Association of Body Water Balance, Nutritional Risk, and Sarcopenia with Outcome in Patients with Acute Ischemic Stroke: A Single-Center Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Definition of Overhydration and Sarcopenia
2.3. Definition of Nutritionally At-Risk
2.4. Statistical Analysis
3. Results
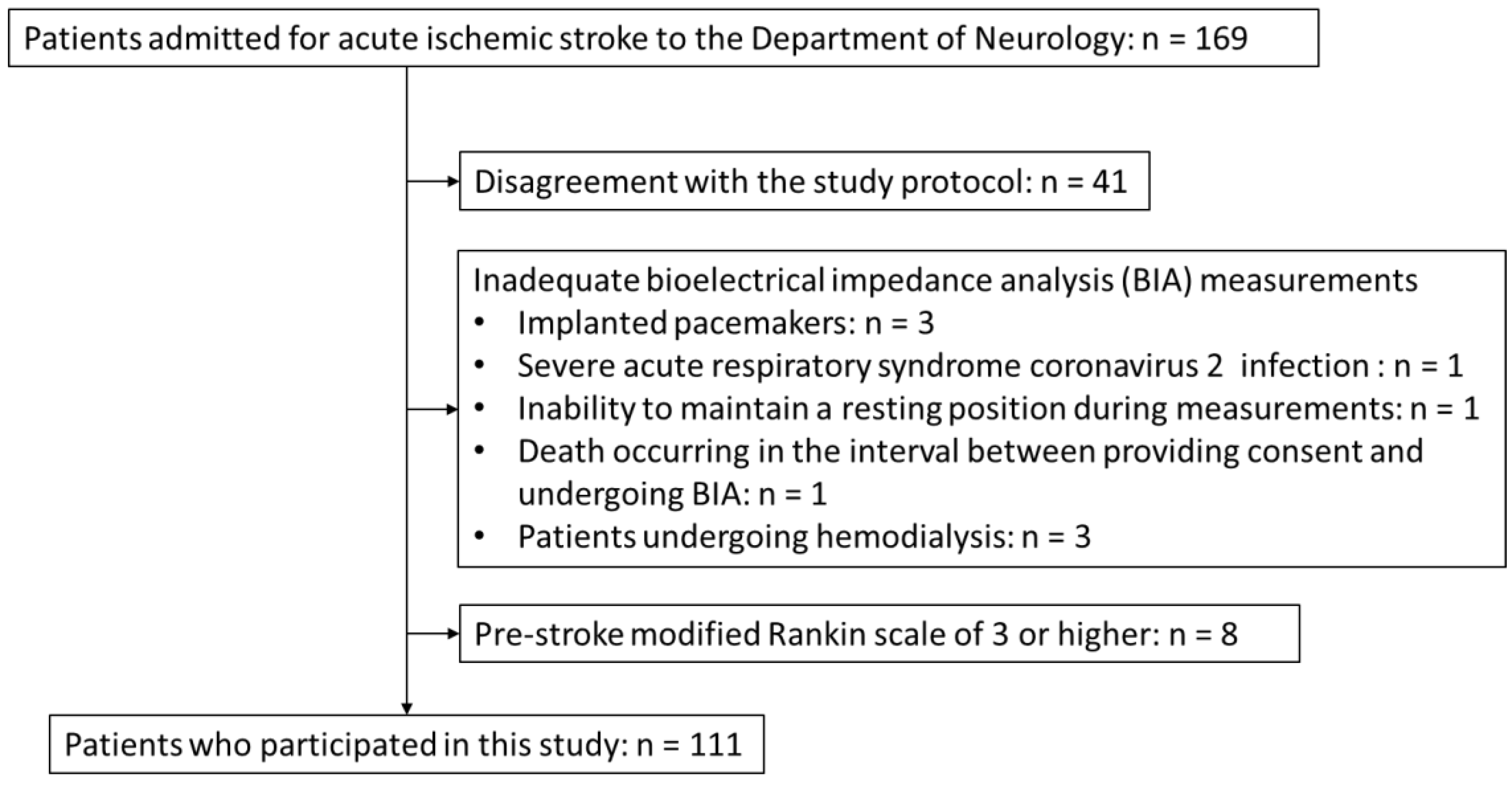
3.1. Study Cohort
3.2. Univariate Comparison between Two Group
3.2.1. Comparison of the Patients with Good and Poor AIS Outcomes
3.2.2. Comparison of AIS Patients with and without Overhydration
3.2.3. Comparison of AIS Patients with and without Nutritional Risk
3.2.4. Comparison of AIS Patients with and without Sarcopenia
3.3. Relationship between the Number of Comorbidities and AIS Outcome
3.4. Multivariate Analysis with Outcome as the Dependent Variable
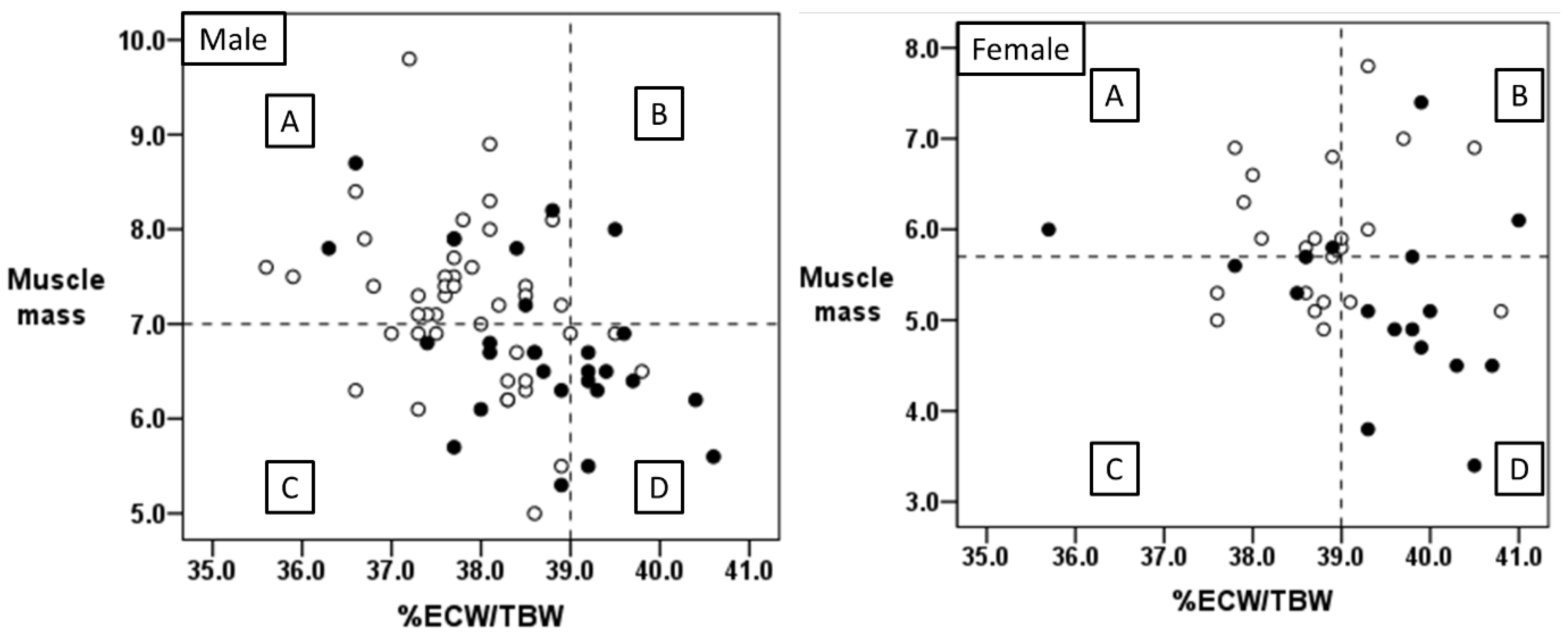
3.5. Relationship between Body Water Balance and Muscle Mass
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luengo-Fernandez, R.; Paul, N.L.; Gray, A.M.; Pendlebury, S.T.; Bull, L.M.; Welch, S.J.; Cuthbertson, F.C.; Rothwell, P.M. Population-based study of disability and institutionalization after transient ischemic attack and stroke: 10-year results of the Oxford Vascular Study. Stroke 2013, 44, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, I.H.; Heo, J.; Baik, M.; Park, H.; Lee, H.S.; Nam, H.S.; Kim, Y.D. Impact of Sarcopenia on Functional Outcomes Among Patients with Mild Acute Ischemic Stroke and Transient Ischemic Attack: A Retrospective Study. Front. Neurol. 2022, 13, 841945. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, H.; Kim, K.Y.; Lee, H.S.; Jung, J.M. Appendicular Skeletal Muscle Mass Associated with Sarcopenia as a Predictor of Poor Functional Outcomes in Ischemic Stroke. Clin. Interv. Aging 2023, 18, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Honda, Y.; Inoue-Umezaki, M.; Makieda, R.; Endo, Y.; Hanayama, K.; Sakaue, H.; Teramoto, F. The association between sarcopenia and functional outcomes in patients undergoing convalescent rehabilitation. J. Med. Investig. 2023, 70, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Deurenberg, P.; Wang, W.; Pietrobelli, A.; Baumgartner, R.N.; Heymsfield, S.B. Hydration of fat-free body mass: New physiological modeling approach. Am. J. Physiol. 1999, 276, E995–E1003. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Ishii, N.; Iwata, Y.; Nakano, C.; Takata, R.; Nishimura, T.; Aizawa, N.; Sakai, Y.; et al. Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis. Nutrients 2018, 10, 1072. [Google Scholar] [CrossRef]
