Biochemical and Anthropometric Outcomes in Paediatric Patients with Heterozygous Familial Hypercholesterolemia after COVID-19 Pandemic Lockdowns: An Exploratory Analysis
Abstract
1. Introduction
2. Materials and Methods
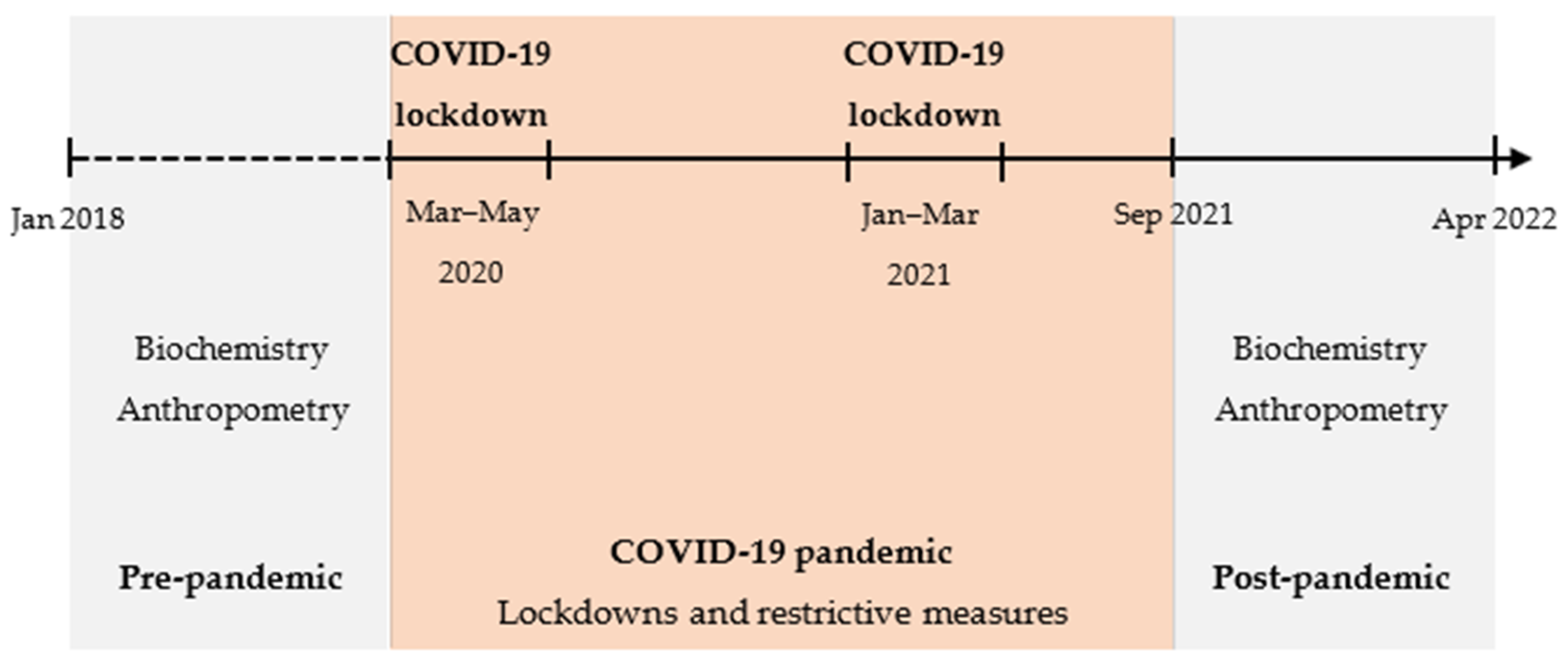
2.1. Study Design
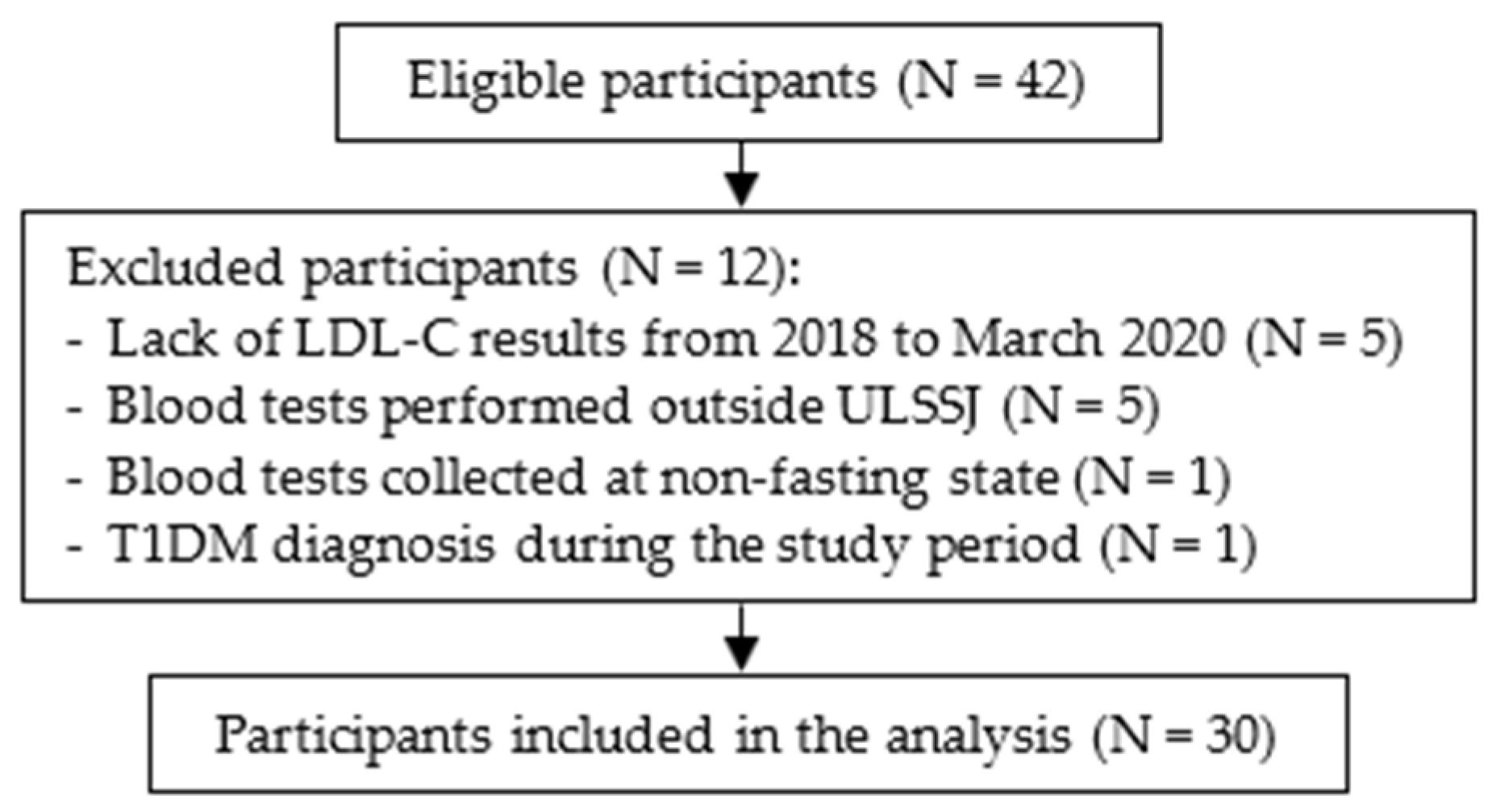
2.2. Participants
2.3. Data Collection
2.3.1. Lipid Profile and Other Biochemical Parameters
2.3.2. Anthropometry
2.4. Statistical Analysis
2.5. Ethical Statement
3. Results
3.1. Sample Characteristics
3.2. Lipid Profile and Other Biochemical Parameters
3.3. Anthropometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watts, G.F.; Gidding, S.; Wierzbicki, A.S.; Toth, P.P.; Alonso, R.; Brown, W.V.; Bruckert, E.; Defesche, J.; Lin, K.K.; Livingston, M.; et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Eur. J. Prev. Cardiol. 2015, 22, 849–854. [Google Scholar] [CrossRef]
- Hu, P.; Dharmayat, K.I.; Stevens, C.A.T.; Sharabiani, M.T.A.; Jones, R.S.; Watts, G.F.; Genest, J.; Ray, K.K.; Vallejo-Vaz, A.J. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients With Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 2020, 141, 1742–1759. [Google Scholar] [CrossRef]
- Beheshti, S.O.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020, 75, 2553–2566. [Google Scholar] [CrossRef] [PubMed]
- Reijman, M.D.; Kusters, D.M.; Wiegman, A. Advances in familial hypercholesterolaemia in children. Lancet Child Adolesc. Health 2021, 5, 652–661. [Google Scholar] [CrossRef]
- Taylor, A.; Wang, D.; Patel, K.; Whittall, R.; Wood, G.; Farrer, M.; Neely, R.D.; Fairgrieve, S.; Nair, D.; Barbir, M.; et al. Mutation detection rate and spectrum in familial hypercholesterolaemia patients in the UK pilot cascade project. Clin. Genet 2010, 77, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Hutter, C.M.; Zimmern, R.L.; Humphries, S.E. Genetic causes of monogenic heterozygous familial hypercholesterolemia: A HuGE prevalence review. Am. J. Epidemiol. 2004, 160, 407–420. [Google Scholar] [CrossRef]
- Jeon, H.; Blacklow, S.C. Structure and physiologic function of the low-density lipoprotein receptor. Annu. Rev. Biochem. 2005, 74, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Defesche, J.C.; Gidding, S.S.; Harada-Shiba, M.; Hegele, R.A.; Santos, R.D.; Wierzbicki, A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Primers 2017, 3, 17093. [Google Scholar] [CrossRef]
