Markers of Intestinal Permeability and Inflammation in Enterally Fed Children with Cerebral Palsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Fecal Sample Collection
2.4. Assessment of Investigated Parameters: Intestinal Barrier Markers and Calprotectin
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, D.; Paget, S.P.; Wimalasundera, N. Current thinking in the health care management of children with cerebral palsy. Med. J. Aust. 2019, 210, 129–135. [Google Scholar] [CrossRef]
- Cans, C. Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev. Med. Child. Neurol. 2000, 42, 816–824. [Google Scholar] [CrossRef]
- Ekawidyani, K.R.; Abdullah, M. Diet, nutrition and intestinal permeability: A mini review. Asia Pac. J. Clin. Nutr. 2023, 32, 8–12. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Heidt, C.; Kämmerer, U.; Fobker, M.; Rüffer, A.; Marquardt, T.; Reuss-Borst, M. Assessment of Intestinal Permeability and Inflammation Bio-Markers in Patients with Rheumatoid Arthritis. Nutrients 2023, 15, 2386. [Google Scholar] [CrossRef]
- Veres-Székely, A.; Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Vannay, Á. Zonulin as a Potential Therapeutic Target in Microbiota-Gut-Brain Axis Disorders: Encouraging Results and Emerging Questions. Int. J. Mol. Sci. 2023, 24, 7548. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Maciejewska, D.; Ryterska, K.; Czerwińka-Rogowska, M.; Jamioł-Milc, D.; Skonieczna-Żydecka, K.; Milkiewicz, P.; Raszeja-Wyszomirska, J.; Stachowska, E. Gut Permeability Might Be Improved by Dietary Fiber in Individuals with Nonalcoholic Fatty Liver Disease (NAFLD) Undergoing Weight Reduction. Nutrients 2018, 10, 1793. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Kim, J.H. Zonulin level, a marker of intestinal Permeability, is increased in association with liver enzymes in young adolescents. Clin. Chim. Acta 2018, 481, 218–224. [Google Scholar] [CrossRef]
- Serek, P.; Oleksy-Wawrzyniak, M. The Effect of Bacterial Infections, Probiotics and Zonulin on Intestinal Barrier Integrity. Int. J. Mol. Sci. 2021, 22, 11359. [Google Scholar] [CrossRef]
- Hałasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- El Asmar, R.; Panigrahi, P.; Bamford, P.; Berti, I.; Not, T.; Coppa, G.V.; Catassi, C.; Fasano, A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 2002, 123, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113, 4435–4440. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Xie, Q.S.; Chen, G.C.; Huang, C.L.; Yu, T.; Chen, Q.K.; Li, J.Y. Impaired intestinal barrier function in type 2 diabetic patients measured by serum LPS, Zonulin and IFABP. J. Diabetes Its Complicat. 2021, 35, 107766. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.; MacKinder, M.; Clark, C.M.; Kountouri, A.; Jere, M.; Ijaz, U.Z.; Hansen, R.; McGrogan, P.; Russell, R.K.; Gerasimidis, K. Intestinal fatty acid binging protein is a disease biomarker in paediatric coeliac disease and Crohn’s Disease. BMC Gastroenterol. 2022, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Sweetser, D.A.; Birkenmeier, E.H.; Klisak, I.J.; Zollman, S.; Sparkes, R.S.; Mohandas, T.; Lusis, A.J.; Gordon, J.I. The Human and Rodent Intestinal Fatty Acid Binging Protein Genes. J. Biol. Chem. 1987, 262, 16060–16071. [Google Scholar] [CrossRef] [PubMed]