- Noda, Y.; Suzuki, H.; Kanai, T.; Samejima, Y.; Nasu, S.; Tanaka, A.; Morishita, N.; Okamoto, N.; Hirashima, T. The Association Between Extracellular Water-to-Total Body Water Ratio and Therapeutic Durability for Advanced Lung Cancer. Anticancer Res. 2020, 40, 3931–3937. [Google Scholar] [CrossRef]
- Berneis, K.; Keller, U. Bioelectrical impedance analysis during acute changes of extracellular osmolality in man. Clin. Nutr. 2000, 19, 361–366. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Andreoli, A.; Matthie, J.; Withers, P. Predicting body cell mass with bioimpedance by using theoretical methods: A technological review. J. Appl. Physiol. 1997, 82, 1542–1558. [Google Scholar] [CrossRef] [PubMed]
- Gudivaka, R.; Schoeller, D.A.; Kushner, R.F.; Bolt, M.J. Single- and multifrequency models for bioelectrical impedance analysis of body water compartments. J. Appl. Physiol. 1999, 87, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Walking Speed: Japanese Data in Chronic Liver Diseases. J. Clin. Med. 2020, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Lupoli, L.; Volpato, S.; Bertani, R.; Coin, A.; Perissinotto, E.; Calliari, I.; Inelmen, E.M.; Busetto, L.; Enzi, G. Body fluid distribution in elderly subjects with congestive heart failure. Ann. Clin. Lab. Sci. 2004, 34, 416–422. [Google Scholar] [PubMed]
- Martins, A.R.; Soares, J.D.P.; Siqueira, J.M.; Pimentel, G.D. Correlation between the SARC-F Score and Hydration Status in Older Gastrointestinal Cancer Outpatients. J. Nutr. Health Aging 2021, 25, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.Z.; Ruan, G.T.; Zhang, Q.; Dong, W.J.; Zhang, X.; Song, M.M.; Zhang, X.W.; Li, X.R.; Zhang, K.P.; Tang, M.; et al. Extracellular water to total body water ratio predicts survival in cancer patients with sarcopenia: A multi-center cohort study. Nutr. Metab. 2022, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kwon, O.; Shin, C.S.; Lee, S.M. Use of bioelectrical impedance analysis for the assessment of nutritional status in critically ill patients. Clin. Nutr. Res. 2015, 4, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, H.; Mizushima, T. Correlation of Body Composition and Nutritional Status with Functional Recovery in Stroke Rehabilitation Patients. Nutrients 2020, 12, 1923. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, T.J.; Kim, Y.; Nam, K.W.; Jeong, H.Y.; Kim, S.K.; Lee, J.S.; Ko, S.B.; Yoon, B.W. Geriatric nutritional risk index predicts poor outcomes in patients with acute ischemic stroke—Automated undernutrition screen tool. PLoS ONE 2020, 15, e0228738. [Google Scholar] [CrossRef]
