Does Serum Uric Acid Mediate Relation between Healthy Lifestyle and Components of Metabolic Syndrome?
Abstract
1. Introduction
2. Materials and Methods
2.1. Data and Participants
2.2. Components of MetS
2.3. Healthy Lifestyle Score and Weighted HLS
2.4. Biochemical and Physical Examination
2.5. Mediating Conceptual Model and Covariates
2.6. Statistical Analyses
3. Results
3.1. Characteristics of Participants
3.2. Associations of the Components of MetS with HLS and Weighted HLS
3.3. Associations of the Components of MetS with SUA, SUA/Cr, and UHR
3.4. Associations between HLS, Weighted HLS, and SUA, SUA/Cr, and UHR
3.5. The Joint Effect of HLS with SUA, SUA/Cr, and UHR on the Components of MetS
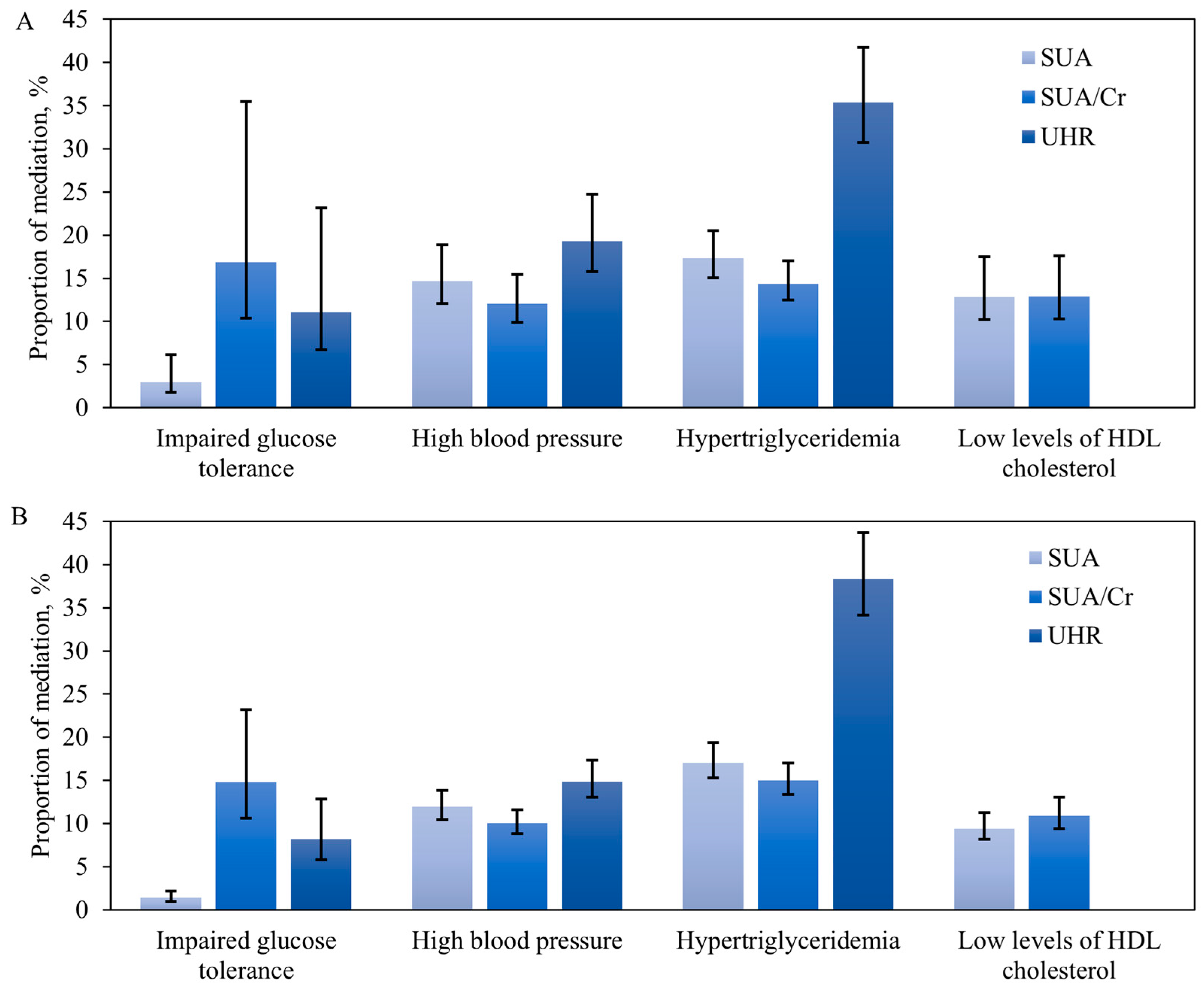
3.6. Role of SUA, SUA/Cr, and UHR Mediating Relationship between Healthy Lifestyle and the Components of MetS
3.7. Subgroup Analyses and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Park, S.; Lee, J.; Seok, J.W.; Park, C.G.; Jun, J. Comprehensive lifestyle modification interventions for metabolic syndrome: A systematic review and meta-analysis. J. Nurs. Scholarsh. 2023, 56, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Pang, D.; Randhawa, G.; Pappas, Y. Risk models and scores for metabolic syndrome: Systematic review protocol. BMJ Open 2019, 9, e027326. [Google Scholar] [CrossRef] [PubMed]
- Galassi, A.; Reynolds, K.; He, J. Metabolic syndrome and risk of cardiovascular disease: A meta-analysis. Am. J. Med. 2006, 119, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cui, Z.; Quan, Z. Effects of Metabolic Syndrome and Its Components on Chronic Kidney Disease and Renal Function: A Two-Sample Mendelian Randomization Study. Metab. Syndr. Relat. Disord. 2023, 22, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Bo, Y.; Zhao, L.; Li, Y.; Ju, L.; Fang, H.; Piao, W.; Yu, D.; Lao, X. Prevalence and Influencing Factors of Metabolic Syndrome among Adults in China from 2015 to 2017. Nutrients 2021, 13, 4475. [Google Scholar] [CrossRef] [PubMed]
- Raya-Cano, E.; Vaquero-Abellán, M.; Molina-Luque, R.; Molina-Recio, G.; Guzmán-García, J.M.; Jiménez-Mérida, R.; Romero-Saldaña, M. Association between Metabolic Syndrome and Leukocytes: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7044. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Ryu, H.K. Association between drinking behaviors and components of metabolic syndrome in subjects in their 20s and 30s: Data obtained from the Korea National Health and Nutrition Examination Survey (2016–2018). Nutr. Res. Pract. 2022, 16, 392–404. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Lee, I. Interactive associations of smoking and physical activity with metabolic syndrome in adult men in Korea. Front. Public Health 2023, 11, 1281530. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Moreno, J.J.; Bodega, P.; de Cos-Gandoy, A.; de Miguel, M.; Santos-Beneit, G.; Fernández-Alvira, J.M.; Fernández-Jiménez, R.; Martínez-Gómez, J.; et al. Metabolic syndrome, adiposity, diet, and emotional eating are associated with oxidative stress in adolescents. Front. Nutr. 2023, 10, 1216445. [Google Scholar] [CrossRef]
