. Fermented Dairy Foods: Impact on Intestinal Microbiota and Health-Linked Biomarkers. Front. Microbiol. 2019, 10, 1046. [Google Scholar] [CrossRef]
- Holt, D.Q.; Strauss, B.J.; Moore, G.T. Patients with Inflammatory Bowel Disease and Their Treating Clinicians Have Different Views Regarding Diet. J. Hum. Nutr. Diet. 2017, 30, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.D.; King, L.; Morgan, M.; Ayis, S.; Direkze, N.; Lomer, M.C.; Lindsay, J.O.; Whelan, K. Food-Related Quality of Life in Inflammatory Bowel Disease: Development and Validation of a Questionnaire. ECCOJC 2016, 10, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Murrells, T.; Morgan, M.; Cummings, F.; Stansfield, C.; Todd, A.; Sebastian, S.; Lobo, A.; Lomer, M.C.E.; Lindsay, J.O.; et al. Food-Related Quality of Life Is Impaired in Inflammatory Bowel Disease and Associated with Reduced Intake of Key Nutrients. Am. J. Clin. Nutr. 2021, 113, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.R.; Clarke, H.; O’Keeffe, M.; Dubois, P.; Irving, P.M.; Lindsay, J.O.; Whelan, K. Nutrient, Fibre, and FODMAP Intakes and Food-Related Quality of Life in Patients with Inflammatory Bowel Disease, and Their Relationship with Gastrointestinal Symptoms of Differing Aetiologies. J. Crohn’s Colitis 2021, 15, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Kärnsund, S.; Lo, B.; Bendtsen, F.; Holm, J.; Burisch, J. Systematic Review of the Prevalence and Development of Osteoporosis or Low Bone Mineral Density and Its Risk Factors in Patients with Inflammatory Bowel Disease. WJG 2020, 26, 5362–5374. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Liu, Y.; Luo, J.; Huang, Z.-D.; Zhang, C.; Fu, Y. Vitamin D Deficiency Associated with Crohn’s Disease and Ulcerative Colitis: A Meta-Analysis of 55 Observational Studies. J. Transl. Med. 2019, 17, 323. [Google Scholar] [CrossRef]
- Fabisiak, N.; Fabisiak, A.; Watala, C.; Fichna, J. Fat-Soluble Vitamin Deficiencies and Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2017, 51, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Walia, C.; Elkadri, A.; Pipkorn, R.; Dunn, R.K.; Sieracki, R.; Goday, P.S.; Cabrera, J.M. A Systematic Review of Micronutrient Deficiencies in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Rempel, J.; Grover, K.; El-Matary, W. Micronutrient Deficiencies and Anemia in Children with Inflammatory Bowel Disease. Nutrients 2021, 13, 236. [Google Scholar] [CrossRef]
- Lambert, K.; Pappas, D.; Miglioretto, C.; Javadpour, A.; Reveley, H.; Frank, L.; Grimm, M.C.; Samocha-Bonet, D.; Hold, G.L. Systematic Review with Meta-analysis: Dietary Intake in Adults with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2021, 54, 742–754. [Google Scholar] [CrossRef]
- Campmans-Kuijpers, M.J.E.; Dijkstra, G. Food and Food Groups in Inflammatory Bowel Disease (IBD): The Design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients 2021, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, A.; Ehrlich, O.G.; Hwang, C.; Issokson, K.; Zapala, S.; Weaver, A.; Siegel, C.A.; Melmed, G.Y. Knowledge, Attitudes, and Beliefs Regarding the Role of Nutrition in IBD Among Patients and Providers. Inflamm. Bowel Dis. 2016, 22, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Park, S.; Liu, Y.; Greenlund, K.J. Dietary Intake Patterns among Adults with Inflammatory Bowel Disease in the United States, 2015. PLoS ONE 2021, 16, e0250441. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempinski, R.; Arabasz, D.; Neubauer, K. Effects of Milk and Dairy on the Risk and Course of Inflammatory Bowel Disease versus Patients’ Dietary Beliefs and Practices: A Systematic Review. Nutrients 2024, 16, 2555. https://doi.org/10.3390/nu16152555
Kempinski R, Arabasz D, Neubauer K. Effects of Milk and Dairy on the Risk and Course of Inflammatory Bowel Disease versus Patients’ Dietary Beliefs and Practices: A Systematic Review. Nutrients. 2024; 16(15):2555. https://doi.org/10.3390/nu16152555
Chicago/Turabian StyleKempinski, Radoslaw, Damian Arabasz, and Katarzyna Neubauer. 2024. "Effects of Milk and Dairy on the Risk and Course of Inflammatory Bowel Disease versus Patients’ Dietary Beliefs and Practices: A Systematic Review" Nutrients 16, no. 15: 2555. https://doi.org/10.3390/nu16152555
APA StyleKempinski, R., Arabasz, D., & Neubauer, K. (2024). Effects of Milk and Dairy on the Risk and Course of Inflammatory Bowel Disease versus Patients’ Dietary Beliefs and Practices: A Systematic Review. Nutrients, 16(15), 2555. https://doi.org/10.3390/nu16152555