- Maruyama, K.; Nakagawa, N.; Koyama, S.; Maruyama, J.I.; Hasebe, N. Malnutrition Increases the Incidence of Death, Cardiovascular Events, and Infections in Patients with Stroke after Rehabilitation. J. Stroke Cerebrovasc. Dis. 2018, 27, 716–723. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshimura, Y.; Abe, T. Nutrition in the First Week after Stroke Is Associated with Discharge to Home. Nutrients 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Kafri, M.W.; Myint, P.K.; Doherty, D.; Wilson, A.H.; Potter, J.F.; Hooper, L. The diagnostic accuracy of multi-frequency bioelectrical impedance analysis in diagnosing dehydration after stroke. Med. Sci. Monit. 2013, 19, 548–570. [Google Scholar] [CrossRef]
- Nalepa, D.; Czarkowska, M.; Załuska, W.; Jakubowska, K.; Chruściel, P. Electrical bioimpedance in patients after ischemic stroke, a civilization disease. Ann. Agric. Environ. Med. 2019, 26, 46–50. [Google Scholar] [CrossRef]
- Wilczyński, J.; Mierzwa-Molenda, M.; Habik-Tatarowska, N. Differences in Body Composition among Patientsafter Hemorrhagic and Ischemic Stroke. Int. J. Environ. Res. Public Health 2020, 17, 4170. [Google Scholar] [CrossRef]
- Park, I.; Lee, J.H.; Jang, D.H.; Kim, J.; Hwang, B.R.; Kim, S.; Lee, J.E.; Jo, Y.H. Assessment of body water distribution in patients with sepsis during fluid resuscitation using multi-frequency direct segmental bioelectrical impedance analysis. Clin. Nutr. 2020, 39, 1826–1831. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, J.H.; Kang, J.; Cho, H.J.; Lee, H.Y. Association Between Changes in Bioelectrical Impedance Analysis (BIA) Parameter and the Clinical Outcomes in Patients with Acute Heart Failure. J. Korean Med. Sci. 2023, 38, e276. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Maries, L.; Manitiu, I. Diagnostic and prognostic values of B-type natriuretic peptides (BNP) and N-terminal fragment brain natriuretic peptides (NT-pro-BNP). Cardiovasc. J. Afr. 2013, 24, 286–289. [Google Scholar] [CrossRef]
- Lyden, P.; Brott, T.; Tilley, B.; Welch, K.M.; Mascha, E.J.; Levine, S.; Haley, E.C.; Grotta, J.; Marler, J. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994, 25, 2220–2226. [Google Scholar] [CrossRef]
