Promoting Glutathione Synthesis: A Possibility for Treating Cardiomyopathy Induced by a Maternal Western Diet
Abstract
1. Introduction
2. Materials and Methods
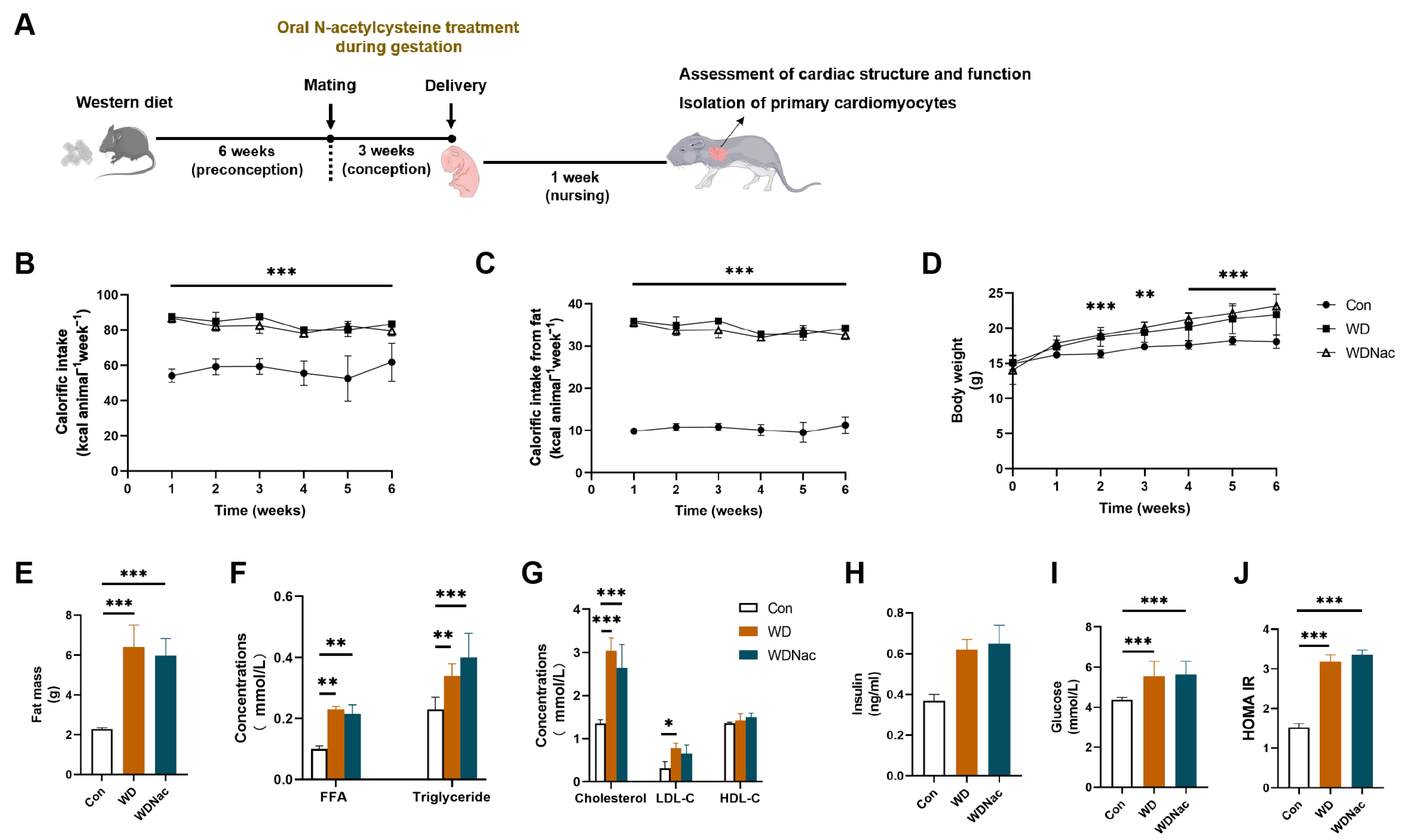
2.1. Animals and Experimental Procedures
2.2. Serological Metabolic Analysis
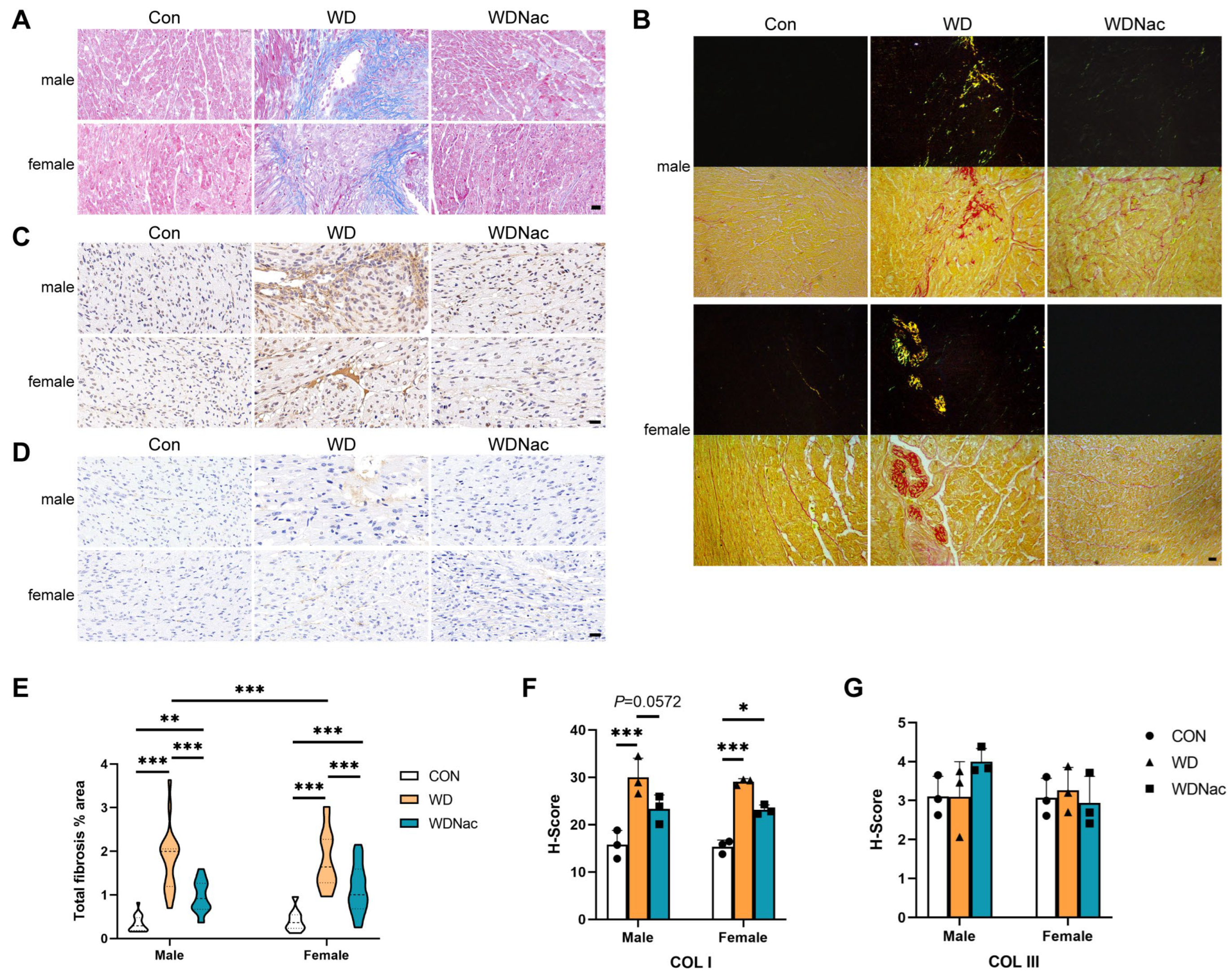
2.3. Histological Analysis
2.4. Echocardiographic Evaluation
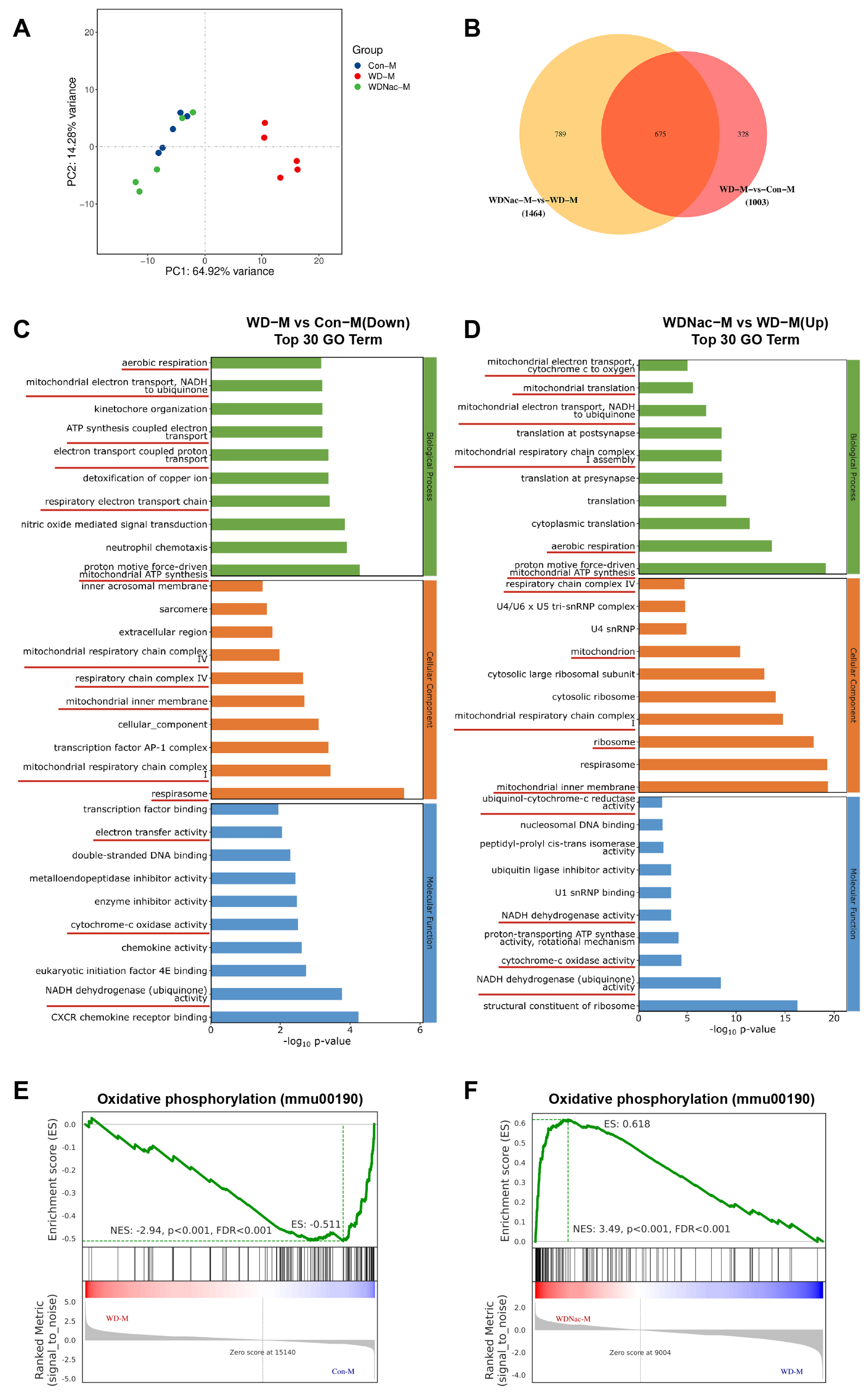
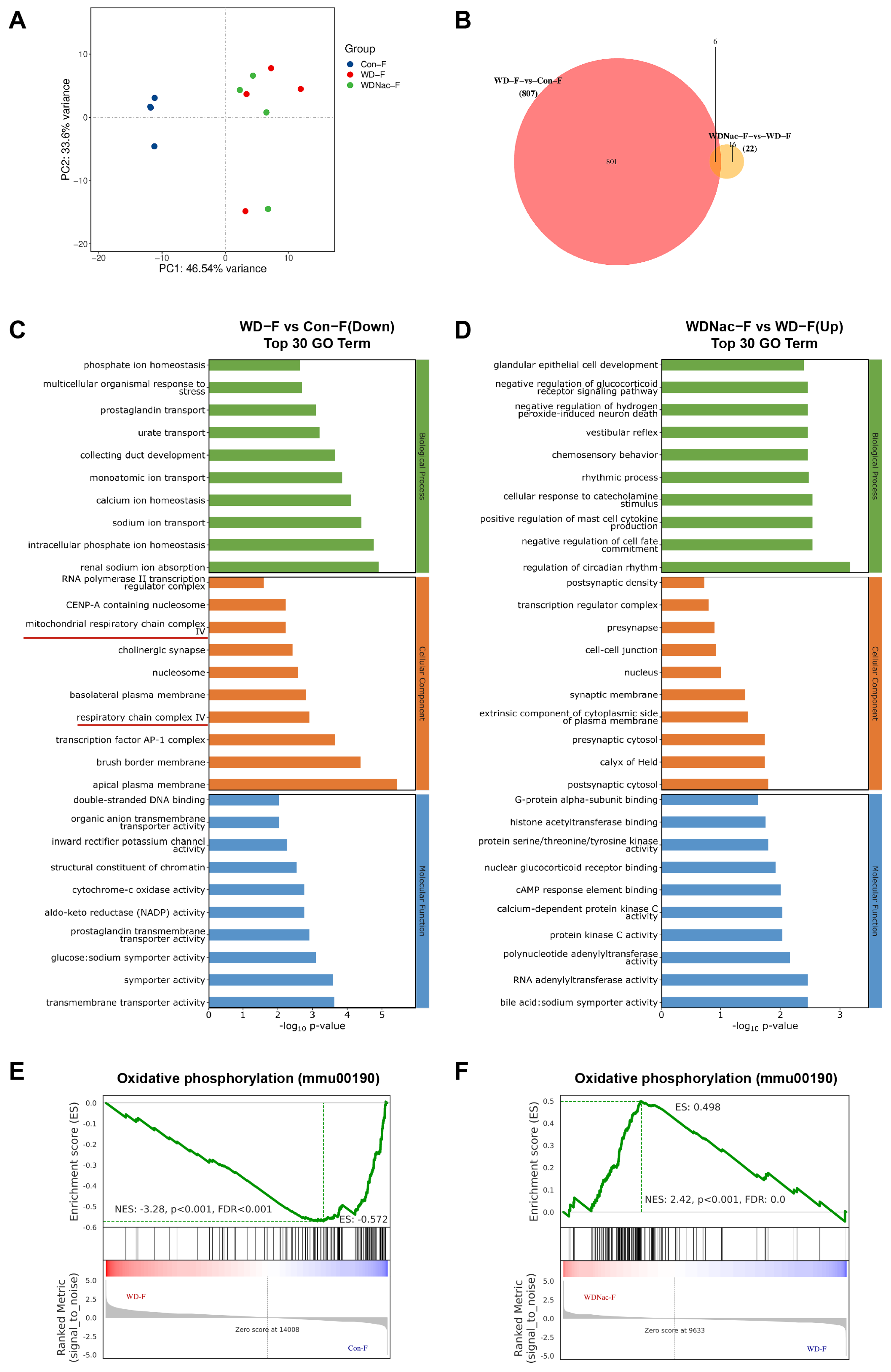
2.5. RNA Sequencing and Functional Enrichment Analysis
2.6. Antioxidant Capacity Assays
2.7. ROS Assay
2.8. Mitochondrial Ultrastructure
2.9. Isolation, Purification, and Culture of Primary Cardiomyocytes
2.10. MitoSOX Assay
2.11. Statistical Analysis
3. Results
3.1. N-Acetylcysteine Restores Heart Abnormalities in Offspring Caused by a Maternal Western Diet
3.2. N-Acetylcysteine Alleviates Myocardial Interstitial Fibrosis and Extracellular Matrix Remodeling Induced by a Maternal Western Diet
3.3. N-Acetylcysteine Attenuates Cardiomyocyte Apoptosis and Dysfunction Resulting from a Maternal Western Diet
3.4. N-Acetylcysteine Recovers Disrupted Oxidative Phosphorylation in Offspring from Dams Fed a Western Diet
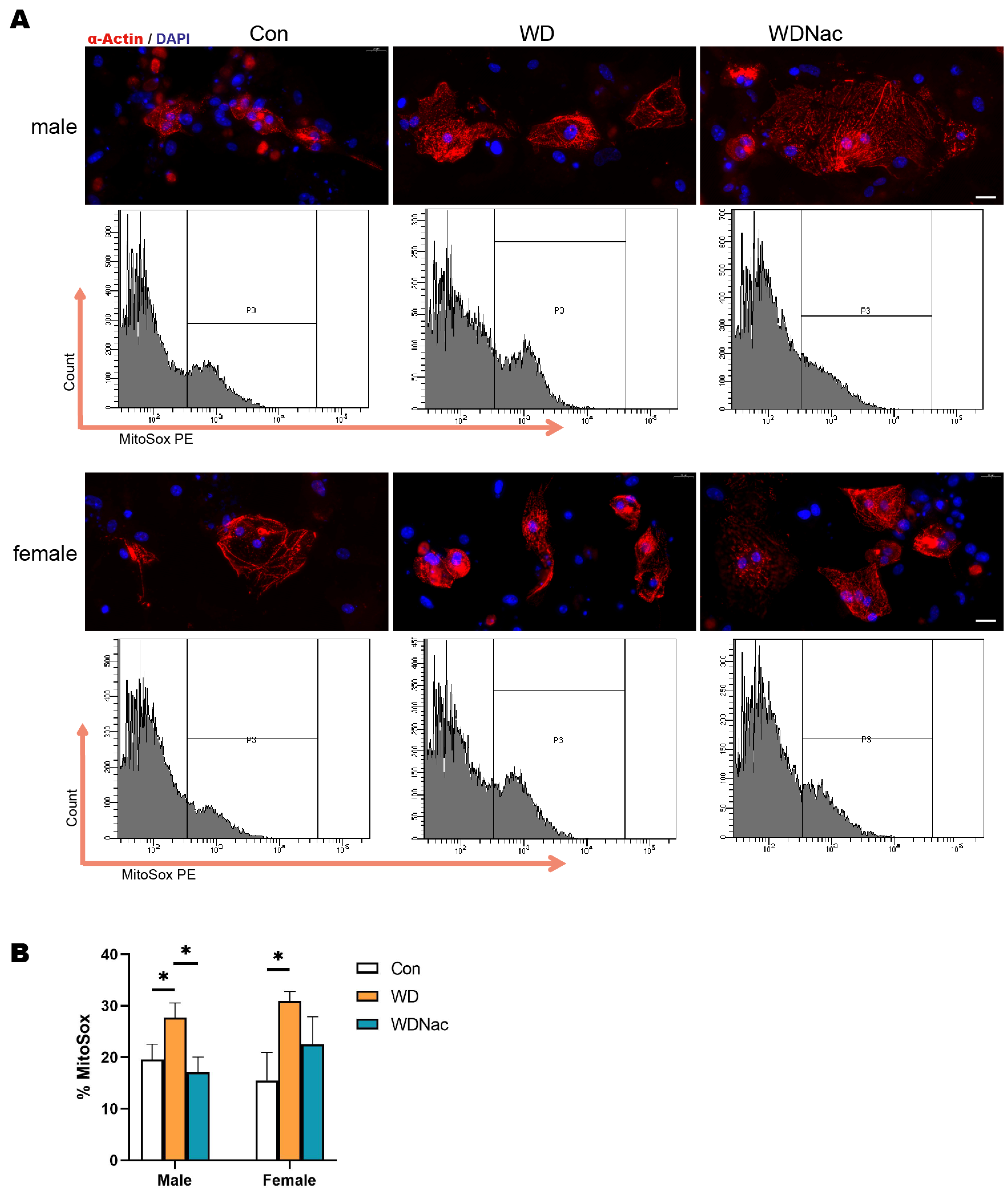
3.5. Boosting GSH Levels and GPx Activity with N-Acetylcysteine Effectively Scavenged Mitohondrial ROS in Male Offspring of Western Diet-Fed Dams
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elmaleh-Sachs, A.; Schwartz, J.L.; Bramante, C.T.; Nicklas, J.M.; Gudzune, K.A.; Jay, M. Obesity Management in Adults: A Review. JAMA 2023, 330, 2000–2015. [Google Scholar] [CrossRef] [PubMed]
- Radlinger, B.; Ress, C.; Folie, S.; Salzmann, K.; Lechuga, A.; Weiss, B.; Salvenmoser, W.; Graber, M.; Hirsch, J.; Holfeld, J.; et al. Empagliflozin protects mice against diet-induced obesity, insulin resistance and hepatic steatosis. Diabetologia 2023, 66, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.; Justo-Méndez, R.; Jiménez-Blasco, D.; Núñez, V.; Calero, I.; Villalba-Orero, M.; Alegre-Martí, A.; Fischer, T.; Gradillas, A.; Sant’anna, V.A.R.; et al. γ-Linolenic acid in maternal milk drives cardiac metabolic maturation. Nature 2023, 618, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Elgazzaz, M.; Berdasco, C.; Garai, J.; Baddoo, M.; Lu, S.; Daoud, H.; Zabaleta, J.; Mauvais-Jarvis, F.; Lazartigues, E. Maternal Western diet programs cardiometabolic dysfunction and hypothalamic inflammation via epigenetic mechanisms predominantly in the male offspring. Mol. Metab. 2024, 80, 101864. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huo, Y.; Zhang, J.; Xu, D.; Bai, F.; Gui, Y. Association between High-Fat Diet during Pregnancy and Heart Weight of the Offspring: A Multivariate and Mediation Analysis. Nutrients 2022, 14, 4237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, L.; Tan, Y.; Zheng, Y.; Gui, Y. N-acetylcysteine protects neonatal mice from ventricular hypertrophy induced by maternal obesity in a sex-specific manner. Biomed. Pharmacother. 2021, 133, 110989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, L.; Wang, W.; Huo, Y.; Zheng, Y.; Wu, F.; Gui, Y. Protective effect of antioxidants on cardiac function in adult offspring exposed to prenatal overnutrition. J. Dev. Orig. Health Dis. 2022, 13, 741–749. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Huo, Y.; Gui, Y. Maternal Obesity and Kawasaki Disease-like Vasculitis: A New Perspective on Cardiovascular Injury and Inflammatory Response in Offspring Male Mice. Nutrients 2023, 15, 3823. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wu, F.; He, J.; Zhang, Y.; Shi, N.; Peng, X.; Ou, Q.; Li, Z.; Jiang, X.; Zhong, J.; et al. Mitochondrial quality control in diabetic cardiomyopathy: From molecular mechanisms to therapeutic strategies. Int. J. Biol. Sci. 2022, 18, 5276–5290. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, K.-A.; Luchian, I.; Goriuc, A.; Antoci, L.-M.; Ciobanu, C.-G.; Popescu, R.; Vlad, C.-E.; Blaj, M.; Foia, L.G. Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef]
