Bovine Milk Fat Globule Membrane Supplementation and Neurocognitive Development: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria
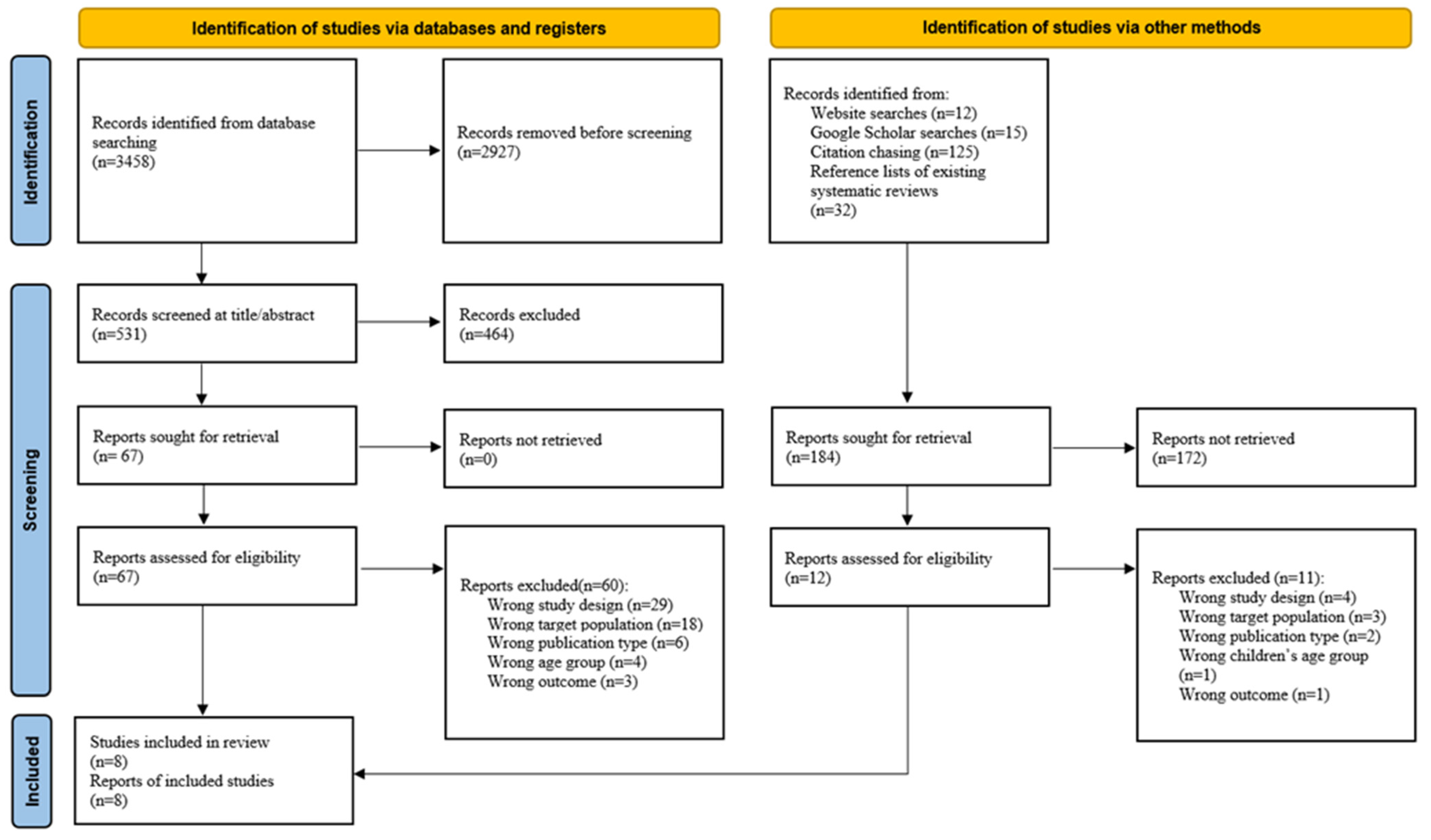
2.4. Study Selection
2.5. Data Extraction
2.6. Statistical Analysis
2.7. Quality Assessment
3. Results
3.1. Included Studies
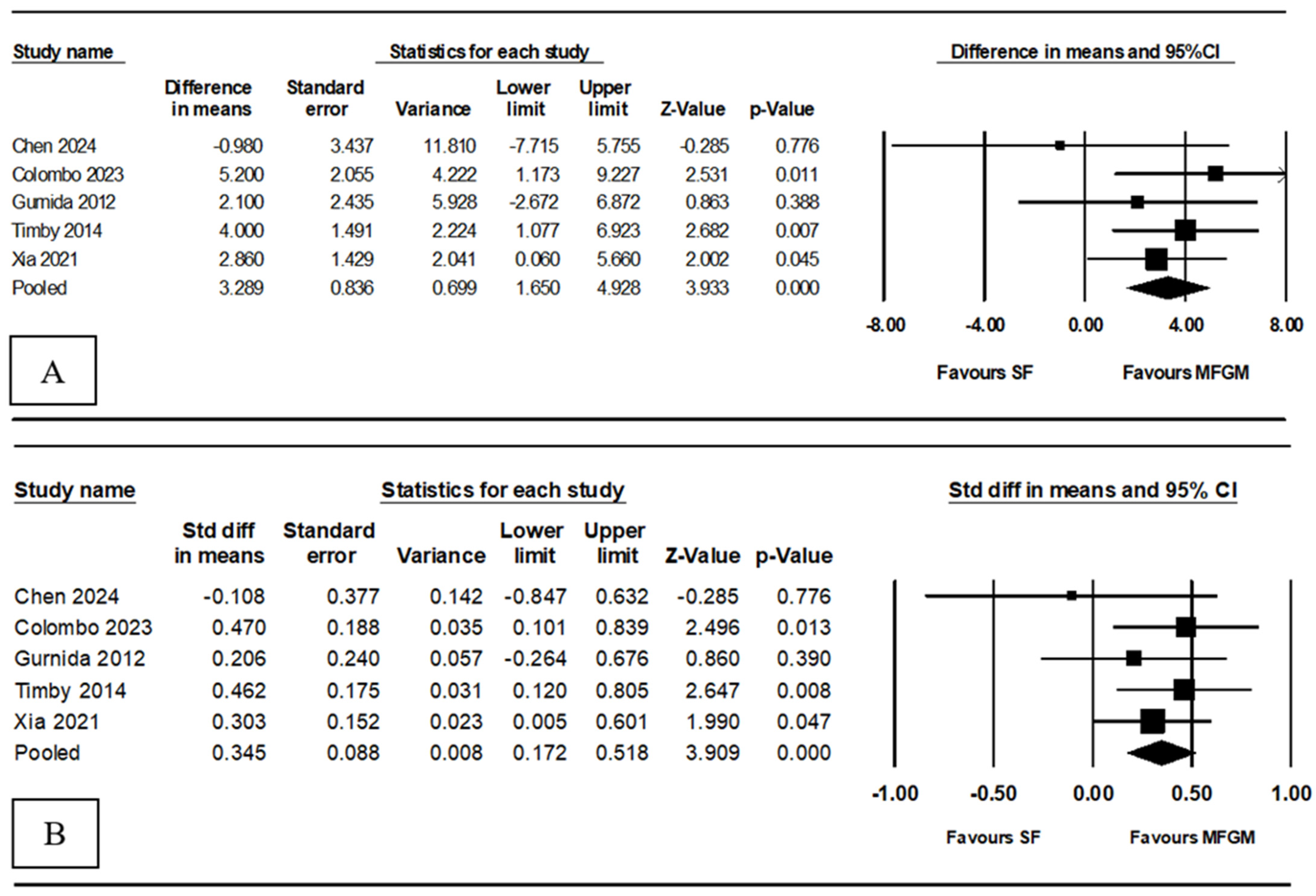
3.2. Meta-Analysis Results: Additional Value to Infant Formula
3.2.1. Cognitive Development
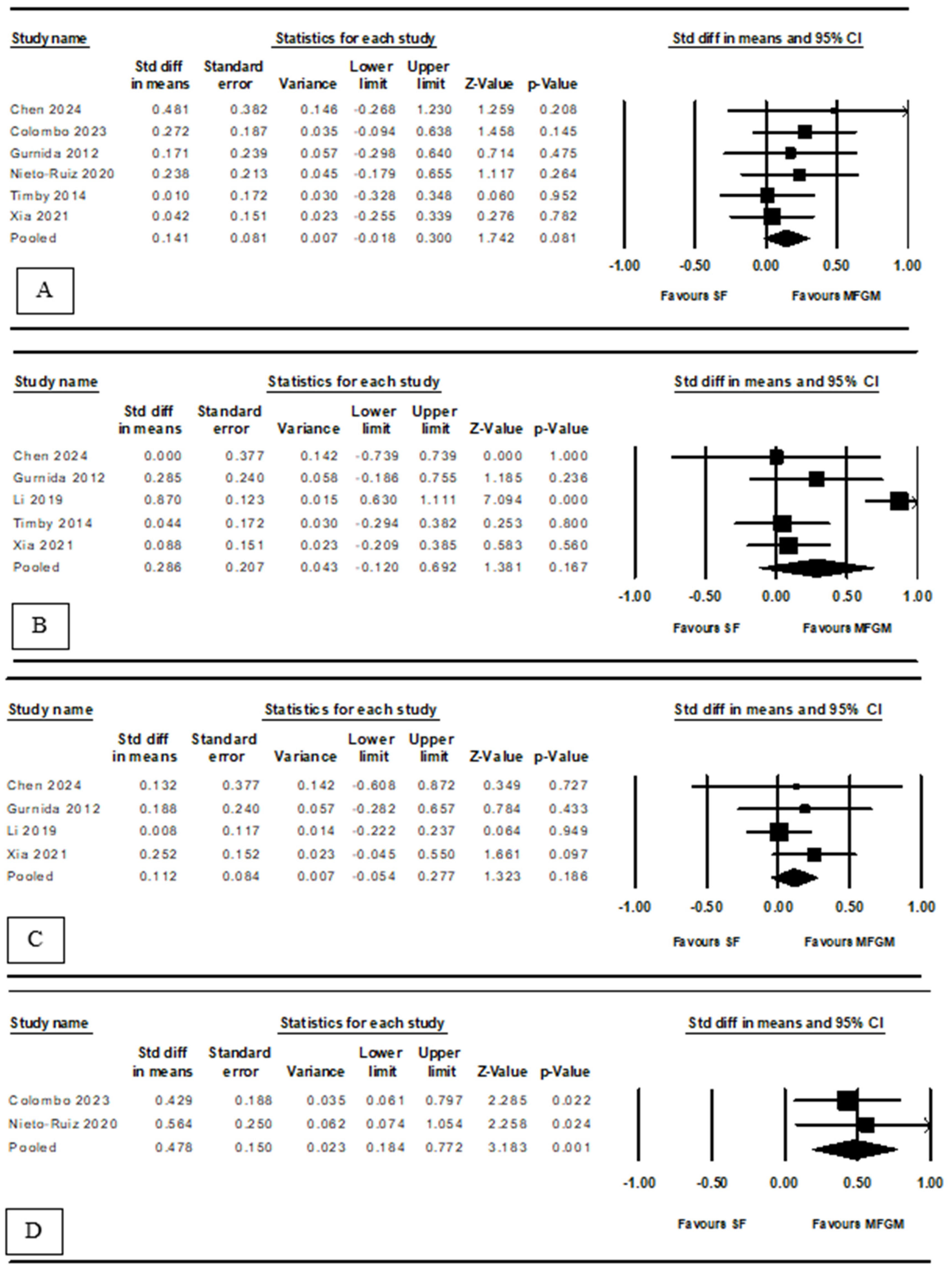
3.2.2. Language, Social–Emotional, Motor, and Executive Function Development
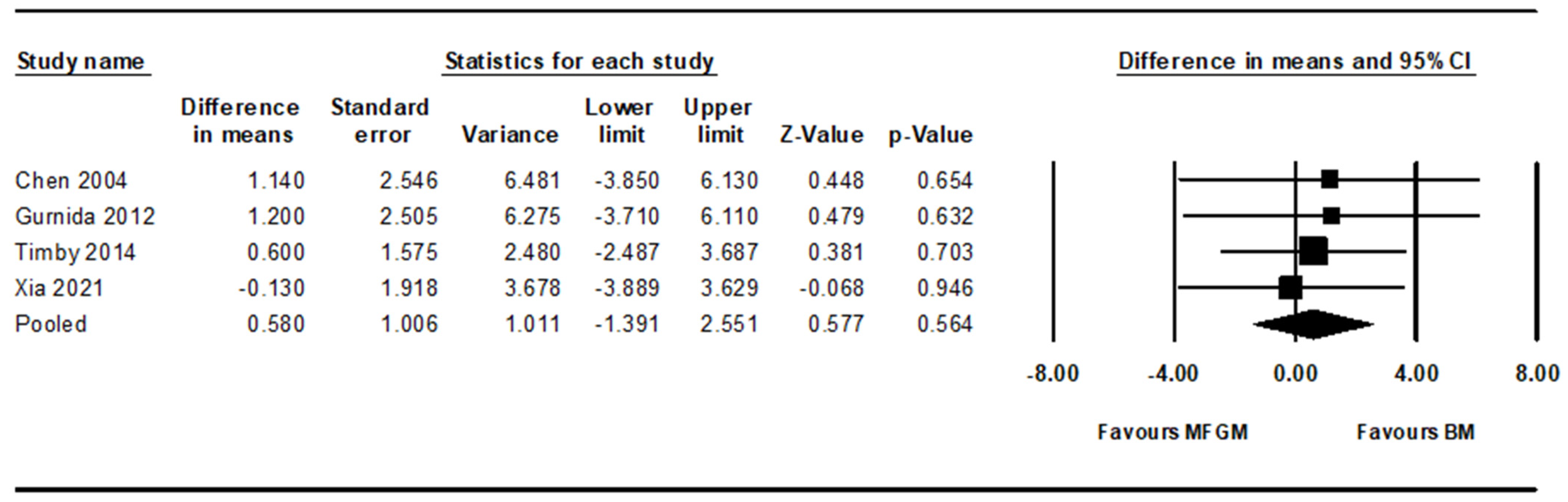
3.3. Secondary Result: Noninferiority to Breast Milk
3.3.1. Cognitive Development
3.3.2. Language, Social–Emotional, and Motor Development