- Shinohara, Y.; Minematsu, K.; Amano, T.; Ohashi, Y. Modified Rankin scale with expanded guidance scheme and interview questionnaire: Interrater agreement and reproducibility of assessment. Cerebrovasc. Dis. 2006, 21, 271–278. [Google Scholar] [CrossRef] [PubMed]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Myers, S.A.; Takiguchi, S.; Yu, M. Stature estimated from knee height in elderly Japanese Americans. J. Am. Geriatr. Soc. 1994, 42, 157–160. [Google Scholar] [CrossRef]
- Masuda, T.; Ohara, K.; Nagayama, I.; Matsuoka, R.; Murakami, T.; Nakagawa, S.; Oka, K.; Asakura, M.; Igarashi, Y.; Fukaya, Y.; et al. Impact of serum albumin levels on the body fluid response to tolvaptan in chronic kidney disease patients. Int. Urol. Nephrol. 2019, 51, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Pek, S.; Liu, Y.L.; Moh, A.; Ang, K.; Tang, W.E.; Lim, Z.; Subramaniam, T.; Sum, C.F.; Lim, C.L.; et al. Higher extracellular water to total body water ratio was associated with chronic kidney disease progression in type 2 diabetes. J. Diabetes Complicat. 2021, 35, 107930. [Google Scholar] [CrossRef]
- Namba, Y.; Yunoki, K.; Nakamura, K.; Ejiri, K.; Oka, T.; Ito, H. Differences in extracellular fluid volume between acute heart failure patients with and without high systolic blood pressure. ESC Heart Fail 2022, 9, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Otani, T.; Tai, R.; Tanaka, Y.; Sakai, K.; Aikawa, A. Assessment of body composition using dry mass index and ratio of total body water to estimated volume based on bioelectrical impedance analysis in chronic kidney disease patients. J. Ren. Nutr. 2013, 23, 28–36. [Google Scholar] [CrossRef]
- Ljunggren, H. Studies on body composition; with special reference to the composition of obesity tissue and non-obesity tissue. Acta Endocrinol. Suppl. 1957, 25, 1–58. [Google Scholar]
- Pierson, R.N., Jr.; Wang, J.; Yang, M.U.; Hashim, S.A.; Van Itallie, T.B. The assessment of human body composition during weight reduction: Evaluation of a new model for clinical studies. J. Nutr. 1976, 106, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.H.; Vaswani, A.N.; Yasumura, S.; Yuen, K.; Ellis, K.J. Improved models for determination of body fat by in vivo neutron activation. Am. J. Clin. Nutr. 1984, 40, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Cusick, A.S.; Bhutta, B.S. Peripheral Edema. In StatPearls; StatPearls Publishing Copyright © 2024; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lang, F.; Busch, G.L.; Ritter, M.; Völkl, H.; Waldegger, S.; Gulbins, E.; Häussinger, D. Functional significance of cell volume regulatory mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.M.D.; Schafer, A.I. Goldman-Cecil Medicine, 26th ed.; Elsevier/Saunders: Amsterdam, The Netherlands, 2020; pp. 1399–1405. [Google Scholar]
- Deurenberg, P.; Tagliabue, A.; Wang, J.; Wolde-Gebriel, Z. Multi-frequency bioelectrical impedance for the prediction of body water compartments: Validation in different ethnic groups. Asia Pac. J. Clin. Nutr. 1996, 5, 217–221. [Google Scholar] [PubMed]

| Total (n = 111) | Good Outcome (mRS Score < 3) (n = 68) | Poor Outcome (mRS Score ≥ 3) (n = 43) | p-Value | |
|---|---|---|---|---|
| Sex (female, %) | 40 (36.0%) | 23 (33.8%) | 17 (39.5%) | 0.341 |
| Age (years) | 77 (19–99) | 73 (19–90) | 81 (51–99) | <0.001 * |
| NIHSS score | 2 (0–30) | 1 (0–7) | 3 (0–30) | <0.001 * |
| Body measurements | ||||
| Height (cm) | 161 (135–180) | 163 (135–177) | 161 (135–180) | 0.277 |
| Body weight (kg) | 60 (34–109) | 61 (34–109) | 58 (35–80) | 0.092 |
| BMI (kg/m2) | 23.5 (14.3–37.7) | 23.9 (18.2–37.7) | 23.1 (14.3–29.8) | 0.263 |
| %ECW/TBW | 38.5 (35.6–41.0) | 38.2 (35.6–40.8) | 39.2 (35.7–41.0) | <0.001 * |
| %ECW/TBW > 0.390 | 31 (27.9%) | 8 (11.8%) | 23 (53.5%) | <0.001 * |
| Muscle mass (kg/m2) | 6.5 (3.4–9.8) | 6.9 (4.9–9.8) | 6.2 (3.4–8.7) | 0.005 * |
| Grip strength (kg) | 22 (0–49) | 25.5 (0–49) | 15 (0–42) | <0.001 * |
| Sarcopenia | 38 (34.2%) | 16 (23.5%) | 28 (65.1%) | <0.001 * |
| Days from admission to evaluation 1 | 8 (1–24) | 7 (1–15) | 8 (0–24) | 0.034 * |
| Laboratory data | ||||
| Albumin (g/dL) | 4.1 (2.7–5.0) | 4.2 (3.3–5) | 3.9 (2.7–5) | <0.001 * |
| Creatinine (mg/dL) | 0.9 (0.5–10.1) | 0.9 (0.5–10.1) | 0.8 (0.5–4.6) | 0.910 |
| CRP (mg/dL) | 0.1 (0.1–10.0) | 0.1 (0.1–3.9) | 0.2 (0.1–10) | 0.202 |
| NT-pro-BNP (pg/mL) | 221.5 (10–9463) | 159 (10–7099) | 345.5 (34–9463) | 0.006 * |
| NT-pro-BNP > 500 pg/mL | 38 (35.8%) | 21 (31.8%) | 17 (40.0%) | 0.183 |
| HbA1c (%) | 6.0 (4.8–10.8) | 6.0 (4.8–10.3) | 6.2 (5.1–10.8) | 0.242 |
| GNRI score | 104.7 (76.4–135.5) | 106.7 (84.5–135.5) | 100.9 (76.4–124.8) | <0.001 * |
| Nutritionally at-risk 2 | 25 (22.5%) | 8 (11.8%) | 17 (40.0%) | <0.001 * |
| Complications | ||||
| Pneumonia | 9 (8.1%) | 1 (1.5%) | 8 (18.6%) | 0.002 * |
| Urinary tract infection | 13 (11.7%) | 3 (4.4%) | 10 (23.3%) | 0.004 * |