- Doukas, P.; Bassett, C.; Krabbe, H.; Frankort, J.; Jacobs, M.J.; Elfeky, M.; Gombert, A. IFABP levels predict visceral malperfusion in the first hours after open thoracoabdominal aortic repair. Front. Cardiovasc. Med. 2023, 10, 1200967. [Google Scholar] [CrossRef] [PubMed]
- Ademuyiwa, A.; Alakaloko, F.; Elebute, O.; Bode, C.; Udenze, I. Serum intestinal fatty-acid binging protein: Predictor of bowel necrosis in pediatric intussusception. J. Ped Surg. 2018, 53, 335–338. [Google Scholar] [CrossRef]
- Pathirana, W.G.W.; Chubb, S.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77–90. [Google Scholar]
- Lężyk-Ciemniak, E.; Tworkiewicz, M.; Wilczyńska, D.; Szaflarska-Popławska, A.; Krogulska, A. Usefulness of Testing for Fecal Calprotectin in Pediatric Gastroenterology Clinical Practice. Med. Princ. Pract. 2021, 30, 311–319. [Google Scholar] [CrossRef]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.S. Diagnostic Accuracy of Fecal Calprotectin for the Detection of Small Bowel Crohn’s Disease through Capsule Endoscopy: An Updated Meta-Analysis and Systematic Review. Gut Liver 2021, 15, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Braegger, C.; Decsi, T.; Dias, J.A.; Hartman, C.; Kolaček, S.; Koletzko, B.; Koletzko, S.; Mihatsch, W.; Moreno, L.; Puntis, J.; et al. ESPGHAN Committee on Nutrition: Practical approach to paediatric enteral nutrition: A comment by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Trivić, I.; Hojsak, I. Evaluation and Treatment of Malnutrition and Associated Gastrointestinal Complications in children with Cerebral Palsy. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Matuszczyk, M.; Meglicka, M.; Wiernicka, A.; Jarzębicka, D.; Osiecki, M.; Kotkowicz-Szczur, M.; Kierkuś, J. Effect on the Crohn’s Disease Exclusion Diet (CDED) on the fecal Calprotectin Level in Children with Active Crohn’s Disease. J. Clin. Med. 2022, 11, 4146. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Corsello, A.; Pugliese, D.; Gasbarrini, A.; Armuzzi, A. Diet and Nutrients in Gastrointestinal Chronic Diseases. Nutrients 2020, 12, 2693. [Google Scholar] [CrossRef] [PubMed]
- Nawarycz, L.O.; Krzyżaniak, A.; Nawarycz, T. Normy rozwojowe dla dzieci i młodzieży w wieku 6–18 lat opracowane na podstawie 4 województw (Developmental Standards for Children and Adolescents Aged 6–18 Developed on the Basis of 4 Voivodeships). In Żywienie i Leczenie Żywieniowe Dzieci i Młodzieży; Szajewska, H., Horvath, A., Eds.; Medycyna Praktyczna: Kraków, Polska, 2017; Volume 1, pp. 474–479. [Google Scholar]
- Kułaga, Z. Normy Rozwojowe OLA/OLAF in Żywienie i Leczenie Żywieniowe Dzieci i Młodzieży (OLA/OLAF Developmental Standards in Nutrition and Nutritional Treatment of Children and Adolescents); Szajewska, H., Horvath, A., Eds.; Medycyna Praktyczna: Kraków, Polska, 2017; Volume 1, pp. 460–467. [Google Scholar]
- Gross Motor Function Classification System. Available online: https://cerebralpalsy.org.au/cerebral-palsy/gross-motor-function-classification-system/ (accessed on 29 December 2023).