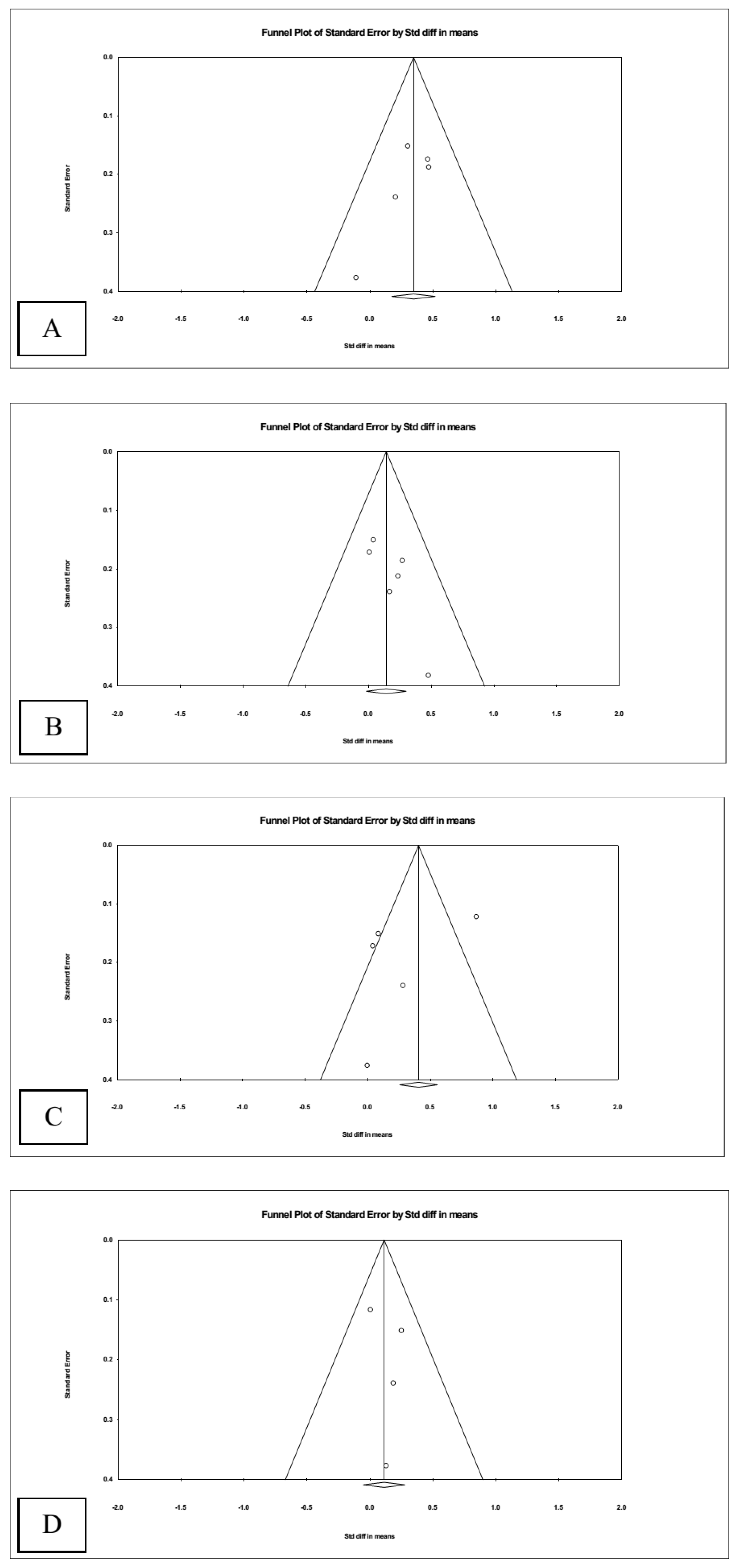
3.4. Publication Bias
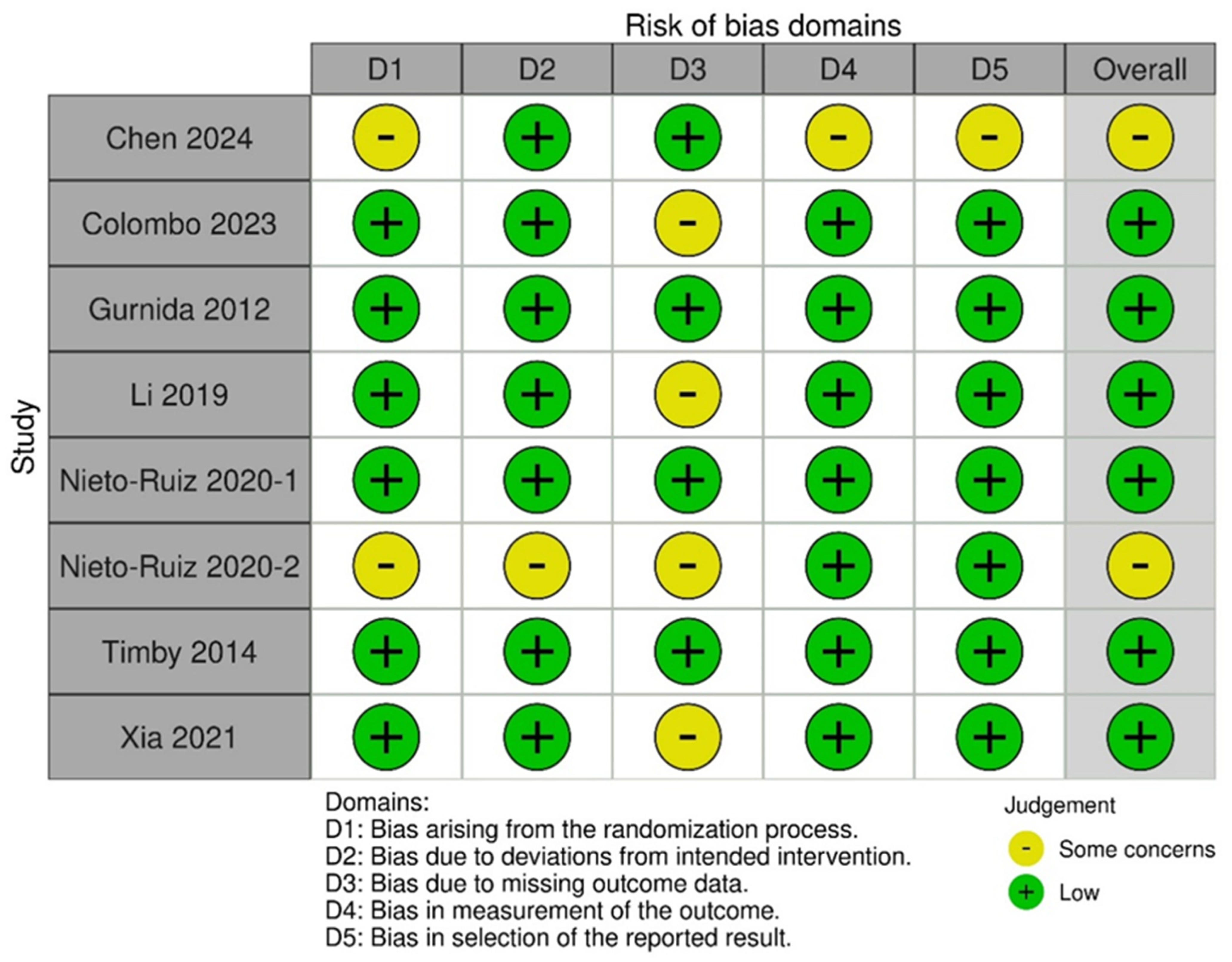
3.5. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meek, J.Y.; Noble, L. Section on breastfeeding. Policy statement: Breastfeeding and the use of human milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, X.; Yang, M.; Acevedo-Fani, A.; Singh, H.; Ye, A. Dynamic in vitro gastric digestion behaviour of commercial infant formulae made with cow, goat and sheep milk. Foods 2024, 13, 1286. [Google Scholar] [CrossRef] [PubMed]
- Demmelmair, H.; Uhl, O.; Zhou, S.J.; Makrides, M.; Gibson, R.A.; Prosser, C.; Gallier, S.; Koletzko, B. Plasma sphingomyelins and carnitine esters of infants