| Cardiovascular | 5 (4.5%) | 2 (2.9%) | 3 (7.0%) | 0.292 |
| Clinical course | ||||
| Length of hospitalization (days) | 15 (4–67) | 12 (4–51) | 21 (12–67) | <0.001 * |
| Normal (n = 80) | Overhydration (n = 31) | p-Value | |
|---|---|---|---|
| Sex (female, %) | 22 (27.5%) | 18 (58.1%) | 0.003 * |
| Age (years) | 74.5 (19–95) | 83 (48–99) | <0.001 * |
| NIHSS score | 1 (0–18) | 3 (0–30) | 0.005 * |
| Body measurements | |||
| Height (cm) | 164 (139–180) | 158 (135–175) | 0.025 * |
| Body weight (kg) | 61 (39–109) | 54 (34–77) | 0.011 * |
| BMI (kg/m2) | 24.1 (14.3–37.7) | 22.5 (16.7–33.8) | 0.054 |
| Muscle mass (kg/m2) | 6.8 (4.9–9.8) | 6.1 (3.4–8) | 0.001 * |
| Grip strength (kg) | 25.5 (0–49) | 11 (0–32) | <0.001 * |
| Sarcopenia | 21 (26.3%) | 23 (74.2%) | <0.001 * |
| Days from admission to evaluation 1 | 7 (1–21) | 8 (0–24) | 0.108 |
| Laboratory data | |||
| Albumin (g/dL) | 4.2 (3.4–5) | 3.7 (2.7–4.8) | <0.001 * |
| Creatinine (mg/dL) | 0.9 (0.5–10.1) | 0.8 (0.5–4.6) | 0.541 |
| CRP (mg/dL) | 0.1 (0.1–3.9) | 0.1 (0.1–10) | 0.253 |
| NT-pro-BNP (pg/mL) | 159 (10–9463) | 1054 (97–6581) | <0.001 * |
| NT-pro-BNP > 500 pg/mL | 18 (23.3%) | 20 (69.0%) | <0.001 * |
| HbA1c (%) | 6.0 (4.8–10.3) | 6.2 (5.3–10.8) | 0.074 |
| GNRI score | 106.0 (88.1–135.5) | 98.9 (76.4–129.5) | <0.001 * |
| Nutritionally at-risk 2 | 11 (13.8%) | 14 (45.2%) | <0.001 * |
| Complications | |||
| Pneumonia | 5 (6.3%) | 4 (12.9%) | 0.217 |
| Urinary tract infection | 6 (7.5%) | 7 (22.6%) | 0.034 * |
| Cardiovascular | 2 (2.5%) | 3 (9.7%) | 0.132 |
| Clinical course | |||
| Length of hospitalization (days) | 14 (4–67) | 18 (10–51) | <0.001 * |
| Poor prognosis | 20 (25.0%) | 23 (74.2%) | <0.001 * |
| Not Nutritionally At-Risk (n = 86) | Nutritionally At-Risk (n = 25) | p-Value | |
|---|---|---|---|
| Sex (female, %) | 29 (33.7%) | 11 (44.0%) | 0.355 |
| Age (years) | 74 (19–96) | 81 (48–99) | 0.008 * |
| NIHSS score | 1 (0–30) | 3 (1–19) | 0.002 * |
| Body measurements | |||
| Height (cm) | 163 (138.3–180) | 159.3 (135–175) | 0.037 * |
| Body weight (kg) | 63 (42.5–109) | 50 (34–63.9) | <0.001 * |
| BMI (kg/m2) | 24.2 (19.2–37.7) | 20.6 (14.3–24.3) | <0.001 * |
| %ECW/TBW | 38.3 (35.7–40.5) | 39.4 (37.6–41.0) | <0.001 * |
| %ECW/TBW > 0.390 | 17 (19.8%) | 14 (56.0%) | <0.001 * |
| Muscle mass (kg/m2) | 6.9 (3.8–9.8) | 5.6 (3.4–6.9) | <0.001 * |
| Grip strength (kg) | 25 (0–49) | 12 (0–31) | <0.001 * |