- Lee, C.-H.; Han, K.-D.; Kwak, M.-S. Physical activity has a more beneficial effect on the risk of all-cause mortality in patients with metabolic syndrome than in those without. Diabetol. Metab. Syndr. 2023, 15, 255. [Google Scholar] [CrossRef] [PubMed]
- Garralda-Del-Villar, M.; Carlos-Chillerón, S.; Diaz-Gutierrez, J.; Ruiz-Canela, M.; Gea, A.; Martínez-González, M.; Bes-Rastrollo, M.; Ruiz-Estigarribia, L.; Kales, S.; Fernández-Montero, A. Healthy Lifestyle and Incidence of Metabolic Syndrome in the SUN Cohort. Nutrients 2018, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Vajdi, M.; Karimi, A.; Farhangi, M.A.; Ardekani, A.M. The association between healthy lifestyle score and risk of metabolic syndrome in Iranian adults: A cross-sectional study. BMC Endocr. Disord. 2023, 23, 16. [Google Scholar] [CrossRef]
- Sotos-Prieto, M.; Bhupathiraju, S.N.; Falcon, L.M.; Gao, X.; Tucker, K.L.; Mattei, J. Association between a Healthy Lifestyle Score and inflammatory markers among Puerto Rican adults. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Tauler, P.; Nivukoski, U.; Niemelä, M.; Bloigu, A.; Bloigu, R.; Aalto, M.; Laatikainen, T.; Niemelä, O. Impacts of unfavourable lifestyle factors on biomarkers of liver function, inflammation and lipid status. PLoS ONE 2019, 14, e0218463. [Google Scholar] [CrossRef]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef] [PubMed]
- van’t Klooster, C.C.; van der Graaf, Y.; Ridker, P.M.; Westerink, J.; Hjortnaes, J.; Sluijs, I.; Asselbergs, F.W.; Bots, M.L.; Kappelle, L.J.; Visseren, F.L.J. The relation between healthy lifestyle changes and decrease in systemic inflammation in patients with stable cardiovascular disease. Atherosclerosis 2020, 301, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.-K.; Wang, M.-H.; Huang, D.-H.; Chiu, H.-T.; Lee, Y.-J.; Lin, J.-D. The Relationship between Serum Uric Acid Level and Metabolic Syndrome: Differences by Sex and Age in Taiwanese. J. Epidemiol. 2010, 20, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Raya-Cano, E.; Vaquero-Abellán, M.; Molina-Luque, R.; De Pedro-Jiménez, D.; Molina-Recio, G.; Romero-Saldaña, M. Association between metabolic syndrome and uric acid: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 18412. [Google Scholar] [CrossRef]
- Nejatinamini, S.; Ataie-Jafari, A.; Qorbani, M.; Nikoohemat, S.; Kelishadi, R.; Asayesh, H.; Hosseini, S. Association between serum uric acid level and metabolic syndrome components. J. Diabetes Metab. Disord. 2015, 14, 70. [Google Scholar] [CrossRef]
