Knee Extensor Muscle Strength Associated with the Onset of Depression in Older Japanese Women: The Otassha Study
Abstract
1. Introduction
2. Methods
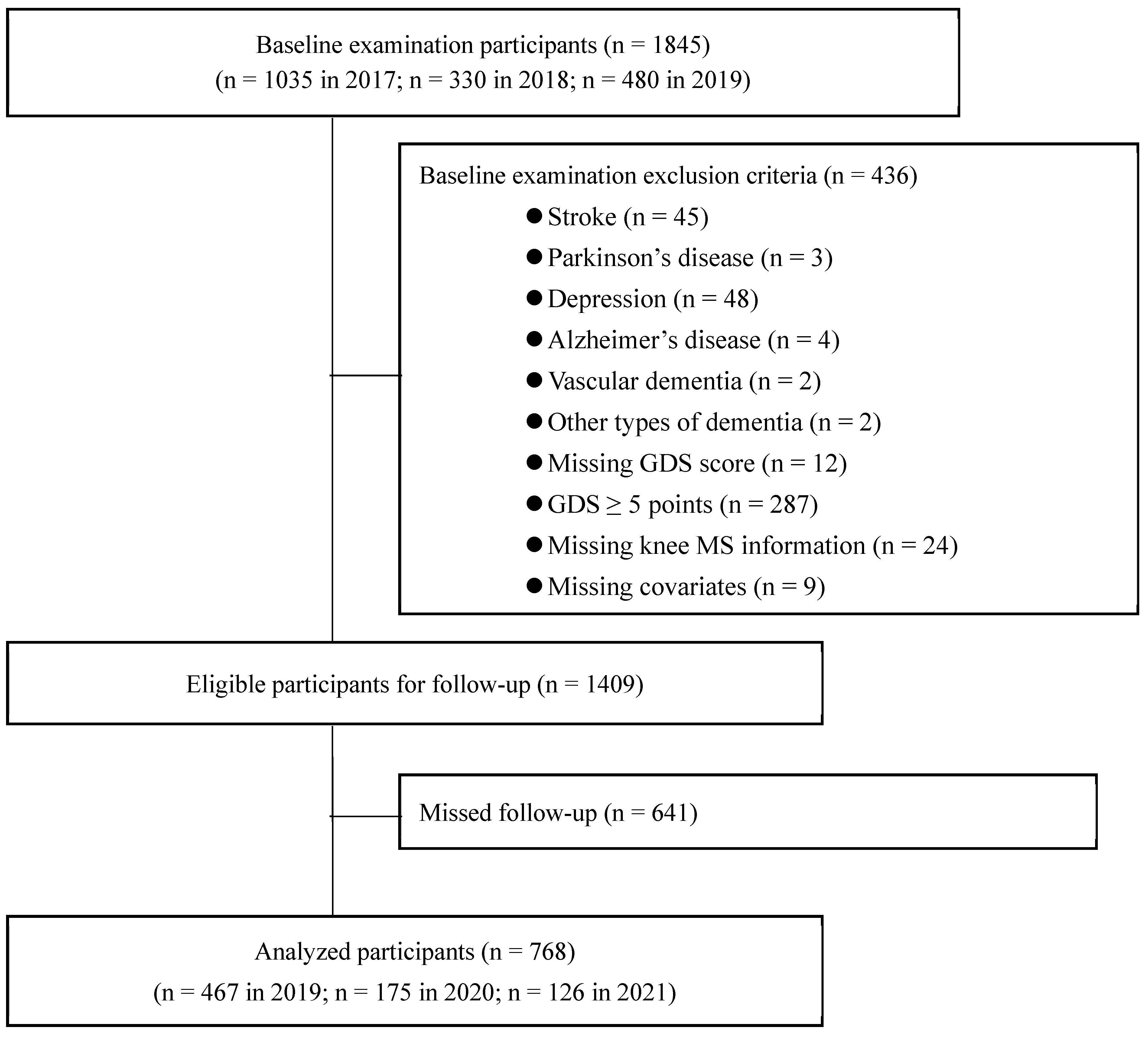
2.1. Study Design and Participants
2.2. Assessment of Knee Extensor Muscle Strength
2.3. The Onset of Geriatric Depressive Symptoms
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexopoulos, G.S. Depression in the elderly. Lancet 2005, 365, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- König, H.-H.; Konnopka, A. The excess costs of depression: A systematic review and meta-analysis. Epidemiology Psychiatr. Sci. 2019, 29, e30. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Haseda, M.; Shiba, K.; Tsuji, T.; Kondo, K.; Kondo, N. Social Isolation and Depressive Symptoms Among Older Adults: A Multiple Bias Analysis Using a Longitudinal Study in Japan. Ann. Epidemiology 2022, 77, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, D.; Sweeny, K.; Sheehan, P.; Rasmussen, B.; Smit, F.; Cuijpers, P.; Saxena, S. Scaling-up treatment of depression and anxiety: A global return on investment analysis. Lancet Psychiatry 2016, 3, 415–424. [Google Scholar] [CrossRef]
- Giovannini, S.; Onder, G.; van der Roest, H.G.; Topinkova, E.; Gindin, J.; Cipriani, M.C.; Denkinger, M.D.; Bernabei, R.; Liperoti, R.; on behalf of the SHELTER Study Investigators. Use of antidepressant medications among older adults in European long-term care facilities: A cross-sectional analysis from the SHELTER study. BMC Geriatr. 2020, 20, 310. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Sánchez, V.; Esteban-Cornejo, I.; Parra-Soto, S.; Petermann-Rocha, F.; Gray, S.R.; Rodríguez-Artalejo, F.; Ho, F.K.; Pell, J.P.; Martínez-Gómez, D.; Celis-Morales, C. Muscle strength and incidence of depression and anxiety: Findings from the UK Biobank prospective cohort study. J. Cachex- Sarcopenia Muscle 2022, 13, 1983–1994. [Google Scholar] [CrossRef]
- Yeung, S.S.; Reijnierse, E.M.; Trappenburg, M.C.; Hogrel, J.-Y.; McPhee, J.S.; Piasecki, M.; Sipila, S.; Salpakoski, A.; Butler-Browne, G.; Pääsuke, M.; et al. Handgrip Strength Cannot Be Assumed a Proxy for Overall Muscle Strength. J. Am. Med Dir. Assoc. 2018, 19, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Rojer, A.G.; D’andrea, L.; Otten, R.H.; Heymans, M.W.; Trappenburg, M.C.; Verlaan, S.; Whittaker, A.C.; Meskers, C.G.; Maier, A.B. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 67, 101266. [Google Scholar] [CrossRef]