- Yan, M.; Li, Y.; Luo, Q.; Zeng, W.; Shao, X.; Li, L.; Wang, Q.; Wang, D.; Zhang, Y.; Diao, H.; et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Mengozzi, A.; Costantino, S.; Paneni, F.; Duranti, E.; Nannipieri, M.; Mancini, R.; Lai, M.; La Rocca, V.; Puxeddu, I.; Antonioli, L.; et al. Targeting SIRT1 Rescues Age- and Obesity-Induced Microvascular Dysfunction in Ex Vivo Human Vessels. Circ. Res. 2022, 131, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Yin, Y.; Ma, X.; Zhang, J.; Pan, W.; Tan, M.; Zhao, Y.; Yang, T.; Jiang, T.; Li, H. Glutathione system enhancement for cardiac protection: Pharmacological options against oxidative stress and ferroptosis. Cell Death Dis. 2023, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [PubMed]
- Moss, H.G.; Brown, T.R.; Wiest, D.B.; Jenkins, D.D. N-Acetylcysteine rapidly replenishes central nervous system glutathione measured via magnetic resonance spectroscopy in human neonates with hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow Metab. 2018, 38, 950–958. [Google Scholar] [CrossRef]
- Nery, F.G.; Tallman, M.J.; Cecil, K.M.; Blom, T.J.; Patino, L.R.; Adler, C.M.; DelBello, M.P. N-acetylcysteine for depression and glutamate changes in the left prefrontal cortex in adolescents and young adults at risk for bipolar disorder: A pilot study. Early Interv. Psychiatry 2022, 16, 195–199. [Google Scholar] [CrossRef]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.-W.; Wintering, N.A.; Bazzan, A.J.; Zhong, L.; Bowens, B.K.; Chervoneva, I.; Intenzo, C.; et al. N-Acetyl Cysteine Is Associated with Dopaminergic Improvement in Parkinson’s Disease. Clin. Pharmacol. Ther. 2019, 106, 884–890. [Google Scholar] [CrossRef]
- Tunster, S.J. Genetic sex determination of mice by simplex PCR. Biol. Sex Differ. 2017, 8, 31. [Google Scholar] [CrossRef]