- Futema, M.; Shah, S.; Cooper, J.A.; Li, K.; Whittall, R.A.; Sharifi, M.; Goldberg, O.; Drogari, E.; Mollaki, V.; Wiegman, A.; et al. Refinement of Variant Selection for the LDL Cholesterol Genetic Risk Score in the Diagnosis of the Polygenic Form of Clinical Familial Hypercholesterolemia and Replication in Samples from 6 Countries. Clin. Chem. 2015, 61, 231–238. [Google Scholar] [CrossRef]
- Talmud, P.J.; Shah, S.; Whittall, R.; Futema, M.; Howard, P.; Cooper, J.A.; Harrison, S.C.; Li, K.; Drenos, F.; Karpe, F.; et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: A case-control study. Lancet 2013, 381, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Viigimaa, M.; Heinsar, S.; Lovic, D.; Katsimardou, A.; Piperidou, A.; Duishvili, D. New Horizons in the Pathogenesis, Pathophysiology and Treatment of Familial Hypercholesterolaemia. Curr. Pharm. Des. 2018, 24, 3599–3604. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Mundal, L.J.; Igland, J.; Veierød, M.B.; Holven, K.B.; Ose, L.; Selmer, R.M.; Wisloff, T.; Kristiansen, I.S.; Tell, G.S.; Leren, T.P.; et al. Impact of age on excess risk of coronary heart disease in patients with familial hypercholesterolaemia. Heart 2018, 104, 1600. [Google Scholar] [CrossRef]
- Banderali, G.; Capra, M.E.; Biasucci, G.; Stracquadaino, R.; Viggiano, C.; Pederiva, C. Detecting Familial hypercholesterolemia in children and adolescents: Potential and challenges. Ital. J. Pediatr. 2022, 48, 115. [Google Scholar] [CrossRef] [PubMed]
- Akioyamen, L.E.; Genest, J.; Chu, A.; Inibhunu, H.; Ko, D.T.; Tu, J.V. Risk factors for cardiovascular disease in heterozygous familial hypercholesterolemia: A systematic review and meta-analysis. J. Clin. Lipidol. 2019, 13, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.B.; Hedegaard, B.S.; Retterstøl, K. Familial hypercholesterolaemia: History, diagnosis, screening, management and challenges. Heart 2020, 106, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Watts, G.F.; Gidding, S.S.; Mata, P.; Pang, J.; Sullivan, D.R.; Yamashita, S.; Raal, F.J.; Santos, R.D.; Ray, K.K. Familial hypercholesterolaemia: Evolving knowledge for designing adaptive models of care. Nat. Rev. Cardiol. 2020, 17, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Mainieri, F.; Tagi, V.M.; Chiarelli, F. Recent Advances on Familial Hypercholesterolemia in Children and Adolescents. Biomedicines 2022, 10, 1043. [Google Scholar] [CrossRef]