- Bao, M.; Chao, J.; Sheng, M.; Cai, R.; Zhang, N.; Chen, H. Longitudinal association between muscle strength and depression in middle-aged and older adults: A 7-year prospective cohort study in China. J. Affect. Disord. 2022, 301, 81–86. [Google Scholar] [CrossRef]
- Iwasaki, M.; Maeda, I.; Kokubo, Y.; Tanaka, Y.; Ueno, T.; Ohara, Y.; Motokawa, K.; Hayakawa, M.; Shirobe, M.; Edahiro, A.; et al. Standard Values and Concurrent Validity of a Newly Developed Occlusal Force-Measuring Device among Community-Dwelling Older Adults: The Otassha Study. Int. J. Environ. Res. Public Health 2022, 19, 5588. [Google Scholar] [CrossRef]
- Kojima, N.; Kim, H.; Saito, K.; Yoshida, H.; Yoshida, Y.; Hirano, H.; Obuchi, S.; Shimada, H.; Suzuki, T. Association of knee-extension strength with instrumental activities of daily living in community-dwelling older adults. Geriatr. Gerontol. Int. 2014, 14, 674–680. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. J. Aging Ment. Health 1986, 5, 165–173. [Google Scholar]
- Mitchell, A.J.; Bird, V.; Rizzo, M.; Meader, N. Diagnostic validity and added value of the geriatric depression scale for depression in primary care: A meta-analysis of GDS30 and GDS15. J. Affect. Disord. 2010, 125, 10–17. [Google Scholar] [CrossRef]
- Shimada, H.; Park, H.; Makizako, H.; Doi, T.; Lee, S.; Suzuki, T. Depressive symptoms and cognitive performance in older adults. J. Psychiatr. Res. 2014, 57, 149–156. [Google Scholar] [CrossRef]
- Chen, F.; Wei, G.; Wang, Y.; Liu, T.; Huang, T.; Wei, Q.; Ma, G.; Wang, D. Risk factors for depression in elderly diabetic patients and the effect of metformin on the condition. BMC Public Health 2019, 19, 1063. [Google Scholar] [CrossRef]
- Plurphanswat, N.; Kaestner, R.; Rodu, B. The Effect of Smoking on Mental Health. Am. J. Health Behav. 2017, 41, 471–483. [Google Scholar] [CrossRef]
- Chang, S.-C.; Pan, A.; Kawachi, I.; Okereke, O.I. Risk factors for late-life depression: A prospective cohort study among older women. Physiol. Behav. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Misawa, J.; Kondo, K. Social factors relating to depression among older people in Japan: Analysis of longitudinal panel data from the AGES project. Aging Ment. Health 2019, 23, 1423–1432. [Google Scholar] [CrossRef]