- Kim, Y.; Kang, J. Association of urinary cotinine-verified smoking status with hyperuricemia: Analysis of population-based nationally representative data. Tob. Induc. Dis. 2020, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Rayamajhi, S.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Gu, Y.; Wang, Y.; Zhang, S.; Wang, X.; et al. Dietary patterns and risk for hyperuricemia in the general population: Results from the TCLSIH cohort study. Nutrition 2022, 93, 111501. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, Y.; Zhang, L.; Liu, X.; Tu, R.; Wang, Y.; Li, R.; Li, L.; Hou, J.; Mao, Z.; et al. Independent and interactive effect of sitting time and physical activity on prevalence of hyperuricemia: The Henan Rural Cohort Study. Arthritis Res. Ther. 2021, 23, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, Y.; Shi, Y.; Song, N.; Fang, Y.; Ding, X. Long-term drinking behavior change patterns and its association with hyperuricemia in chinese adults: Evidence from China Health and Nutrition Survey. BMC Public Health 2022, 22, 1230. [Google Scholar] [CrossRef]
- She, D.; Xu, W.; Liu, J.; Zhang, Z.; Fang, P.; Li, R.; Kong, D.; Xuan, M.; Liu, Q.; Pan, M.Y.; et al. Serum Uric Acid to Creatinine Ratio and Risk of Metabolic Syndrome in Patients with Overweight/Obesity. Diabetes Metab. Syndr. Obes. 2023, 16, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Kosekli, M.A.; Kurtkulagii, O.; Kahveci, G.; Duman, T.T.; Tel, B.M.A.; Bilgin, S.; Demirkol, M.E.; Aktas, G. The association between serum uric acid to high density lipoprotein-cholesterol ratio and non-alcoholic fatty liver disease: The abund study. Rev. Assoc. Médica Bras. 2021, 67, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Y.; Shi, G.; Mi, B.; Zeng, L.; Li, Q.; Shen, Y.; Zhao, Y.; Pei, L.; Kang, Y.; et al. Cohort Profile: Regional Ethnic Cohort Study in Northwest China. Int. J. Epidemiol. 2022, 51, e18–e26. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Verde, L.; Simancas-Racines, D.; Zambrano, A.K.; Frias-Toral, E.; Colao, A.; Savastano, S.; Muscogiuri, G. Adherence to the Mediterranean diet as a possible additional tool to be used for screening the metabolically unhealthy obesity (MUO) phenotype. J. Transl. Med. 2023, 21, 675. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Shao, W.; Yu, S.; Yu, J.; Huang, C.; Ren, H.; Liu, C.; Xu, Y.; Zhu, Y. Healthy lifestyle scores associate with incidence of type 2 diabetes mediated by uric acid. Nutr. Metab. 2023, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Sun, D.; Zhou, T.; Heianza, Y.; Lv, J.; Li, L.; Qi, L. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: A prospective study of 385 292 UK biobank participants. Eur. Heart J. 2020, 41, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Chen, J.; Hou, R.; Sun, Y.; Xiao, Q.; Pan, X.; Zhu, X. Emerging healthy lifestyle factors and all-cause mortality among people with metabolic syndrome and metabolic syndrome-like characteristics in NHANES. J. Transl. Med. 2023, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Hackett, R.A.; Vo, T.T.; Vansteelandt, S.; Davies-Kershaw, H. The role of loneliness on hearing ability and dementia: A novel mediation approach. J. Am. Geriatr. Soc. 2023, 71, 2834–2844. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Liu, J.; Xu, Y. Effects of Uric Acid on Diabetes Mellitus and Its Chronic Complications. Int. J. Endocrinol. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Shahin, L.; Patel, K.M.; Heydari, M.K.; Kesselman, M.M. Hyperuricemia and Cardiovascular Risk. Cureus 2021, 13, e14855. [Google Scholar] [CrossRef] [PubMed]