- Mumolo, M.G.; Bertani, L.; Ceccarelli, L.; Laino, G.; Di Fluri, G.; Albano, E.; Tapete, G.; Costa, F. From bench to bedside: Fecal calprotectin in inflammatory bowel diseases clinical setting. World J. Gastroenterol. 2018, 24, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, Ö.Y.; Canan, O.; Hoşnut, F.Ö.; Akçay, E.; Özçay, F. Fecal calprotectin levels in Helicobacter pylori gastritis in children. Turk. J. Pediatr. 2020, 62, 986–993. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Fernandez-Banares, F.; Sato, T.; Pardi, D.S. Microscopic colitis: Etiopathology, diagnosis, and rational management. eLife 2022, 11, e79397. [Google Scholar] [CrossRef]
- Albanna, E.A.; Ahmed, H.S.; Awad, H.A. Stool calprotectin in necrotizing enterocolitis. J. Clin. Neonatol. 2014, 3, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Vaos, G.; Kostakis, I.D.; Zavras, N.; Chatzemichael, A. The role of calprotectin in pediatric disease. Biomed. Res. Int. 2013, 2013, 542363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, Y.; Jeong, S.J. Is fecal calprotectin always normal in children with irritable bowel syndrome? Intest. Res. 2019, 17, 546–553. [Google Scholar] [CrossRef]
- Xiong, L.-J.; Xie, X.-L.; Li, Y.; Deng, X.-Z. Current status of fecal calprotectin as a diagnostic or monitoring biomarker for cow’s milk protein allergy in children: A scoping review. World J. Pediatr. 2021, 17, 63–70. [Google Scholar] [CrossRef]
- Stríz, I.; Trebichavský, I. Calprotectin—A pleiotropic molecule in acute and chronic inflammation. Physiol. Res. 2004, 53, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Orfei, M.; Gasparetto, M.; Hensel, K.O.; Zellweger, F.; Heuschkel, R.B.; Zilbauer, M. Guidance on the interpretation of faecal calprotectin levels in children. PLoS ONE 2021, 16, e0246091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sipponen, T.; Kolho, K.L. Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 74–80. [Google Scholar] [CrossRef]
- Ge, C.; Lu, Y.; Shen, H.; Zhu, L. Monitoring of intestinal inflammation and prediction of recurrence in ulcerative colitis. Scand. J. Gastroenterol. 2022, 57, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Szczubełek, M.; Pomorska, K.; Korólczyk-Kowalczyk, M.; Lewandowski, K.; Kaniewska, M.; Rydzewska, G. Effectiveness of Crohn’s Disease Exclusion Diet for Induction of Remission in Crohn’s Disease Adult Patients. Nutrients 2021, 13, 4112. [Google Scholar] [CrossRef] [PubMed]