- Luirink, I.K.; Wiegman, A.; Kusters, D.M.; Hof, M.H.; Groothoff, J.W.; de Groot, E.; Kastelein, J.J.P.; Hutten, B.A. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N. Engl. J. Med. 2019, 381, 1547–1556. [Google Scholar] [CrossRef]
- Tada, H.; Kojima, N.; Yamagami, K.; Nomura, A.; Nohara, A.; Usui, S.; Sakata, K.; Takamura, M.; Kawashiri, M.-a. Early diagnosis and treatments in childhood are associated with better prognosis in patients with familial hypercholesterolemia. Am. J. Prev. Cardiol. 2022, 12, 100434. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B. 3.20 Hypercholesterolemia. In Pediatric Nutrition in Practice, 3rd ed.; Koletzko, B., Bhutta, Z.A., Cai, W., Dhansay, M.A., Duggan, C.P., Makrides, M., Orsi, M., Eds.; World Review of Nutrition and Dietetics; Karger: Basel, Switzerland, 2022; Volume 124, pp. 362–367. [Google Scholar]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 March 2023).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Anumudu, C.K.; Al-Sharify, Z.T.; Egele-Godswill, E.; Mbaegbu, P. COVID-19 pandemic: A review of the global lockdown and its far-reaching effects. Sci. Prog. 2021, 104, 00368504211019854. [Google Scholar] [CrossRef]
- Wang, D.; Mao, Z. A comparative study of public health and social measures of COVID-19 advocated in different countries. Health Policy 2021, 125, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Ferreira da Silva, R.; Macedo, M.; Conceição, J. A pandemia de COVID-19 em Portugal: Evolução, Vacinação e Farmacovigilância. RevistaMultidisciplinar 2022, 4, 135–154. [Google Scholar] [CrossRef]
- Capra, M.E.; Stanyevic, B.; Giudice, A.; Monopoli, D.; Decarolis, N.M.; Esposito, S.; Biasucci, G. The Effects of COVID-19 Pandemic and Lockdown on Pediatric Nutritional and Metabolic Diseases: A Narrative Review. Nutrients 2022, 15, 88. [Google Scholar] [CrossRef]
- Sharma, M.; Idele, P.; Manzini, A.; Aladro, C.; Ipince, A.; Olsson, G.; Banati, P.; Anthony, D. Life in Lockdown: Child and Adolescent Mental Health and Well-Being in the Time of COVID-19; UNICEF Office of Research—Innocenti: Florence, Italy, 2021. [Google Scholar]