consuming whole goat or cow milk-based infant formulas or human milk. J. Nutr. 2024, 154, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Yang, M.; Liu, W.; Luo, X.; Yue, X. Proteomics and peptidomics as a tool to compare the proteins and endogenous peptides in human, cow, and donkey milk. J. Agric. Food Chem. 2023, 71, 16435–16451. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Rao, S.C.; Schulzke, S.M.; Patole, S.K.; Simmer, K. Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst. Rev. 2016, 12, CD000375. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 3, CD000376. [Google Scholar] [CrossRef]
- Rosenfeld, E.; Beyerlein, A.; Hadders-Algra, M.; Kennedy, K.; Singhal, A.; Fewtrell, M.; Lucas, A.; Koletzko, B.; Von Kries, R. IPD meta-analysis shows no effect of LC-PUFA supplementation on infant growth at 18 months. Acta Paediatr. 2009, 98, 91–97. [Google Scholar] [CrossRef]
- Agostoni, C.; Guz-Mark, A.; Marderfeld, L.; Milani, G.P.; Silano, M.; Shamir, R. The long-term effects of dietary nutrient intakes during the first 2 years of life in healthy infants from developed countries: An umbrella review. Adv. Nutr. 2019, 10, 489–501. [Google Scholar] [CrossRef]
- Lönnerdal, B. Bioactive proteins in human milk: Health, nutrition, and implications for infant formulas. J. Pediatr. 2016, 173, S4–S9. [Google Scholar] [CrossRef]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, production, and clinical treatments