- Gerasimidis, K.; Nikolaou, C.K.; Edwards, C.A.; McGrogan, P. Serial fecal calprotectin changes in children with Crohn’s disease on treatment with exclusive enteral nutrition: Associations with disease activity, treatment response, and prediction of a clinical relapse. J. Clin. Gastroenterol. 2011, 45, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Burgess, L. Exclusive enteral nutrition and induction of remission of active Crohn’s Disease in children. Expert. Rev. Clin. Immunol. 2013, 9, 375–383; quiz 384. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-M.; He, L.-W.; Yan, T.; Guo, X.-F.; Hu, P.-J.; Peng, J.-S.; Cheng, W.-J.; Li, L.-L.; He, Q. Oral exclusive enteral nutrition induces mucosal and transmural healing in patients with Crohn’s Disease. Gastroenterol. Rep. 2019, 7, 176–184. [Google Scholar] [CrossRef]
- MacLellan, A.; Connors, J.; Grant, S.; Cahill, L.; Langille, M.G.I.; Van Limbergen, J. The Impact of Exclusive Enteral Nutrition (EEN) on the Gut Microbiome in Crohn’s Disease: A Review. Nutrients 2017, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Colson, S.B.; Siparsky, G.L.; Capocelli, K.E.; Pan, Z.; Sokol, R.J.; Hoffenberg, E.J. Inflammatory bowel disease in pediatric Patients with cerebral palsy. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 6. [Google Scholar] [CrossRef] [PubMed]
- Łoniewska, B.; Adamek, K.; Węgrzyn, D.; Kaczmarczyk, M.; Skonieczna-Żydecka, K.; Clark, J.; Adler, G.; Tousty, J.; Uzar, I.; Tousty, P.; et al. Analysis of Faecal Zonulin and Calprotectin Concentrations in Healthy Children During the First Two Years of Life. An Observational Prospective Cohort Study. J. Clin. Med. 2020, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, E. New noninvasive biomarkers of intestinal inflammation and increased intestinal permeability in pediatric inflammatory bowel diseases and their correlation with fecal calprotectin: A pilot study. Minerva Gastroenterol (Torino) 2023, 69, 504–510. [Google Scholar] [CrossRef]
- Parkhomenko, L.K.; Strashok, L.A.; Khomenko, M.A. The role of zonulin in the development of liver fibrosis in obese adolescents. Wiad. Lek. 2021, 74, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, S.; Setty, M. Celiac disease. Curr. Opin. Gastroenterol. 2008, 24, 707–712. [Google Scholar] [CrossRef]
- Tarko, A.; Suchojad, A.; Michalec, M.; Majcherczyk, M.; Brzozowska, A.; Maruniak-Chudek, I. Zonulin: A Potential Marker of Intestine Injury in Newborns. Dis. Markers 2017, 2017, 2413437. [Google Scholar] [CrossRef]
- Olivieri, F.; Maguolo, A.; Corradi, M.; Zusi, C.; Huber, V.; Fornari, E.; Morandi, A.; Maffeis, C. Serum zonulin as an index of glucose dysregulation in children and adolescents with overweight and obesity. Pediatr. Obes. 2022, 17, e12946. [Google Scholar] [CrossRef]
- Esnafoglu, E.; Cırrık, S.; Ayyıldız, S.N.; Erdil, A.; Ertürk, E.Y.; Daglı, A.; Noyan, T. Increased Serum Zonulin Levels as an Intestinal Permeability Marker in Autistic Subjects. J. Pediatr. 2017, 188, 240–244. [Google Scholar] [CrossRef]