- Du, C.; Zhao, Y.; Wang, K.; Nan, X.; Chen, R.; Xiong, B. Effects of Milk-Derived Extracellular Vesicles on the Colonic Transcriptome and Proteome in Murine Model. Nutrients 2022, 14, 3057. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Huang, Q.; Yi, X.; Qin, F.; Zhong, Z.; Lin, L.; Yang, H.; Gong, G.; Wu, W. Hypoxia Acclimation Protects against Heart Failure Postacute Myocardial Infarction via Fundc1-Mediated Mitophagy. Oxidative Med. Cell. Longev. 2022, 2022, 8192552. [Google Scholar] [CrossRef]
- Louwagie, E.J.; Larsen, T.D.; Wachal, A.L.; Gandy, T.C.; Eclov, J.A.; Rideout, T.C.; Kern, K.A.; Cain, J.T.; Anderson, R.H.; Mdaki, K.S.; et al. Age and Sex Influence Mitochondria and Cardiac Health in Offspring Exposed to Maternal Glucolipotoxicity. iScience 2020, 23, 101746. [Google Scholar] [CrossRef]
- Talbot, C.P.J.; Dolinsky, V.W. Sex differences in the developmental origins of cardiometabolic disease following exposure to maternal obesity and gestational diabetes. Appl. Physiol. Nutr. Metab. 2019, 44, 687–695. [Google Scholar] [CrossRef]
- Chen, Y.; Lüttmann, F.F.; Schoger, E.; Schöler, H.R.; Zelarayán, L.C.; Kim, K.-P.; Haigh, J.J.; Kim, J.; Braun, T. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science 2021, 373, 1537–1540. [Google Scholar] [CrossRef]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef]
- Mahmoud, A.I.; Kocabas, F.; Muralidhar, S.A.; Kimura, W.; Koura, A.S.; Thet, S.; Porrello, E.R.; Sadek, H.A. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 2013, 497, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Mitrić, A.; Castellano, I. Targeting gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic. Biol. Med. 2023, 208, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Alborzinia, H.; Flórez, A.F.; Kreth, S.; Brückner, L.M.; Yildiz, U.; Gartlgruber, M.; Odoni, D.I.; Poschet, G.; Garbowicz, K.; Shao, C.; et al. MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat. Cancer 2022, 3, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chen, A.; Li, L.; Liang, Q.; Wang, S.; Dong, Q.; Fu, M.; Lan, Z.; Li, Y.; Liu, X.; et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022, 102, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Tieu, S.; Charchoglyan, A.; Paulsen, L.; Wagter-Lesperance, L.C.; Shandilya, U.K.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. N-Acetylcysteine and Its Immunomodulatory Properties in Humans and Domesticated Animals. Antioxidants 2023, 12, 1867. [Google Scholar] [CrossRef] [PubMed]
- Safe, I.P.; Amaral, E.P.; Araújo-Pereira, M.; Lacerda, M.V.G.; Printes, V.S.; Souza, A.B.; Beraldi-Magalhães, F.; Monteiro, W.M.; Sampaio, V.S.; Barreto-Duarte, B.; et al. Adjunct N-Acetylcysteine Treatment in Hospitalized Patients With HIV-Associated Tuberculosis Dampens the Oxidative Stress in Peripheral Blood: Results from the RIPENACTB Study Trial. Front. Immunol. 2020, 11, 602589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, Y.; Fu, W.; Wang, R.; Cao, J.; Li, S.; Tian, X.; Li, Z.; Hua, C.; Zhai, Y.; et al. PLEKHM2 deficiency induces impaired mitochondrial clearance elevated ROS levels in, human iPSC-derived cardiomyocytes. Cell Death Discov. 2024, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Chen, Z.; Yu, Y.; Shi, H.; Yu, Y.; Wang, Y.; Chen, R.; Ge, J. Mitochondrial calpain-1 activates NLRP3 inflammasome by cleaving ATP5A1 and inducing mitochondrial ROS in CVB3-induced myocarditis. Basic Res. Cardiol. 2022, 117, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Song, K.; Tu, B.; Lin, L.-C.; Sun, H.; Zhou, Y.; Li, R.; Shi, Y.; Yang, J.-J.; Zhang, Y.; et al. Crosstalk between oxidative stress and epigenetic marks: New roles and therapeutic implications in cardiac fibrosis. Redox Biol. 2023, 65, 102820. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Liu, X.; Zhu, Q.; Liu, Z.; Yang, C.; Wu, H.; Zhang, L.; Xia, X.; Wang, M.; Hao, H.; et al. N-Acetylcysteine Enhances the Recovery of Ischemic Limb in Type-2 Diabetic Mice. Antioxidants 2022, 11, 1097. [Google Scholar] [CrossRef]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs 2018, 18, 283–298. [Google Scholar] [CrossRef]