of milk fat globule membrane for infant nutrition and well-being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, J.; Liu, Q.; Liu, Y.; Tian, X.; Qiao, W.; Zhao, Y.; Liu, Y.; Chen, L. Human milk sphingomyelin: Function, metabolism, composition and mimicking. Food Chem. 2024, 447, 138991. [Google Scholar] [CrossRef] [PubMed]
- Kalinichenko, L.S.; Gulbins, E.; Kornhuber, J.; Müller, C.P. Sphingolipid control of cognitive functions in health and disease. Prog. Lipid Res. 2022, 86, 101162. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.J.J.; Lee, E.K.H.; Woo, K.C.K.; Sarvananthan, R.; Lee, Y.Y.; Mohd Hussin, Z.A. Brain-immune-gut benefits with early life supplementation of milk fat globule membrane. JGH Open 2022, 6, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fil, J.E.; Fleming, S.A.; Chichlowski, M.; Gross, G.; Berg, B.M.; Dilger, R.N. Evaluation of dietary bovine milk fat globule membrane supplementation on growth, serum cholesterol and lipoproteins, and neurodevelopment in the young pig. Front. Pediatr. 2019, 7, 417. [Google Scholar] [CrossRef] [PubMed]
- Ambrożej, D.; Dumycz, K.; Dziechciarz, P.; Ruszczyński, M. Milk fat globule membrane supplementation in children: Systematic review with meta-analysis. Nutrients 2021, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis, Version 4; Biostat: Englewood, NJ, USA, 2021; Available online: https://meta-analysis.com/ (accessed on 14 February 2024).