- Cenni, S.; Casertano, M.; Trani, M.; Pacella, D.; Martinelli, M.; Staiano, A.; Miele, E.; Strisciuglio, C. The use of calgranulin-C (S100A12) and fecal zonulin as possible non-invasive markers in children with inflammatory bowel disease: A clinical study. Eur. J. Pediatr. 2023, 182, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, M.; Ergül, B.; Doğan, Z.; Filik, L.; Can, M.; Arslan, L. Intestinal fatty acid binding protein (I-FABP) as a promising test for Crohn’s disease: A preliminary study. Clin. Lab. 2015, 61, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Bodelier, A.G.; Pierik, M.J.; Lenaerts, K.; de Boer, E.; Olde Damink, S.W.; Hameeteman, W.M.; Masclee, A.A.; Jonkers, D.M. Plasma intestinal fatty acid-binding protein fails to predict endoscopic disease activity in inflammatory bowel disease patients. Eur. J. Gastroenterol. Hepatol. 2016, 28, 807–813. [Google Scholar] [CrossRef] [PubMed]
| Type of Intestinal Barrier | Component |
|---|---|
| Mechanical | Mucus epithelial layer (tight junctions) |
| Humoral | Defensins, immunoglobulin A |
| Immunological | Lymphocytes, innate immune cells |
| Muscular | Smooth muscles |
| Neurological | Enteric nervous system |
| Group | Presence of Cerebral Palsy | Enteral Nutrition |
|---|---|---|
| CPEN | + | + |
| CPC | + | − |
| HC | − | − |
| Characteristics | CPEN (n = 30) | CPC (n = 23) | HC (n = 24) |
|---|---|---|---|
| Age | 10.80 (±3.96) | 8.96 (±4.38) | 8.08 (±4.34) |
| Length/Height (cm) | 131.18 (±16.65) | 122.96 (±25.30) | 127.56 (±29.31) |
| Body mass (kg) | 22.92 (±5.95) | 22.69 (±13.66) | 32.30 (±21.90) |
| BMI | 13.30 (±2.28) | 13.93 (±3.30) | 17.50 (±3.90) |
| GMFCS | 4.767 (±0.50) | 4.21 (±0.88) | NA |
| Dysphagia | 56.67% (n = 17) | 4.34% (n = 1) | 0% (n = 0) |
| GERD | 46.67% (n = 14) | 21.73% (n = 5) | 16.66% (n = 4) |
| Constipation | 73.33% (n = 22) | 26.08% (n = 6) | 20.83% (n = 5) |
| Abdominal pain | 30% (n = 9) | 39.13% (n = 9) | 16.66% (n = 4) |
| Level | Description |
|---|---|
| Level I | Walks without limitations |
| Level II | Walks with limitations |
| Level III | Walks with a handheld mobility device |
| Level IV | Requires physical assistance or uses powered mobility |
| Level V | Transported in a manual wheelchair in all settings |
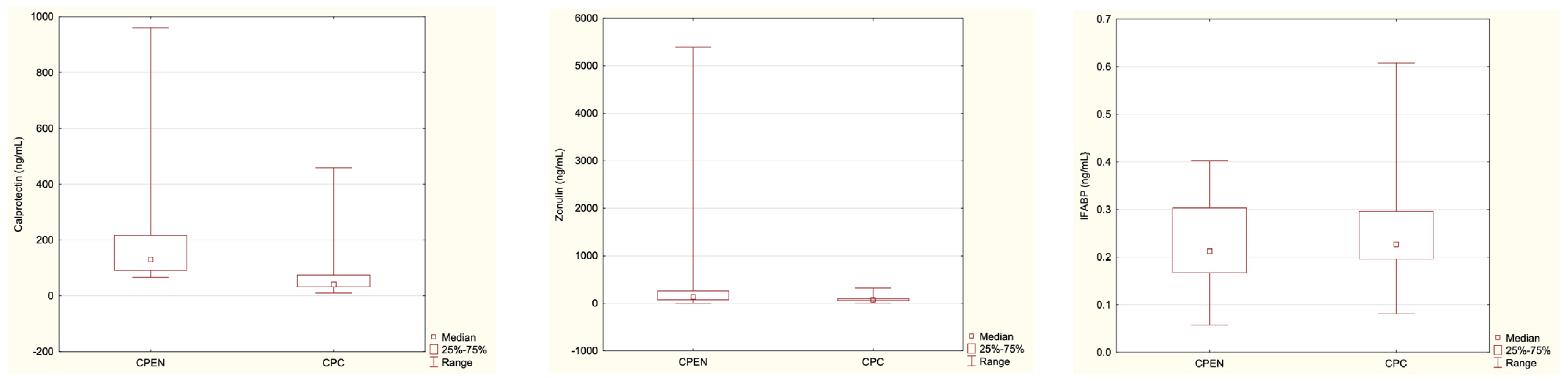
| Parameter | CPEN (n = 30) | CPC (n = 23) | HC (n = 24) |
|---|---|---|---|
| Calprotectin [ng/mL] | 96.23 | 41.15 | 32.18 |
| Median range | 8.02–960.60 | 9.76–458.95 | 10.31–229.25 |
| Mean ± SD | 152.43 ± 202.54 | 84.39 ± 114.10 | 47.05 ± 50.84 |
| p-value | p = 0.012 | ||
| p = 0.07 | |||
| Zonulin [ng/mL] | 137.88 | 71.2 | 122.43 |