- Shimosawa, T.; Viazzi, F.; Piscitelli, P.; Giorda, C.; Ceriello, A.; Genovese, S.; Russo, G.; Guida, P.; Fioretto, P.; De Cosmo, S.; et al. Metabolic syndrome, serum uric acid and renal risk in patients with T2D. PLoS ONE 2017, 12, e0176058. [Google Scholar] [CrossRef]
- Xu, A.; Yan, D.; Tu, Y.; Jiang, F.; Wang, J.; Zhang, R.; Sun, X.; Wang, T.; Wang, S.; Bao, Y.; et al. Uric Acid Is Independently Associated with Diabetic Kidney Disease: A Cross-Sectional Study in a Chinese Population. PLoS ONE 2015, 10, e0129797. [Google Scholar] [CrossRef]
- Yu, X.; Sun, F.; Ming, J.; Liang, S.; Zhang, W.; Wang, L.; Li, Q.; Xu, Q.; Wang, L.; Shi, L.; et al. Serum uric acid to high-density lipoprotein cholesterol ratio is a promising marker for identifying metabolic syndrome in nondiabetic Chinese men. Postgrad. Med. 2023, 135, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Jensen, T.; Solak, Y.; Le, M.; Roncal-Jimenez, C.; Rivard, C.; Lanaspa, M.A.; Nakagawa, T.; Johnson, R.J. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur. J. Intern. Med. 2016, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Copur, S.; Demiray, A.; Kanbay, M. Uric acid in metabolic syndrome: Does uric acid have a definitive role? Eur. J. Intern. Med. 2022, 103, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lu, J.; Cao, X.; Shi, T.T.; Feng, J.P.; Yang, J.K. An Increase in Normal SUA Level Within the Normal Range Predicts Risk of Metabolic Syndrome, Especially in Women: A Cross-Sectional Study. Horm. Metab. Res. 2017, 49, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Chen, F.; Ma, G.; Wang, D. Measurement of the Combined Levels of Serum Uric Acid and Alanine Aminotransferase and the Risk of Metabolic Syndrome in a Population Aged 60 Years or More in Northeastern China. Med. Sci. Monit. 2020, 26, e916459-1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Mao, Z.; Liu, X.; Zhang, X.; Yang, K.; Liu, R.; Qian, X.; Zhang, H.; Jiang, J.; et al. Sex-specific associations of serum uric acid with metabolic syndrome in Chinese rural population: The RuralDiab study. Clin. Chim. Acta 2018, 480, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Hayashi, T.; Tsumura, K.; Endo, G.; Fujii, S.; Okada, K. Serum uric acid and the risk for hypertension and Type 2 diabetes in Japanese men: The Osaka Health Survey. J. Hypertens. 2001, 19, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, P.; Shankar, A. Association between Serum Uric Acid Levels and Diabetes Mellitus. Int. J. Endocrinol. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Alberti, K.G. Physical activity, metabolic factors, and the incidence of coronary heart disease and type 2 diabetes. Arch. Intern. Med. 2000, 160, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Karim, M.N.; Hébert, J.R.; Shivappa, N.; de Courten, B. Association between Diet Quality Indices and Incidence of Type 2 Diabetes in the Melbourne Collaborative Cohort Study. Nutrients 2021, 13, 4162. [Google Scholar] [CrossRef]