- Anderson, J.W.; Johnstone, B.M.; Remley, D.T. Breast-feeding and cognitive development: A meta-analysis. Am. J. Clin. Nutr. 1999, 70, 525–535. [Google Scholar] [CrossRef]
- Chen, B.; Jia, Q.; Chen, Z.; You, Y.; Liu, Y.; Zhao, J.; Chen, L.; Ma, D.; Xing, Y. Comparative evaluation of enriched formula milk powder with OPO and MFGM vs. breastfeeding and regular formula milk powder in full-term infants: A comprehensive study on gut microbiota, neurodevelopment, and growth. Food Funct. 2024, 15, 1417–1430. [Google Scholar] [CrossRef]
- Colombo, J.; Harris, C.L.; Wampler, J.L.; Zhuang, W.; Shaddy, D.J.; Liu, B.Y.; Wu, S.S. Improved neurodevelopmental outcomes at 5.5 years of age in children who received bovine milk fat globule membrane and lactoferrin in infant formula through 12 months: A randomized controlled trial. J. Pediatr. 2023, 261, 113483. [Google Scholar] [CrossRef] [PubMed]
- Gurnida, D.A.; Rowan, A.M.; Idjradinata, P.; Muchtadi, D.; Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 2012, 88, 595–601. [Google Scholar] [CrossRef]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved neurodevelopmental outcomes associated with bovine milk fat globule membrane and lactoferrin in infant formula: A randomized, controlled trial. J. Pediatr. 2019, 215, 24–31.e8. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Ruiz, A.; Diéguez, E.; Sepúlveda-Valbuena, N.; Herrmann, F.; Cerdó, T.; López-Torrecillas, F.; De-Castellar, R.; Jiménez, J.; Pérez-García, M.; Miranda, M.T.; et al. The effects of an infant formula enriched with milk fat globule membrane, long-chain polyunsaturated fatty acids and synbiotics on child behavior up to 2.5 years old: The COGNIS study. Nutrients 2020, 12, 3825. [Google Scholar] [CrossRef]
- Nieto-Ruiz, A.; Diéguez, E.; Sepúlveda-Valbuena, N.; Catena, E.; Jiménez, J.; Rodríguez-Palmero, M.; Catena, A.; Miranda, M.T.; García-Santos, J.A.; Bermúdez, M.G.; et al. Influence of a functional nutrients-enriched infant formula on language development in healthy children at four years old. Nutrients 2020, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Timby, N.; Domellöf, E.; Hernell, O.; Lönnerdal, B.; Domellöf, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 99, 860–868. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, B.; Zhou, L.; Ma, J.; Yang, L.; Wang, F.; Liu, H.; Zhang, N.; Li, X.; Petocz, P.; et al. Neurodevelopmental outcomes of healthy Chinese term infants fed infant formula enriched in bovine milk fat globule membrane for 12 months: A randomized controlled trial. Asia Pac. J. Clin. Nutr. 2021, 30, 401–414. [Google Scholar] [CrossRef]
- Verfuerden, M.L.; Dib, S.; Jerrim, J.; Fewtrell, M.; Gilbert, R.E. Effect of long-chain polyunsaturated fatty acids in infant formula on long-term cognitive function in childhood: A systematic review and meta-analysis of randomised controlled trials. PLoS ONE 2020, 15, e0241800. [Google Scholar] [CrossRef]
- European Union Commission. Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 Supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Infant Formula and Follow-On Formula and as Regards Requirements on Information Relating to Infant and Young Child Feeding; OJEC: Aberdeen, UK, 2016; Available online: https://eur-lex.europa.eu/eli/reg_del/2016/127/oj (accessed on 30 January 2024).