| Median range | 0.45–5395.05 | 3.25–322.75 | 32.85–1193.9 |
| Mean ± SD | 530.95 ± 1091.59 | 89.97 ± 67.34 | 176.41 ± 235.94 |
| p-value | p = 0.0025 | ||
| p = 0.04 | |||
| IFABP [ng/mL] | 0.21 | 0.23 | 0.27 |
| Median range | 0.06–0.40 | 0.08–0.61 | 0.17–0.577 |
| Mean ± SD | 0.23 ± 0.09 | 0.25 ± 0.11 | 0.29 ± 0.10 |
| p-value | p = 0.62 | ||
| p = 0.10 | |||
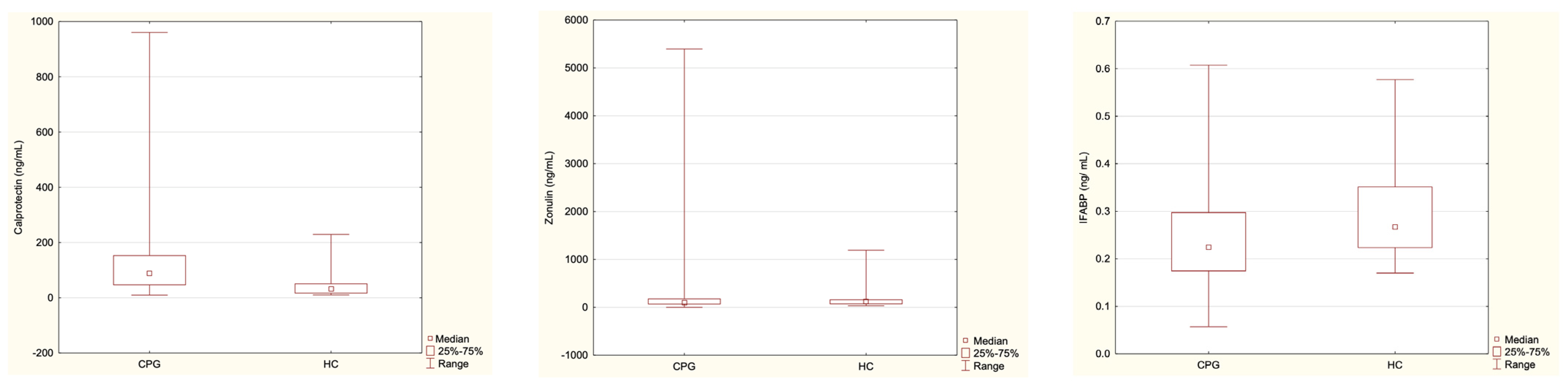
| Parameter | CPG (n = 53) | HC (n = 24) |
|---|---|---|
| Calprotectin | 57.72 | 32.18 |
| Median range | 8.02–960.59 | 10.31–229.25 |
| Mean ± SD | 122.88 ± 171.89 | 47.05 ± 50.84 |
| p-value | p = 0.000018 | |
| Zonulin | 93.25 | 122.43 |
| Median range | 0.45–5395.05 | 32.85–1193.9 |
| Mean ± SD | 339.58 ± 845.66 | 176.41 ± 235.94 |
| p-value | p = 0.54 | |
| IFABP | 0.22 | 0.27 |
| Median range | 0.06–0.61 | 0.17–0.58 |
| Mean ± SD | 0.24 ± 0.1 | 0.29 ± 0.1 |
| p-value | 0.021 | |
| Range (ng/mL) | CPEN (n = 30) | CPC (n = 23) | HC (n = 24) |
|---|---|---|---|
| 0–50 | - | 60.87% (n = 14) | 75% (n = 18) |
| 50–250 | 80% (n = 24) | 30.43% (n = 7) | 25% (n = 6) |
| 250–600 | 13.33% (n = 4) | 8.7% (n = 2) | - |
| 600–1000 | 6.67% (n = 2) | - | - |
| Range (ng/mL) | CPEN (n = 30) | CPC (n = 23) | HC (n = 24) |
|---|---|---|---|
| 0–60 | 23.33% (n = 7) | 26.09% (n = 6) | 20.83% (n = 5) |
| >60 | 76.67% (n = 23) | 73.91% (n = 17) | 79.17% (n = 19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickiewicz-Góra, D.; Sznurkowska, K.; Skonieczna-Żydecka, K.; Drozd, A.; Borkowska, A.; Zagierski, M.; Troch, J.; Szlagatys-Sidorkiewicz, A. Markers of Intestinal Permeability and Inflammation in Enterally Fed Children with Cerebral Palsy. Nutrients 2024, 16, 2447. https://doi.org/10.3390/nu16152447
Mickiewicz-Góra D, Sznurkowska K, Skonieczna-Żydecka K, Drozd A, Borkowska A, Zagierski M, Troch J, Szlagatys-Sidorkiewicz A. Markers of Intestinal Permeability and Inflammation in Enterally Fed Children with Cerebral Palsy. Nutrients. 2024; 16(15):2447. https://doi.org/10.3390/nu16152447
Chicago/Turabian StyleMickiewicz-Góra, Dorota, Katarzyna Sznurkowska, Karolina Skonieczna-Żydecka, Arleta Drozd, Anna Borkowska, Maciej Zagierski, Joanna Troch, and Agnieszka Szlagatys-Sidorkiewicz. 2024. "Markers of Intestinal Permeability and Inflammation in Enterally Fed Children with Cerebral Palsy" Nutrients 16, no. 15: 2447. https://doi.org/10.3390/nu16152447
APA StyleMickiewicz-Góra, D., Sznurkowska, K., Skonieczna-Żydecka, K., Drozd, A., Borkowska, A., Zagierski, M., Troch, J., & Szlagatys-Sidorkiewicz, A. (2024). Markers of Intestinal Permeability and Inflammation in Enterally Fed Children with Cerebral Palsy. Nutrients, 16(15), 2447. https://doi.org/10.3390/nu16152447