- Di Fazio, N.; Morena, D.; Delogu, G.; Volonnino, G.; Manetti, F.; Padovano, M.; Scopetti, M.; Frati, P.; Fineschi, V. Mental Health Consequences of COVID-19 Pandemic Period in the European Population: An Institutional Challenge. Int. J. Environ. Res. Public Health 2022, 19, 9347. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.; Carducci, B.; Klein, J.D.; Bhutta, Z.A. Indirect effects of COVID-19 on child and adolescent mental health: An overview of systematic reviews. BMJ Glob. Health 2022, 7, e010713. [Google Scholar] [CrossRef]
- Samji, H.; Wu, J.; Ladak, A.; Vossen, C.; Stewart, E.; Dove, N.; Long, D.; Snell, G. Review: Mental health impacts of the COVID-19 pandemic on children and youth—A systematic review. Child Adolesc. Ment. Health 2022, 27, 173–189. [Google Scholar] [CrossRef]
- Saulle, R.; De Sario, M.; Bena, A.; Capra, P.; Culasso, M.; Davoli, M.; De Lorenzo, A.; Lattke, L.S.; Marra, M.; Mitrova, Z.; et al. School closures and mental health, wellbeing and health behaviours among children and adolescents during the second COVID-19 wave: A systematic review of the literature. Epidemiol. Prev. 2022, 46, 333–352. [Google Scholar] [CrossRef]
- Medrano, M.; Cadenas-Sanchez, C.; Oses, M.; Arenaza, L.; Amasene, M.; Labayen, I. Changes in lifestyle behaviours during the COVID-19 confinement in Spanish children: A longitudinal analysis from the MUGI project. Pediatr. Obes. 2020, 16, e12731. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; de Carvalho Padilha, P.; Mantilla-Escalante, D.C.; Ulloa, N.; Brun, P.; Acevedo-Correa, D.; Arantes Ferreira Peres, W.; Martorell, M.; Aires, M.T.; de Oliveira Cardoso, L.; et al. COVID-19 Confinement and Changes of Adolescent’s Dietary Trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients 2020, 12, 1807. [Google Scholar] [CrossRef]
- Brakspear, L.; Boules, D.; Nicholls, D.; Burmester, V. The Impact of COVID-19-Related Living Restrictions on Eating Behaviours in Children and Adolescents: A Systematic Review. Nutrients 2022, 14, 3657. [Google Scholar] [CrossRef]
- World Health Organization—European Region. Nutrition, Physical Activity, Well-Being and COVID-19—Results from 13 Countries Participating in Round 6 of the Childhood Obesity Surveillance Initiative Study; WHO: Geneva, Switzerland, 2023.