| Sarcopenia | 23 (26.7%) | 21 (84.0%) | <0.001 * |
| Days from admission to evaluation 1 | 8 (0–21) | 7 (2–24) | 0.406 |
| Laboratory data | |||
| Albumin (g/dL) | 4.2 (3.4–5.0) | 3.6 (2.7–4.1) | <0.001 * |
| Creatinine (mg/dL) | 0.9 (0.49–2.12) | 0.9 (0.49–10.1) | 0.382 |
| CRP (mg/dL) | 0.1 (0.1–8.2) | 0.3 (0.1–10) | 0.002 * |
| NT-pro-BNP (pg/mL) | 162 (10–3916) | 551 (133–9463) | <0.001 * |
| NT-pro-BNP > 500 pg/mL | 25 (30.1%) | 13 (56.5%) | 0.019 * |
| HbA1c (%) | 6 (4.8–10.3) | 5.8 (5.2–8.6) | 0.935 |
| GNRI score | 106.7 (98.3–136.5) | 93.6 (76.4–97.8) | <0.001 * |
| Complications | |||
| Pneumonia | 6 (7.0%) | 3 (12%) | 0.419 |
| Urinary tract infection | 7 (8.1%) | 6 (24%) | 0.070 |
| Cardiovascular | 3 (3.5%) | 2 (8.0%) | 0.314 |
| Clinical course | |||
| Length of hospitalization (days) | 14 (4–51) | 18 (7–67) | 0.009 * |
| Poor prognosis | 26 (30.2%) | 17 (68.0%) | <0.001 * |
| Non-Sarcopenia (n = 67) | Sarcopenia (n = 44) | p-Value | |
|---|---|---|---|
| Sex (female, %) | 24 (35.8%) | 16 (36.4%) | 0.556 |
| Age (years) | 72 (19–95) | 81 (31–99) | <0.001 * |
| NIHSS score | 1 (0–18) | 3 (0–30) | 0.001 * |
| Body measurements | |||
| Height (cm) | 164 (139–180) | 159 (135–175) | 0.028 * |
| Body weight (kg) | 64 (43–109) | 53 (34–72) | <0.001 * |
| BMI (kg/m2) | 24.2 (19.0–37.7) | 21.7 (14.3–26.6) | <0.001 * |
| %ECW/TBW | 38.1 (35.6–41.0) | 39.2 (36.6–40.8) | <0.001 * |
| %ECW/TBW > 0.390 | 8 (11.9%) | 23 (52.3%) | <0.001 * |
| Muscle mass (kg/m2) | 7.1 (5.1–9.8) | 5.9 (3.4–6.9) | <0.001 * |
| Grip strength (kg) | 29 (0–49) | 16 (0–27) | <0.001 * |
| Days from admission to evaluation 1 | 7 (1–21) | 8 (0–24) | 0.058 |
| Laboratory data | |||
| Albumin (g/dL) | 4.2 (3.4–5) | 3.9 (2.7–5) | <0.001 * |
| Creatinine (mg/dL) | 0.9 (0.5–10.1) | 0.9 (0.5–4.6) | 0.787 |
| CRP (mg/dL) | 0.1 (0.1–3) | 0.2 (0.1–10) | 0.080 |
| NT-pro-BNP (pg/mL) | 159 (10–7099) | 406 (34–9463) | 0.001 * |
| NT-pro-BNP > 500 pg/mL | 19 (29.2%) | 19 (46.3%) | 0.057 |
| HbA1c (%) | 5.9 (4.8–10.3) | 6.2 (5.3–10.8) | 0.323 |
| GNRI score | 107.3 (93.3–135.5) | 98.5 (76.4–124.8) | <0.001 * |
| Nutritionally at-risk 2 | 4 (6.0%) | 21 (47.7%) | <0.001 * |
| Complications | |||
| Pneumonia | 2 (3%) | 7 (15.9%) | 0.019 * |
| Urinary tract infection | 5 (7.5%) | 8 (18.2%) | 0.080 |
| Cardiovascular | 4 (6%) | 1 (2.3%) | 0.338 |
| Clinical course | |||
| Length of hospitalization (days) | 13 (6–51) | 18 (4–67) | 0.001 * |