- Dludla, P.V.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Tiano, L.; Marcheggiani, F.; Cirilli, I.; Louw, J.; Nkambule, B.B. The beneficial effects of N-acetyl cysteine (NAC) against obesity associated complications: A systematic review of pre-clinical studies. Pharmacol. Res. 2019, 146, 104332. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; An, N.; Shen, J.; Chen, H.; Chen, Y.; Sun, J.; Hu, Z.; Qiu, J.; Jin, C.; He, S.; et al. Fibroblast growth factor 18 alleviates stress-induced pathological cardiac hypertrophy in male mice. Nat. Commun. 2023, 14, 1235. [Google Scholar] [CrossRef] [PubMed]
- Vendrov, A.E.; Xiao, H.; Lozhkin, A.; Hayami, T.; Hu, G.; Brody, M.J.; Sadoshima, J.; Zhang, Y.-Y.; Runge, M.S.; Madamanchi, N.R. Cardiomyocyte NOX4 regulates resident macrophage-mediated inflammation and diastolic dysfunction in stress cardiomyopathy. Redox Biol. 2023, 67, 102937. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yao, S.Y.; Wang, Z.; Shen, L.; Guo, D.; Zhu, Y.; Yang, X.; Yu, Q.; Gao, C. A Sequential Dual Functional Supramolecular Hydrogel with Promoted Drug Release to Scavenge ROS and Stabilize HIF-1alpha for Myocardial Infarction Treatment. Adv. Healthc. Mater. 2024, 13, e2302940. [Google Scholar] [CrossRef] [PubMed]
- Florido, J.; Martinez-Ruiz, L.; Rodriguez-Santana, C.; López-Rodríguez, A.; Hidalgo-Gutiérrez, A.; Cottet-Rousselle, C.; Lamarche, F.; Schlattner, U.; Guerra-Librero, A.; Aranda-Martínez, P.; et al. Melatonin drives apoptosis in head and neck cancer by increasing mitochondrial ROS generated via reverse electron transport. J. Pineal Res. 2022, 73, e12824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Wang, S.; Cheng, H.; Xu, L.; Pei, G.; Wang, Y.; Fu, C.; Jiang, Y.; He, C.; et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct. Target. Ther. 