- Neville, R.D.; Lakes, K.D.; Hopkins, W.G.; Tarantino, G.; Draper, C.E.; Beck, R.; Madigan, S. Global Changes in Child and Adolescent Physical Activity During the COVID-19 Pandemic: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022, 176, 886–894. [Google Scholar] [CrossRef]
- Anderson, L.N.; Yoshida-Montezuma, Y.; Dewart, N.; Jalil, E.; Khattar, J.; De Rubeis, V.; Carsley, S.; Griffith, L.E.; Mbuagbaw, L. Obesity and weight change during the COVID-19 pandemic in children and adults: A systematic review and meta-analysis. Obes. Rev. 2023, 24, e13550. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Chen, Y.-C.; Chen, W.-Y.; Chen, C.-Y.; Hsu, W.-Y.; Chou, Y.; Chang, Y.-H. Weight Gain Associated with COVID-19 Lockdown in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3668. [Google Scholar] [CrossRef]
- Departamento de Alimentação e Nutrição. Infográfico—Obesidade Infantil 2022; Instituto Nacional de Saúde Doutor Ricardo Jorge: Lisbon, Portugal, 2023.
- Kayikcioglu, M.; Tokgozoglu, L.; Tuncel, O.K.; Pirildar, S.; Can, L. Negative impact of COVID-19 pandemic on the lifestyle and management of patients with homozygous familial hypercholesterolemia. J. Clin. Lipidol. 2020, 14, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Scicali, R.; Piro, S.; Ferrara, V.; Di Mauro, S.; Filippello, A.; Scamporrino, A.; Romano, M.; Purrello, F.; Di Pino, A. Direct and Indirect Effects of SARS-CoV-2 Pandemic in Subjects with Familial Hypercholesterolemia: A Single Lipid-Center Real-World Evaluation. J. Clin. Med. 2021, 10, 4363. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Leung, K.S.K.; Garg, T.; Mazzoleni, A.; Miteu, G.D.; Zakariya, F.; Awuah, W.A.; Yin, E.T.S.; Haroon, F.; Hussain, Z.; et al. Barriers and shortcomings in access to cardiovascular management and prevention for familial hypercholesterolemia during the COVID-19 pandemic. Clin. Cardiol. 2023, 46, 831–844. [Google Scholar] [CrossRef]
- Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ 1991, 303, 893–896. [Google Scholar] [CrossRef]
- Wiegman, A.; Gidding, S.S.; Watts, G.F.; Chapman, M.J.; Ginsberg, H.N.; Cuchel, M.; Ose, L.; Averna, M.; Boileau, C.; Borén, J.; et al. Familial hypercholesterolaemia in children and adolescents: Gaining decades of life by optimizing detection and treatment. Eur. Heart J. 2015, 36, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. BMI-for-Age (0–5 years). Available online: https://www.who.int/childgrowth/standards/bmi_for_age/en/ (accessed on 22 September 2022).
- World Health Organization. BMI-for-Age (5–19 years). Available online: http://www.who.int/growthref/who2007_bmi_for_age/en/ (accessed on 22 September 2022).
- Heidari-Beni, M.; Bemanalizadeh, M.; Heshmat, R.; Qorbani, M.; Kelishadi, R. Changes in Lifestyle Behaviors of Children and Adolescents during the COVID-19 Pandemic and the Impact on the Development of Non-Communicable Diseases: A Narrative Review. Med. J. Islam. Repub. Iran 2022, 36, 165. [Google Scholar] [CrossRef]
- Ilesanmi, O.; Afolabi, A.; Kwaghe, A. A scope review on the global impact of COVID-19 lockdown on adolescents’ health. Afr. Health Sci. 2021, 21, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Panchal, U.; Salazar de Pablo, G.; Franco, M.; Moreno, C.; Parellada, M.; Arango, C.; Fusar-Poli, P. The impact of COVID-19 lockdown on child and adolescent mental health: Systematic review. Eur. Child Adolesc. Psychiatry 2021, 32, 1151–1177. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Shah, N.; Mbeledogu, C.; Garstang, J. Child wellbeing in the United Kingdom following the COVID-19 lockdowns. Paediatr. Child Health 2021, 31, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Caroppo, E.; Mazza, M.; Sannella, A.; Marano, G.; Avallone, C.; Claro, A.E.; Janiri, D.; Moccia, L.; Janiri, L.; Sani, G. Will Nothing Be the Same Again?: Changes in Lifestyle during COVID-19 Pandemic and Consequences on Mental Health. Int. J. Environ. Res. Public Health 2021, 18, 8433. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.M.; Chandrasekaran, B. Estimating the Impact of the Pandemic on Children’s Physical Health: A Scoping Review. J. Sch. Health 2021, 91, 936–947. [Google Scholar] [CrossRef]