- Fukunaga, R.; Abe, Y.; Nakagawa, Y.; Koyama, A.; Fujise, N.; Ikeda, M. Living alone is associated with depression among the elderly in a rural community in Japan. Psychogeriatrics 2012, 12, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Friberg, A.S.; Moustsen, I.R.; Larsen, S.B.; Hartung, T.; Andersen, E.W.; Olsen, M.H.; Tjønneland, A.; Kjaer, S.K.; Johansen, C.; Brasso, K.; et al. Educational level and the risk of depression after prostate cancer. Acta Oncol. 2019, 58, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Cano-Ibáñez, N.; Serra-Majem, L.; Martín-Peláez, S.; Martínez-González, M.; Salas-Salvadó, J.; Corella, D.; Lassale, C.; Martínez, J.A.; Alonso-Gómez, M.; Wärnberg, J.; et al. Dietary diversity and depression: Cross-sectional and longitudinal analyses in Spanish adult population with metabolic syndrome. Findings from PREDIMED-Plus trial. Public Health Nutr 2023, 26, 598–610. [Google Scholar] [CrossRef]
- Evans, J.R.; Fletcher, A.E.; Wormald, R.P.L. Depression and Anxiety in Visually Impaired Older People. Ophthalmology 2007, 114, 283–288. [Google Scholar] [CrossRef]
- Fukumori, N.; Yamamoto, Y.; Takegami, M.; Yamazaki, S.; Onishi, Y.; Sekiguchi, M.; Otani, K.; Kikuchi, S.-I.; Fukuhara, S. Association between hand-grip strength and depressive symptoms: Locomotive Syndrome and Health Outcomes in Aizu Cohort Study (LOHAS). Age and Ageing 2015, 44, 592–598. [Google Scholar] [CrossRef]
- Hamer, M.; Batty, G.D.; Kivimaki, M. Sarcopenic obesity and risk of new onset depressive symptoms in older adults: English Longitudinal Study of Ageing. Int. J. Obes. 2015, 39, 1717–1720. [Google Scholar] [CrossRef]
- Stessman, J.; Rottenberg, Y.; Fischer, M.; Hammerman-Rozenberg, A.; Jacobs, J.M. Handgrip Strength in Old and Very Old Adults: Mood, Cognition, Function, and Mortality. J. Am. Geriatr. Soc. 2017, 65, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Van Milligen, B.A.; Vogelzangs, N.; Smit, J.H.; Penninx, B.W.J.H. Physical function as predictor for the persistence of depressive and anxiety disorders. J. Affect. Disord. 2012, 136, 828–832. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Trevisan, C.; Bolzetta, F.; De Rui, M.; Solmi, M.; Sartori, L.; Musacchio, E.; Zambon, S.; Perissinotto, E.; et al. Poor Physical Performance Predicts Future Onset of Depression in Elderly People: Progetto Veneto Anziani Longitudinal Study. Phys. Ther. 2017, 97, 659–668. [Google Scholar] [CrossRef]