- Wei, S.; Brejnrod, A.D.; Trivedi, U.; Mortensen, M.S.; Johansen, M.Y.; Karstoft, K.; Vaag, A.A.; Ried-Larsen, M.; Sørensen, S.J. Impact of intensive lifestyle intervention on gut microbiota composition in type 2 diabetes: A post-hoc analysis of a randomized clinical trial. Gut Microbes 2021, 14, 2005407. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Sun, S.; Huang, Y.; Gao, Q.; Xie, X.; Wang, P.; Li, J.; Liang, L.; He, X.; Jiang, Y.; et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. npj Biofilms Microbiomes 2021, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I.; Daimon, T. Associations of Blood Urate Level with Glycemic Status and Other Cardiometabolic Risk Factors in Middle-Aged Women. Women’s Health Rep. 2021, 2, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I. Inverse associations between serum urate and glycemic status in a general population and in persons with diabetes mellitus. Diabetol. Metab. Syndr. 2020, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Dohi, O.; Yoshida, N.; Kamada, K.; et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J. Gastroenterol. 2018, 54, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, T.; Li, Y.; Tang, Y.; Huang, Z. Gut microbiota are associated with sex and age of host: Evidence from semi-provisioned rhesus macaques in southwest Guangxi, China. Ecol. Evol. 2021, 11, 8096–8122. [Google Scholar] [CrossRef] [PubMed]
- Poursafa, P.; Kamali, Z.; Fraszczyk, E.; Boezen, H.M.; Vaez, A.; Snieder, H. DNA methylation: A potential mediator between air pollution and metabolic syndrome. Clin. Epigenetics 2022, 14, 82. [Google Scholar] [CrossRef] [PubMed]
| Impaired Glucose Tolerance | High Blood Pressure | Hypertriglyceridemia | Low Levels of HDL Cholesterol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | OR (95%CI) | p | n (%) | OR (95%CI) | p | n (%) | OR (95%CI) | p | n (%) | OR (95%CI) | p | |
| HLS | ||||||||||||
| 0~1 | 100 (11.0) | Reference | 444 (48.7) | Reference | 471 (51.6) | Reference | 281 (30.8) | Reference | ||||
| 2 | 176 (10.8) | 0.929 (0.705~1.225) | 0.602 | 637 (39.0) | 0.704 (0.590~0.840) | <0.001 | 642 (39.3) | 0.702 (0.592~0.831) | <0.001 | 538 (32.9) | 0.890 (0.744~1.065) | 0.205 |
| 3 | 191 (7.7) | 0.605 (0.453~0.807) | 0.001 | 802 (32.3) | 0.579 (0.484~0.692) | <0.001 | 645 (26.0) | 0.453 (0.381~0.538) | <0.001 | 808 (32.6) | 0.661 (0.551~0.792) | <0.001 |
| 4~5 | 131 (5.8) | 0.564 (0.408~0.778) | <0.001 | 477 (21.1) | 0.392 (0.321~0.478) | <0.001 | 377 (16.7) | 0.306 (0.252~0.372) | <0.001 | 715 (31.6) | 0.527 (0.434~0.641) | <0.001 |