- Barbosa, A.O.; Silva, J.; Silva, D.J.; Cabral, T.G.; Jesus, F.M.; Mendonça, G.; Prazeres Filho, A.; Moura, I.R.D.; Cristina da Costa Silva, E.; Rocha, S.; et al. Longitudinal association between moderate to vigorous physical activity and lipid profile indicators in adolescents. Eur. J. Sport. Sci. 2023, 23, 1405–1414. [Google Scholar] [CrossRef]
- Gil-Campos, M.; Pérez-Ferreirós, A.; Llorente-Cantarero, F.J.; Anguita-Ruiz, A.; Bedoya-Carpente, J.J.; Kalén, A.; Moreno, L.A.; Bueno, G.; Gil, Á.; Aguilera, C.M.; et al. Association of Diet, Physical Activity Guidelines and Cardiometabolic Risk Markers in Children. Nutrients 2021, 13, 2954. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.G.; Janssen, I. Dose-response relationship between physical activity and dyslipidemia in youth. Can. J. Cardiol. 2010, 26, 201–205. [Google Scholar] [CrossRef]
- Perrone, M.A.; Feola, A.; Pieri, M.; Donatucci, B.; Salimei, C.; Lombardo, M.; Perrone, A.; Parisi, A. The Effects of Reduced Physical Activity on the Lipid Profile in Patients with High Cardiovascular Risk during COVID-19 Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 8858. [Google Scholar] [CrossRef] [PubMed]
- Lia, L.; Ricci, E.; Colaprico, C.; Di Legge, E.; Faticoni, A.; Donini, L.M.; La Torre, G. Assessment of the Impact of COVID-19 Lockdown on the Nutritional Status and Lipid Profile of Employees in a Teaching Hospital in Rome: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 4549. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Manent, J.I.; Altisench Jané, B.; Sanchís Cortés, P.; Busquets-Cortés, C.; Arroyo Bote, S.; Masmiquel Comas, L.; López González, Á.A. Impact of COVID-19 Lockdown on Anthropometric Variables, Blood Pressure, and Glucose and Lipid Profile in Healthy Adults: A before and after Pandemic Lockdown Longitudinal Study. Nutrients 2022, 14, 1237. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Xu, X.; Mei, Y.; Rahmani, J.; Seraj, S.S.; Qi, X. COVID-19 Pandemic Impact on Cardiometabolic Markers in Adults in Chongqing, China: A Retrospective Cohort Study. Front. Public Health 2022, 10, 859488. [Google Scholar] [CrossRef]
- Samur, B.M.; Samur, T.G.; Gul-Sir, U.; Hatipoglu, N. Vicious cycle between severity of childhood obesity and pandemic: Potential impact of metformin. Obes. Med. 2022, 33, 100433. [Google Scholar] [CrossRef]
- Valenzise, M.; D’Amico, F.; Cucinotta, U.; Lugarà, C.; Zirilli, G.; Zema, A.; Wasniewska, M.; Pajno, G.B. The lockdown effects on a pediatric obese population in the COVID-19 era. Ital. J. Pediatr. 2021, 47, 209. [Google Scholar] [CrossRef] [PubMed]
- Hickman, T.B.; Briefel, R.R.; Carroll, M.D.; Rifkind, B.M.; Cleeman, J.I.; Maurer, K.R.; Johnson, C.L. Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: Data from the Third National Health and Nutrition Examination Survey. Prev. Med. 1998, 27, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Auinger, P.; Huang, T.T.K. Growth Curves for Cardio-Metabolic Risk Factors in Children and Adolescents. J. Pediatr. 2009, 155, S6.e15–S16.e26. [Google Scholar] [CrossRef]
- Tamir, I.; Heiss, G.; Glueck, C.J.; Christensen, B.; Kwiterovich, P.; Rifkind, B.M. Lipid and lipoprotein distributions in white children ages 6-19 yr. The lipid research clinics program prevalence study. J. Chronic. Dis. 1981, 34, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Tagi, V.M.; Samvelyan, S.; Chiarelli, F. An update of the consensus statement on insulin resistance in children 2010. Front. Endocrinol. 2022, 13, 1061524. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Arayess, L.; Knockaert, N.; Winkens, B.; Lubrecht, J.W.; Verweij, M.; Vreugdenhil, A.C.E. The Side-Effects of the COVID-19 Pandemic: Increased BMI z-Score in Children with Overweight and Obesity in a Personalised Lifestyle Intervention One Year after the Start of the Pandemic in The Netherlands. Nutrients 2022, 14, 1942. [Google Scholar] [CrossRef] [PubMed]