- Choi, H.; Irwin, M.R.; Cho, H.J. Impact of social isolation on behavioral health in elderly: Systematic review. World J. Psychiatry 2015, 5, 432–438. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O.; King, A.C.; Macera, C.A.; Castaneda-Sceppa, C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Au, B.; Ollis, L.; Schmitz, N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Exp. Gerontol. 2018, 102, 109–132. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Milaneschi, Y.; Lamers, F.; Vogelzangs, N. Understanding the somatic consequences of depression: Biological mechanisms and the role of depression symptom profile. BMC Med. 2013, 11, 129. [Google Scholar] [CrossRef]
- Tuttle, C.S.; Thang, L.A.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Kavazis, A.N.; DeRuisseau, K.C. Mechanisms of disuse muscle atrophy: Role of oxidative stress. Am. J. Physiol. Integr. Comp. Physiol. 2005, 288, R337–R344. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Loaiza-Martínez, D.A.; Sánchez-Sánchez, J.; Rubio-Arias, J.Á.; Alacid, F.; Prats-Moya, S.; Martínez-Olcina, M.; Yáñez-Sepúlveda, R.; Asencio-Mas, N.; Marcos-Pardo, P.J. Psychological, Physiological, and Physical Effects of Resistance Training and Personalized Diet in Celiac Women. Front. Nutr. 2022, 9, 838364. [Google Scholar] [CrossRef]
- Fukunaga, M.; Ishimura, N.; Fukuyama, C.; Izumi, D.; Ishikawa, N.; Araki, A.; Oka, A.; Mishiro, T.; Ishihara, S.; Maruyama, R.; et al. Celiac disease in non-clinical populations of Japan. J. Gastroenterol. 2018, 53, 208–214. [Google Scholar] [CrossRef]
| Overall (n = 768) | Muscle Strength | |||
|---|---|---|---|---|
| Lowest (n = 256) | Middle (n = 256) | Highest (n = 256) | ||
| Number of cases | 96 (12.5) | 49 (16.4) | 28 (9.8) | 19 (7.5) |
| Age, years | 72.7 (4.9) | 73.7 (4.9) | 72.9 (5.0) | 71.6 (4.7) |
| 65–69, n (%) | 238 (37.1) | 58 (28.5) | 79 (38.3) | 101 (44.4) |
| 70–74, n (%) | 257 (40.0) | 88 (43.9) | 86 (37.9) | 83 (38.3) |
| 75–79, n (%) | 200 (20.2) | 77 (23.4) | 65 (21.0) | 58 (16.4) |
| 80–84, n (%) | 66 (2.6) | 30 (4.2) | 23 (2.8) | 13 (0.9) |
| >85, n (%) | 7 (0.6) | 3 (1.1) | 3 (1.2) | 1 (0.4) |
| Height, cm | 152.1 (5.3) | 151.9 (5.0) | 151.9 (5.3) | 152.7 (5.6) |
| Weight, kg | 55.3 (8.0) | 55.3 (9.0) | 51.5 (7.4) | 50.5 (6.6) |
| Body mass index, kg/m2 * | 22.7 (3.2) | 24.0 (3.6) | 22.3 (2.9) | 21.7 (2.7) |
| Lean | 56 (7.8) | 10 (3.7) | 20 (8.4) | 26 (11.2) |
| Normal | 556 (71.5) | 156 (61.2) | 194 (74.3) | 206 (79.0) |