| Ptrend | <0.001 | Ptrend | <0.001 | Ptrend | <0.001 | Ptrend | <0.001 | |||||
| Weighted HLS | ||||||||||||
| Q1 | 281 (12.6) | Reference | 883 (44.9) | Reference | 1021 (47.4) | Reference | 611 (37.0) | Reference | ||||
| Q2 | 138 (9.3) | 0.807 (0.642~1.015) | 0.067 | 672 (43.6) | 0.884 (0.761~1.028) | 0.110 | 518 (34.2) | 0.704 (0.608~0.815) | <0.001 | 743 (36.3) | 0.774 (0.672~0.891) | <0.001 |
| Q3 | 108 (5.2) | 0.494 (0.386~0.633) | <0.001 | 440 (23.0) | 0.443 (0.380~0.516) | <0.001 | 319 (19.0) | 0.326 (0.279~0.380) | <0.001 | 321 (25.1) | 0.423 (0.357~0.502) | <0.001 |
| Q4 | 71 (4.8) | 0.481 (0.361~0.641) | <0.001 | 365 (19.6) | 0.337 (0.285~0.398) | <0.001 | 277 (14.3) | 0.289 (0.243~0.343) | <0.001 | 667 (28.8) | 0.430 (0.369~0.501) | <0.001 |
| Ptrend | <0.001 | Ptrend | <0.001 | Ptrend | <0.001 | Ptrend | <0.001 | |||||
| n | Impaired Glucose Tolerance | High Blood Pressure | Hypertriglyceridemia | Low Levels of HDL Cholesterol | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | ||
| SUA | |||||||||
| Continuous variable | 1.001 (0.999~1.002) | 0.270 | 1.004 (1.004~1.005) | <0.001 | 1.007 (1.007~1.008) | <0.001 | 1.003 (1.002~1.004) | <0.001 | |
| Q1 | 1736 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1783 | 1.406 (1.039~1.904) | 0.027 | 1.284 (1.071~1.541) | 0.007 | 2.024 (1.658~2.471) | <0.001 | 1.203 (1.039~1.393) | 0.013 |
| Q3 | 1828 | 1.443 (1.061~1.963) | 0.019 | 1.789 (1.483~2.158) | <0.001 | 3.203 (2.616~3.923) | <0.001 | 1.547 (1.316~1.819) | <0.001 |
| Q4 | 1940 | 1.360 (0.988~1.871) | 0.059 | 2.594 (2.129~3.161) | <0.001 | 6.060 (4.908~7.482) | <0.001 | 2.048 (1.713~2.448) | <0.001 |
| SUA/Cr | |||||||||
| Continuous variable | 1.284 (1.199~1.375) | <0.001 | 1.278 (1.221~1.337) | <0.001 | 1.496 (1.429~1.567) | <0.001 | 1.223 (1.174~1.275) | <0.001 | |
| Q1 | 1794 | Reference | Reference | Reference | Reference | ||||
| Q2 | 1832 | 1.060 (0.808~1.390) | 0.674 | 1.134 (0.962~1.337) | 0.133 | 1.444 (1.216~1.715) | <0.001 | 1.217 (1.049~1.410) | 0.009 |
| Q3 | 1827 | 1.110 (0.848~1.453) | 0.446 | 1.553 (1.322~1.824) | <0.001 | 2.313 (1.961~2.729) | <0.001 | 1.398 (1.208~1.619) | <0.001 |
| Q4 | 1834 | 1.984 (1.546~2.546) | <0.001 | 2.101 (1.792~2.464) | <0.001 | 3.628 (3.082~4.270) | <0.001 | 1.883 (1.630~2.175) | <0.001 |
| UHR | |||||||||
| Continuous variable | 1.035 (1.015~1.056) | 0.001 | 1.086 (1.072~1.101) | <0.001 | 1.262 (1.241~1.283) | <0.001 | — | — | |
| Q1 | 1701 | Reference | Reference | Reference | — | ||||
| Q2 | 1854 | 1.451 (1.052~2.001) | 0.023 | 1.485 (1.234~1.788) | <0.001 | 3.749 (2.923~4.809) | <0.001 | — | — |
| Q3 | 1839 | 1.904 (1.380~2.626) | <0.001 | 2.125 (1.751~2.579) | <0.001 | 8.668 (6.739~11.148) | <0.001 | — | — |
| Q4 | 1893 | 1.850 (1.317~2.598) | <0.001 | 3.123 (2.542~3.838) | <0.001 | 25.137 (19.317~32.711) | <0.001 | — | — |