- Pietrobelli, A.; Fearnbach, N.; Ferruzzi, A.; Vrech, M.; Heo, M.; Faith, M.; Pecoraro, L.; Zoller, T.; Antoniazzi, F.; Piacentini, G.; et al. Effects of COVID-19 lockdown on lifestyle behaviors in children with obesity: Longitudinal study update. Obes. Sci. Pract. 2022, 8, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Zachurzok, A.; Wójcik, M.; Gawlik, A.; Starzyk, J.B.; Mazur, A. An Attempt to Assess the Impact of Pandemic Restrictions on the Lifestyle, Diet, and Body Mass Index of Children with Endocrine Diseases-Preliminary Results. Nutrients 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, L.G.; Adelborg, K.; Baker, J.L. Change in body mass index from childhood onwards and risk of adult cardiovascular disease. Trends Cardiovasc. Med. 2020, 30, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.; Barros, H.; Ramos, E.; Li, L. Trajectories of total and central adiposity throughout adolescence and cardiometabolic factors in early adulthood. Int. J. Obes. 2016, 40, 1899–1905. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Litvinova, L.; Poggio, P.; Orekhov, A.N.; Melnichenko, A.A. Familial Hypercholesterolaemia as a Predisposing Factor for Atherosclerosis. Biomedicines 2022, 10, 2639. [Google Scholar] [CrossRef]
- Alonso, R.; Mata, N.; Castillo, S.; Fuentes, F.; Saenz, P.; Muñiz, O.; Galiana, J.; Figueras, R.; Diaz, J.L.; Gomez-Enterría, P.; et al. Cardiovascular disease in familial hypercholesterolaemia: Influence of low-density lipoprotein receptor mutation type and classic risk factors. Atherosclerosis 2008, 200, 315–321. [Google Scholar] [CrossRef]
- Besseling, J.; Kindt, I.; Hof, M.; Kastelein, J.J.; Hutten, B.A.; Hovingh, G.K. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: A study of a cohort of 14,000 mutation carriers. Atherosclerosis 2014, 233, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Buscot, M.J.; Thomson, R.J.; Juonala, M.; Sabin, M.A.; Burgner, D.P.; Lehtimäki, T.; Hutri-Kähönen, N.; Viikari, J.S.A.; Raitakari, O.T.; Magnussen, C.G. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur. Heart J. 2018, 39, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, Y.; Sun, C.; Zhou, Z. The impact of COVID lockdown on glycaemic control in paediatric patients with type 1 diabetes: A systematic review and meta-analysis of 22 observational studies. Front. Endocrinol. 2022, 13, 1069559. [Google Scholar] [CrossRef]
- Pugh, P.; Hemingway, P.; Christian, M.; Higginbottom, G. Children’s, parents’, and other stakeholders’ perspectives on the factors influencing the initiation of early dietary change in the management of childhood chronic disease: A mixed studies systematic review using a narrative synthesis. Patient Educ. Couns. 2021, 104, 844–857. [Google Scholar] [CrossRef]
- Smith, B.M.; Sharma, R.; Das, A.; Aboumatar, H.; Pitts, S.I.; Day, J.; Holzhauer, K.; Bass, E.; Bennett, W.L. Patient and family engagement strategies for children and adolescents with chronic diseases: A review of systematic reviews. Patient Educ. Couns. 2021, 104, 2213–2223. [Google Scholar] [CrossRef]
- Carman, K.L.; Dardess, P.; Maurer, M.; Sofaer, S.; Adams, K.; Bechtel, C.; Sweeney, J. Patient and family engagement: A framework for understanding the elements and developing interventions and policies. Health Aff. 2013, 32, 223–231. [Google Scholar] [CrossRef]