| Obese | 156 (20.7) | 90 (35.0) | 42 (17.3) | 24 (9.8) |
| Knee extensor strength, N | 4.31 (1.04) | 3.16 (0.56) | 4.32 (0.26) | 5.44 (0.55) |
| Medication, n (%) | ||||
| Hypertension | 268 (32.6) | 113 (42.5) | 88 (32.7) | 64 (22.4) |
| Diabetes | 78 (10.0) | 37 (12.6) | 24 (9.3) | 17 (7.9) |
| Dyslipidemia | 281 (34.3) | 109 (39.3) | 92 (33.2) | 80 (30.4) |
| Osteoporosis | 182 (21.0) | 68 (23.8) | 60 (19.2) | 54 (20.1) |
| Heart disease | 88 (10.0) | 44 (13.6) | 22 (9.8) | 22 (6.5) |
| Cancer | 99 (12.9) | 37 (13.1) | 28 (12.6) | 34 (13.1) |
| Smoking, n (%) | 47 (6.5) | 18 (6.5) | 14 (5.6) | 15 (7.5) |
| Alcohol, n (%) | 325 (43.8) | 95 (41.6) | 121 (43.9) | 109 (45.8) |
| Job engagement, n (%) | ||||
| ≥35 h/week | 65 (65.4) | 26 (66.4) | 13 (68.7) | 26 (61.2) |
| <35 h/week | 185 (25.4) | 52 (23.4) | 64 (24.3) | 69 (28.5) |
| None | 518 (9.2) | 178 (10.3) | 179 (7.0) | 161 (10.3) |
| Living alone (yes, %) | 199 (22.6) | 70 (26.2) | 61 (22.0) | 68 (19.6) |
| Hobby or lesson (yes, %) | 483 (62.5) | 148 (57.5) | 165 (66.4) | 170 (63.6) |
| Volunteering (yes, %) | 296 (38.5) | 91 (38.3) | 114 (43.5) | 91 (33.6) |
| Education level, years | ||||
| ≤9 | 90 (10.1) | 48 (14.5) | 18 (7.5) | 24 (8.4) |
| <9, ≤12 | 369 (47.7) | 114 (48.1) | 129 (44.9) | 126 (50.0) |
| >12 | 309 (42.2) | 94 (37.4) | 109 (47.7) | 106 (41.6) |
| Dietary patterns | 11.11 (1.2) | 10.97 (1.39) | 11.13 (1.21) | 11.23 (1.10) |
| Variables | Participants | Number of Cases | Incident Ratio (%) | OR (95% CIs) | p Value |
|---|---|---|---|---|---|
| Age, years | 768 | 96 | 12.5 | 1.07 (1.03, 1.12) | <0.001 |
| Body mass index, kg/m2 | |||||
| Lean | 56 | 5 | 8.9 | 0.67 (0.26, 1.74) | 0.41 |
| Normal | 556 | 71 | 12.8 | Ref | |
| Obese | 156 | 20 | 12.8 | 1.01 (0.59, 1.71) | 0.99 |
| Knee extensor strength | |||||
| Lowest | 256 | 49 | 19.1 | Ref | |
| Middle | 256 | 28 | 10.9 | 0.52 (0.31, 0.86) | 0.01 |
| Highest | 256 | 19 | 7.4 | 0.34 (0.19, 0.59) | <0.001 |
| Medication, yes | |||||
| Hypertension | 268 | 40 | 14.9 | 1.39 (0.90, 2.15) | 0.14 |
| Diabetes | 78 | 10 | 12.8 | 1.03 (0.51, 2.08) | 0.93 |
| Dyslipidemia | 281 | 39 | 13.9 | 1.22 (0.79, 1.88) | 0.38 |
| Osteoporosis | 182 | 26 | 14.3 | 1.23 (0.76, 1.99) | 0.41 |
| Heart disease | 88 | 14 | 15.9 | 1.38 (0.75, 2.56) | 0.31 |
| Cancer | 99 | 18 | 18.2 | 1.68 (0.86, 2.96) | 0.07 |
| Smoking, yes | 47 | 9 | 19.1 | 1.73 (0.81, 3.69) | 0.16 |