| Poor prognosis | 15 (22.4%) | 28 (63.6%) | <0.001 * |
| Number of Comorbidities | Comorbidities | Good Outcome (n = 68) | Poor Outcome (n = 43) | p-Value |
|---|---|---|---|---|
| 0 | None | 46 (67.6%) | 10 (23.3%) | <0.001 * |
| 1 | Overhydration | 4 | 3 | |
| Being nutritionally at-risk | 2 | 1 | ||
| Sarcopenia | 9 | 4 | ||
| Total | 15 (22.1%) | 8 (18.6%) | ||
| 2 | Overhydration + being nutritionally at-risk | 0 | 1 | |
| Overhydration + sarcopenia | 1 | 9 | ||
| Being nutritionally at-risk + sarcopenia | 3 | 5 | ||
| Total | 4 (5.9%) | 15 (34.9%) | ||
| 3 | All | 3 (4.4%) | 10 (23.3%) |
| B | Standard Error | p-Value | Exp(B) | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Age | 0.060 | 0.026 | 0.020 * | 1.062 | 1.010–1.117 |
| NIHSS on admission | 0.582 | 0.160 | <0.001 * | 1.790 | 1.307–2.451 |
| Overhydration | 1.705 | 0.594 | 0.004 * | 5.504 | 1.717–17.648 |
| Good Outcome (n = 68) | Poor Outcome (n = 43) | p-Value | |
|---|---|---|---|
| Group A (normal %ECW/TBW and muscle mass) | 39 | 9 | |
| Group B (high %ECW/TBW and normal muscle mass) | 4 | 4 | |
| Group C (normal %ECW/TBW and low muscle mass) | 21 | 11 | |
| Non-Group D | 64 | 24 | p < 0.001 * |
| Group D (high %ECW/TBW and low muscle mass) | 4 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimoto, T.; Tasaki, K.; Ishihara, M.; Hara, M.; Nakajima, H. Association of Body Water Balance, Nutritional Risk, and Sarcopenia with Outcome in Patients with Acute Ischemic Stroke: A Single-Center Prospective Study. Nutrients 2024, 16, 2165. https://doi.org/10.3390/nu16132165
Akimoto T, Tasaki K, Ishihara M, Hara M, Nakajima H. Association of Body Water Balance, Nutritional Risk, and Sarcopenia with Outcome in Patients with Acute Ischemic Stroke: A Single-Center Prospective Study. Nutrients. 2024; 16(13):2165. https://doi.org/10.3390/nu16132165
Chicago/Turabian StyleAkimoto, Takayoshi, Kenta Tasaki, Masaki Ishihara, Makoto Hara, and Hideto Nakajima. 2024. "Association of Body Water Balance, Nutritional Risk, and Sarcopenia with Outcome in Patients with Acute Ischemic Stroke: A Single-Center Prospective Study" Nutrients 16, no. 13: 2165. https://doi.org/10.3390/nu16132165
APA StyleAkimoto, T., Tasaki, K., Ishihara, M., Hara, M., & Nakajima, H. (2024). Association of Body Water Balance, Nutritional Risk, and Sarcopenia with Outcome in Patients with Acute Ischemic Stroke: A Single-Center Prospective Study. Nutrients, 16(13), 2165. https://doi.org/10.3390/nu16132165