- Ellis, K.L.; Pang, J.; Chan, D.C.; Hooper, A.J.; Bell, D.A.; Burnett, J.R.; Watts, G.F. Familial combined hyperlipidemia and hyperlipoprotein(a) as phenotypic mimics of familial hypercholesterolemia: Frequencies, associations and predictions. J. Clin. Lipidol. 2016, 10, 1329–1337.e1323. [Google Scholar] [CrossRef]


| Baseline Characteristics | N (%) Median [P25, P75] |
|---|---|
| Gender | |
| Male | 13 (43%) |
| Female | 17 (57%) |
| Age (years) | 11 [9, 13] |
| Age at follow-up initiation (years) | 8 [5, 11] |
| Time in follow-up (years) | 3 [1, 5] |
| Pre-treatment LDL-C (mg/dL) | 158 [136, 197] |
| Genetic testing (yes, n) | 12 (40%) |
| No mutations identified | 6 (20%) |
| Pathogenic LDLR mutation | 3 (10%) |
| LDLR variant with likely pathogenicity | 3 (10%) |
| Relatives with same FH mutation (yes, n) | 5 (17%) |
| Family history of premature CHD/sudden death (yes, n) | 8 (27%) |
| Family members with hypercholesterolemia (n) | |
| 0 | 3 (10%) |
| 1–2 | 14 (47%) |
| ≥3 | 13 (43%) |
| On lipid-lowering medication (yes, n) | 8 (27%) |
| Statin only | 6 (20%) |
| Rosuvastatin | 4 (13%) |
| Pravastatin | 2 (7%) |
| Statin + ezetimibe | 2 (7%) |
| N | Baseline | Post-Pandemic | p * | |
|---|---|---|---|---|
| Age (years) | 30 | 11 [9, 13] | 14 [12, 16] | - |
| Biochemical parameters | ||||
| LDL-C (mg/dL) | 30 | 125 [112, 150] | 125 [100, 147] | 0.894 |
| HDL-C (mg/dL) | 30 | 58 [52, 65] | 56 [51, 61] | 0.107 |
| TG (mg/dL) | 30 | 64 [44, 86] | 59 [42, 86] | 0.178 |
| TC (mg/dL) | 10 | 197 [178, 228] | 211 [157, 244] | 0.919 |
| Lp(a) (mg/dL) | 11 | 21 [4, 47] | 20 [6, 35] | 0.341 |
| apoA (mg/dL) | 15 | 143 [136, 154] | 141 [133, 161] | 0.887 |
| apoB (mg/dL) | 14 | 88 [80, 96] | 82 [68, 101] | 0.490 |
| Glucose (mg/dL) | 16 | 92 [86, 94] | 91 [88, 98] | 0.178 |
| ALT (U/L) | 17 | 16 [14, 26] | 16 [14, 20] | 0.850 |
| AST (U/L) | 19 | 21 [20, 29] | 21 [17, 24] | 0.238 |
| Anthropometry | ||||
| Weight (kg) | 28 | 38.7 [28.2, 50.4] | 50.9 [37.7, 58.7] | <0.001 |
| Height (cm) | 28 | 146.8 [130.5, 158.0] | 158.4 [141.9, 166.0] | <0.001 |
| Height z-score | 28 | −0.72 [−1.14, 0.56] | −0.54 [−1.17, 0.90] | 0.097 |
| BMI (kg/m2) | 28 | 17.9 [16.8, 19.9] | 20.3 [17.7, 21.7] | <0.001 |
| BMI z-score | 28 | 0.19 [−0.58, 0.89] | 0.30 [−0.48, 1.10] | 0.524 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, M.; Moreira-Rosário, A.; Padeira, G.; Gaspar Silva, P.; Correia, C.; Nunes, A.; Garcia, E.; Faria, A.; Teixeira, D.; Calhau, C.; et al. Biochemical and Anthropometric Outcomes in Paediatric Patients with Heterozygous Familial Hypercholesterolemia after COVID-19 Pandemic Lockdowns: An Exploratory Analysis. Nutrients 2024, 16, 2170. https://doi.org/10.3390/nu16132170
Peres M, Moreira-Rosário A, Padeira G, Gaspar Silva P, Correia C, Nunes A, Garcia E, Faria A, Teixeira D, Calhau C, et al. Biochemical and Anthropometric Outcomes in Paediatric Patients with Heterozygous Familial Hypercholesterolemia after COVID-19 Pandemic Lockdowns: An Exploratory Analysis. Nutrients. 2024; 16(13):2170. https://doi.org/10.3390/nu16132170
Chicago/Turabian StylePeres, Maria, André Moreira-Rosário, Gonçalo Padeira, Patrícia Gaspar Silva, Carla Correia, Andreia Nunes, Elisabete Garcia, Ana Faria, Diana Teixeira, Conceição Calhau, and et al. 2024. "Biochemical and Anthropometric Outcomes in Paediatric Patients with Heterozygous Familial Hypercholesterolemia after COVID-19 Pandemic Lockdowns: An Exploratory Analysis" Nutrients 16, no. 13: 2170. https://doi.org/10.3390/nu16132170
APA StylePeres, M., Moreira-Rosário, A., Padeira, G., Gaspar Silva, P., Correia, C., Nunes, A., Garcia, E., Faria, A., Teixeira, D., Calhau, C., Pereira-da-Silva, L., Ferreira, A. C., & Rocha, J. C. (2024). Biochemical and Anthropometric Outcomes in Paediatric Patients with Heterozygous Familial Hypercholesterolemia after COVID-19 Pandemic Lockdowns: An Exploratory Analysis. Nutrients, 16(13), 2170. https://doi.org/10.3390/nu16132170