| Alcohol, yes | 325 | 39 | 12.0 | 0.92 (0.60, 1.43) | 0.72 |
| Job engagement | |||||
| ≥35 h/week | 65 | 6 | 9.2 | 1.00 (0.38, 2.64) | 0.99 |
| <35 h/week | 185 | 17 | 9.2 | 1.61 (0.67, 3.87) | 0.28 |
| None | 518 | 73 | 14.1 | Ref | |
| Living alone, yes | 199 | 28 | 14.1 | 1.21 (0.75, 1.94) | 0.44 |
| Hobby or lesson, yes | 483 | 50 | 10.4 | 0.60 (0.39, 0.92) | 0.02 |
| Volunteering, yes | 296 | 34 | 11.5 | 0.86 (0.55, 1.34) | 0.50 |
| Education level, years | |||||
| ≤9 | 90 | 17 | 18.9 | Ref | |
| <9, ≤12 | 369 | 45 | 12.2 | 0.60 (0.32, 1.10) | 0.10 |
| >12 | 309 | 34 | 11.0 | 0.53 (0.28, 1.00) | 0.05 |
| Dietary pattern | 768 | 96 | 12.5 | 0.91 (0.78, 1.07) | 0.27 |
| Knee Extensor Muscle Strength (N) | p for Trend | |||
|---|---|---|---|---|
| Lowest | Middle | Highest | ||
| Crude adjusted | 1.00 (reference) | 0.52 (0.31, 0.86) | 0.34 (0.19, 0.59) | <0.001 |
| Age adjusted * | 1.00 (reference) | 0.54 (0.32, 0.91) | 0.37 (0.21, 0.67) | <0.001 |
| Multivariable adjusted ** | 1.00 (reference) | 0.68 (0.39, 1.20) | 0.48 (0.26, 0.91) | 0.022 |
| Knee Extensor Muscle Strength (N) | p for Trend | |||
|---|---|---|---|---|
| Lowest | Middle | Highest | ||
| Crude adjusted | 1.00 (reference) | 0.57 (0.31, 1.05) | 0.27 (0.13, 0.59) | <0.001 |
| Age adjusted * | 1.00 (reference) | 0.59 (0.31, 1.12) | 0.30 (0.13, 0.67) | 0.002 |
| Multivariable adjusted ** | 1.00 (reference) | 0.75 (0.38, 1.45) | 0.37 (0.16, 0.86) | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, T.; Kojima, N.; Osuka, Y.; Sasai, H. Knee Extensor Muscle Strength Associated with the Onset of Depression in Older Japanese Women: The Otassha Study. Nutrients 2024, 16, 2179. https://doi.org/10.3390/nu16142179
Ohta T, Kojima N, Osuka Y, Sasai H. Knee Extensor Muscle Strength Associated with the Onset of Depression in Older Japanese Women: The Otassha Study. Nutrients. 2024; 16(14):2179. https://doi.org/10.3390/nu16142179
Chicago/Turabian StyleOhta, Takahisa, Narumi Kojima, Yosuke Osuka, and Hiroyuki Sasai. 2024. "Knee Extensor Muscle Strength Associated with the Onset of Depression in Older Japanese Women: The Otassha Study" Nutrients 16, no. 14: 2179. https://doi.org/10.3390/nu16142179
APA StyleOhta, T., Kojima, N., Osuka, Y., & Sasai, H. (2024). Knee Extensor Muscle Strength Associated with the Onset of Depression in Older Japanese Women: The Otassha Study. Nutrients, 16(14), 2179. https://doi.org/10.3390/nu16142179