- Hughes, H.K.; Landa, M.M.; Sharfstein, J.M. Marketing claims for infant formula: The need for evidence. JAMA Pediatr. 2017, 171, 105–106. [Google Scholar] [CrossRef]
- Raza, G.S.; Herzig, K.H.; Leppäluoto, J. Invited review: Milk fat globule membrane—A possible panacea for neurodevelopment, infections, cardiometabolic diseases, and frailty. J. Dairy Sci. 2021, 104, 7345–7363. [Google Scholar] [CrossRef]
- Parisi, C.; Hesse, N.; Tacke, U.; Rocamora, S.P.; Blaschek, A.; Hadders-Algra, M.; Black, M.J.; Heinen, F.; Müller-Felber, W. Analysis of motor development within the first year of life: 3-D motion tracking without markers for early detection of developmental disorders. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2020, 63, 881–890. (In German) [Google Scholar] [CrossRef] [PubMed]
- Al-Abdi, S.; Aljughaiman, A.; Alrashidi, J.; Aldarwish, M.; Zekri, A.; Alshamari, F. A systematic comparison between infant formula compositions using the Bray-Curtis Similarity Index. Int. J. Pediatr. Adolesc. Med. 2020, 7, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Borewicz, K.; Brück, W.M. Supplemented infant formula and human breast milk show similar patterns in modulating infant microbiota composition and function in vitro. Int. J. Mol. Sci. 2024, 25, 1806. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | N | Population | Intervention | Comparison | Outcome |
|---|---|---|---|---|---|---|
| Chen et al., 2024 [21] | China | 79 MFGM = 17 SF = 12 BF = 50 | Healthy, term infants | Enriched formula milk powder containing 1,3-dioleoyl-2-palmitoylglycerol and MFGM | SF, BF | At 4 and 6 months old CNBS-R2016 |
| Colombo et al., 2023 [22] | China | 116 MFGM = 57 SF = 59 | Healthy, term neonates and infants | MFGM + lactoferrin From 10–14 days of age until 365 days of age | SF | At 5.5 years old WPPSI-IV, CBCL, Stroop Task, DCCS |
| Gurnida et al., 2012 [23] | Indonesia | 110 MFGM = 35 SF = 35 BF = 40 | Healthy, term neonates and infants | MFGM from enrollment (2–8 weeks of age) up to 6 months (24 weeks of age) | SF, BF | At 6 months old GMDS |
| Li et al., 2019 [24] | China | 191 MFGM = 143 SF = 148 | Healthy, term neonates and infants | MFGM + lactoferrin From 10–14 days of age up to 365 days of age | SF | At day 365 and 545 BSID |
| Nieto-Ruiz et al., 2020-1 [25] | Spain | 103 MFGM = 41 SF = 29 BF = 33 | Healthy 0–2-month-old full-term neonates, infants, and children | LCPUFAs AA, DHA, MFGM, symbiotics, gangliosides, nucleotides, and sialic acid From 0–2 months of age up to 18 months of age | SF, BF | At 1.5 and 2.5 years old CBCL |
| Nieto-Ruiz et al., 2020-2 [26] | Spain | 122 MFGM = 43 SF = 46 BF = 33 | Healthy 0–2-month-old full-term neonates, infants, and children | LCPUFAs AA, DHA, MFGM, symbiotics, gangliosides, nucleotides, and sialic acid From 0–2 months of age up to 18 months of age | SF, BF | At 4 years old PLON-R |
| Timby et al., 2014 [27] | Sweden | 213 MFGM = 73 SF = 68 BF = 72 | Healthy neonates and infants | MFGM from enrollment < 2 months to 6 months of age, followed up until 12 months of age | SF, BF | At 12 months old BSID |
| Xia et al., 2021 [28] | China | 418 MFGM = 108 SF = 104 BF = 206 | Healthy neonates and infants | MFGM from enrollment (<14 days of age) until 6 months of age | SF, BF | At 6 and 12 months old BSID |
| Child Development Domain | Mean Difference | 95% Lower Bound | 95% Upper Bound | Noninferior |
|---|---|---|---|---|
| Cognitive | 0.58 | –1.39 | 2.55 | Yes |
| Language | 1.91 | –2.046 | 4.074 | Inconclusive |
| Motor | 1.20 | –0.66 | 3.06 | Inconclusive |
| Social–emotional | 3.41 | –4.65 | 11.47 | Inconclusive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongseiratch, T.; Kittisakmontri, K.; Chandeying, N. Bovine Milk Fat Globule Membrane Supplementation and Neurocognitive Development: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2374. https://doi.org/10.3390/nu16142374
Thongseiratch T, Kittisakmontri K, Chandeying N. Bovine Milk Fat Globule Membrane Supplementation and Neurocognitive Development: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(14):2374. https://doi.org/10.3390/nu16142374
Chicago/Turabian StyleThongseiratch, Therdpong, Kulnipa Kittisakmontri, and Nutthaporn Chandeying. 2024. "Bovine Milk Fat Globule Membrane Supplementation and Neurocognitive Development: A Systematic Review and Meta-Analysis" Nutrients 16, no. 14: 2374. https://doi.org/10.3390/nu16142374
APA StyleThongseiratch, T., Kittisakmontri, K., & Chandeying, N. (2024). Bovine Milk Fat Globule Membrane Supplementation and Neurocognitive Development: A Systematic Review and Meta-Analysis. Nutrients, 16(14), 2374. https://doi.org/10.3390/nu16142374