2022, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Rajgarhia, A.; Ayasolla, K.R.; Zaghloul, N.; Da Re, J.M.L.; Miller, E.J.; Ahmed, M. Extracellular Superoxide Dismutase (EC-SOD) Regulates Gene Methylation and Cardiac Fibrosis During Chronic Hypoxic Stress. Front. Cardiovasc. Med. 2021, 8, 669975. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Liu, T.; Zhai, P.; Chen, W.; Li, H.; Molkentin, J.D.; Vatner, S.F.; Sadoshima, J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 2008, 133, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Yano, N.; Wang, H.; Zhao, Y.T.; Dubielecka, P.M.; Zhuang, S.; Chin, Y.E.; Qin, G.; Zhao, T.C. Sodium Butyrate Protects -Against High Fat Diet-Induced Cardiac Dysfunction and Metabolic Disorders in Type II Diabetic Mice. J. Cell Biochem. 2017, 118, 2395–2408. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, G.; Liu, J.; Peng, Y.; Xu, X.; Zhao, F.; Shi, Y.; Jin, C.; Zhang, J.; Wei, B. The role of histone deacetylases in cardiac energy metabolism in heart diseases. Metabolism 2023, 142, 155532. [Google Scholar] [CrossRef]
- Yamawaki, H.; Haendeler, J.; Berk, B.C. Thioredoxin: A key regulator of cardiovascular homeostasis. Circ. Res. 2003, 93, 1029–1033. [Google Scholar] [CrossRef]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Sircar, E.; Bhattacharyya, C.; Choudhuri, A.; Mishra, A.; Dutta, S.; Bhatta, S.; Sachin, K.; Sengupta, R. S-Denitrosylation: A Crosstalk between Glutathione and Redoxin Systems. Antioxidants 2022, 11, 1921. [Google Scholar] [CrossRef] [PubMed]



| Con | WD | WDNac | p Value | |
|---|---|---|---|---|
| Male | n = 9 | n = 10 | n = 9 | |
| LVPW;s | 0.81 ± 0.08 | 0.92 ± 0.13 | 0.85 ± 0.22 | 0.2977 |
| LVPW;d | 0.48 ± 0.05 | 0.61 ± 0.09 | 0.55 ± 0.17 | 0.1425 |
| LVID;s | 0.85 ± 0.16 | 1.02 ± 0.17 | 0.94 ± 0.21 | 0.1539 |
| LVID;d | 1.73 ± 0.21 | 2.01 ± 0.26 | 1.86 ± 0.30 | 0.1062 |
| IVS;s | 0.80 ± 0.08 | 1.01 ± 0.12 a,b | 0.77 ± 0.13 | 0.0006 |