| HLS | n | Impaired Glucose Tolerance | High Blood Pressure | Hypertriglyceridemia | Low Levels of HDL Cholesterol | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (99%CI) | p | OR (99%CI) | p | OR (99%CI) | p | OR (99%CI) | p | |||
| SUA | ||||||||||
| Higher | Lower | 1072 | Reference | Reference | Reference | Reference | ||||
| Higher | Higher | 747 | 0.672 (0.426~1.061) | 0.025 | 0.670 (0.511~0.879) | <0.001 | 0.581 (0.449~0.752) | <0.001 | 0.722 (0.549~0.948) | 0.002 |
| Lower | Lower | 1473 | 1.127 (0.787~1.612) | 0.391 | 0.545 (0.433~0.686) | <0.001 | 0.412 (0.330~0.514) | <0.001 | 0.688 (0.546~0.866) | <0.001 |
| Lower | Higher | 3995 | 0.669 (0.461~0.970) | 0.005 | 0.367 (0.291~0.463) | <0.001 | 0.210 (0.167~0.264) | <0.001 | 0.455 (0.360~0.574) | <0.001 |
| SUA/SCr | ||||||||||
| Higher | Lower | 752 | Reference | Reference | Reference | Reference | ||||
| Higher | Higher | 1069 | 0.698 (0.456~1.068) | 0.029 | 0.594 (0.446~0.790) | <0.001 | 0.562 (0.428~0.738) | <0.001 | 0.733 (0.560~0.959) | 0.003 |
| Lower | Lower | 1793 | 0.562 (0.391~0.808) | <0.001 | 0.522 (0.410~0.666) | <0.001 | 0.441 (0.349~0.558) | <0.001 | 0.672 (0.529~0.853) | <0.001 |
| Lower | Higher | 3673 | 0.355 (0.246~0.512) | <0.001 | 0.362 (0.284~0.461) | <0.001 | 0.226 (0.179~0.286) | <0.001 | 0.444 (0.351~0.561) | <0.001 |
| UHR | ||||||||||
| Higher | Lower | 1094 | Reference | Reference | Reference | — | ||||
| Higher | Higher | 727 | 0.790 (0.513~1.218) | 0.161 | 0.664 (0.506~0.871) | <0.001 | 0.623 (0.481~0.807) | <0.001 | — | — |
| Lower | Lower | 1451 | 1.034 (0.725~1.475) | 0.808 | 0.550 (0.437~0.691) | <0.001 | 0.236 (0.188~0.297) | <0.001 | — | — |
| Lower | Higher | 4015 | 0.575 (0.396~0.835) | <0.001 | 0.374 (0.297~0.472) | <0.001 | 0.121 (0.096~0.154) | <0.001 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Jing, H.; Wang, Z.; Li, Z.; Chacha, S.; Teng, Y.; Mi, B.; Zhang, B.; Liu, Y.; Li, Q.; et al. Does Serum Uric Acid Mediate Relation between Healthy Lifestyle and Components of Metabolic Syndrome? Nutrients 2024, 16, 2137. https://doi.org/10.3390/nu16132137
Huang Y, Jing H, Wang Z, Li Z, Chacha S, Teng Y, Mi B, Zhang B, Liu Y, Li Q, et al. Does Serum Uric Acid Mediate Relation between Healthy Lifestyle and Components of Metabolic Syndrome? Nutrients. 2024; 16(13):2137. https://doi.org/10.3390/nu16132137
Chicago/Turabian StyleHuang, Yan, Hui Jing, Ziping Wang, Zongkai Li, Samuel Chacha, Yuxin Teng, Baibing Mi, Binyan Zhang, Yezhou Liu, Qiang Li, and et al. 2024. "Does Serum Uric Acid Mediate Relation between Healthy Lifestyle and Components of Metabolic Syndrome?" Nutrients 16, no. 13: 2137. https://doi.org/10.3390/nu16132137
APA StyleHuang, Y., Jing, H., Wang, Z., Li, Z., Chacha, S., Teng, Y., Mi, B., Zhang, B., Liu, Y., Li, Q., Shen, Y., Yang, J., Qu, Y., Wang, D., Yan, H., & Dang, S. (2024). Does Serum Uric Acid Mediate Relation between Healthy Lifestyle and Components of Metabolic Syndrome? Nutrients, 16(13), 2137. https://doi.org/10.3390/nu16132137