| IVS;d | 0.52 ± 0.08 | 0.66 ± 0.10 a,b | 0.47 ± 0.08 | 0.0008 |
| LV Mass | 13.07 ± 2.92 | 23.67 ± 8.45 a,b | 14.83 ± 6.69 | 0.0093 |
| LVEF | 65.02 ± 4.36 | 63.01 ± 3.07 | 63.39 ± 3.40 | 0.4901 |
| LVFS | 31.44 ± 5.35 | 29.27 ± 3.53 | 31.80 ± 8.31 | 0.6556 |
| Female | n = 7 | n = 8 | n = 10 | |
| LVPW;s | 0.78 ± 0.12 | 0.98 ± 0.28 | 0.98 ± 0.20 | 0.1234 |
| LVPW;d | 0.49 ± 0.08 | 0.66 ± 0.27 | 0.62 ± 0.16 | 0.1974 |
| LVID;s | 0.85 ± 0.18 | 0.89 ± 0.17 | 0.93 ± 0.14 | 0.6165 |
| LVID;d | 1.71 ± 0.22 | 1.85 ± 0.23 | 1.92 ± 0.27 | 0.2376 |
| IVS;s | 0.77 ± 0.19 | 1.03 ± 0.22 a | 1.00 ± 0.17 | 0.0330 |
| IVS;d | 0.48 ± 0.08 | 0.67 ± 0.18 a | 0.61 ± 0.12 | 0.0310 |
| LV Mass | 11.99 ± 2.83 | 23.22 ± 12.70 | 21.10 ± 7.53 | 0.0498 |
| LVEF | 64.54 ± 4.28 | 64.68 ± 7.65 | 64.32 ± 6.38 | 0.9926 |
| LVFS | 30.79 ± 5.07 | 31.80 ± 8.31 | 31.18 ± 7.13 | 0.9613 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, J.; Xu, D.; Gui, Y.; Bai, F.; Huo, Y.; Cao, L.; Gui, Y. Promoting Glutathione Synthesis: A Possibility for Treating Cardiomyopathy Induced by a Maternal Western Diet. Nutrients 2024, 16, 2520. https://doi.org/10.3390/nu16152520
Zhang J, Wang J, Xu D, Gui Y, Bai F, Huo Y, Cao L, Gui Y. Promoting Glutathione Synthesis: A Possibility for Treating Cardiomyopathy Induced by a Maternal Western Diet. Nutrients. 2024; 16(15):2520. https://doi.org/10.3390/nu16152520
Chicago/Turabian StyleZhang, Jialing, Jiayu Wang, Da Xu, Yiting Gui, Fan Bai, Yu Huo, Li Cao, and Yonghao Gui. 2024. "Promoting Glutathione Synthesis: A Possibility for Treating Cardiomyopathy Induced by a Maternal Western Diet" Nutrients 16, no. 15: 2520. https://doi.org/10.3390/nu16152520
APA StyleZhang, J., Wang, J., Xu, D., Gui, Y., Bai, F., Huo, Y., Cao, L., & Gui, Y. (2024). Promoting Glutathione Synthesis: A Possibility for Treating Cardiomyopathy Induced by a Maternal Western Diet. Nutrients, 16(15), 2520. https://doi.org/10.3390/nu16152520








