Longitudinal Three-Year Associations of Dietary Fruit and Vegetable Intake with Serum hs-C-Reactive Protein in Adults with and without Type 1 Diabetes
Abstract
1. Introduction
2. Methods
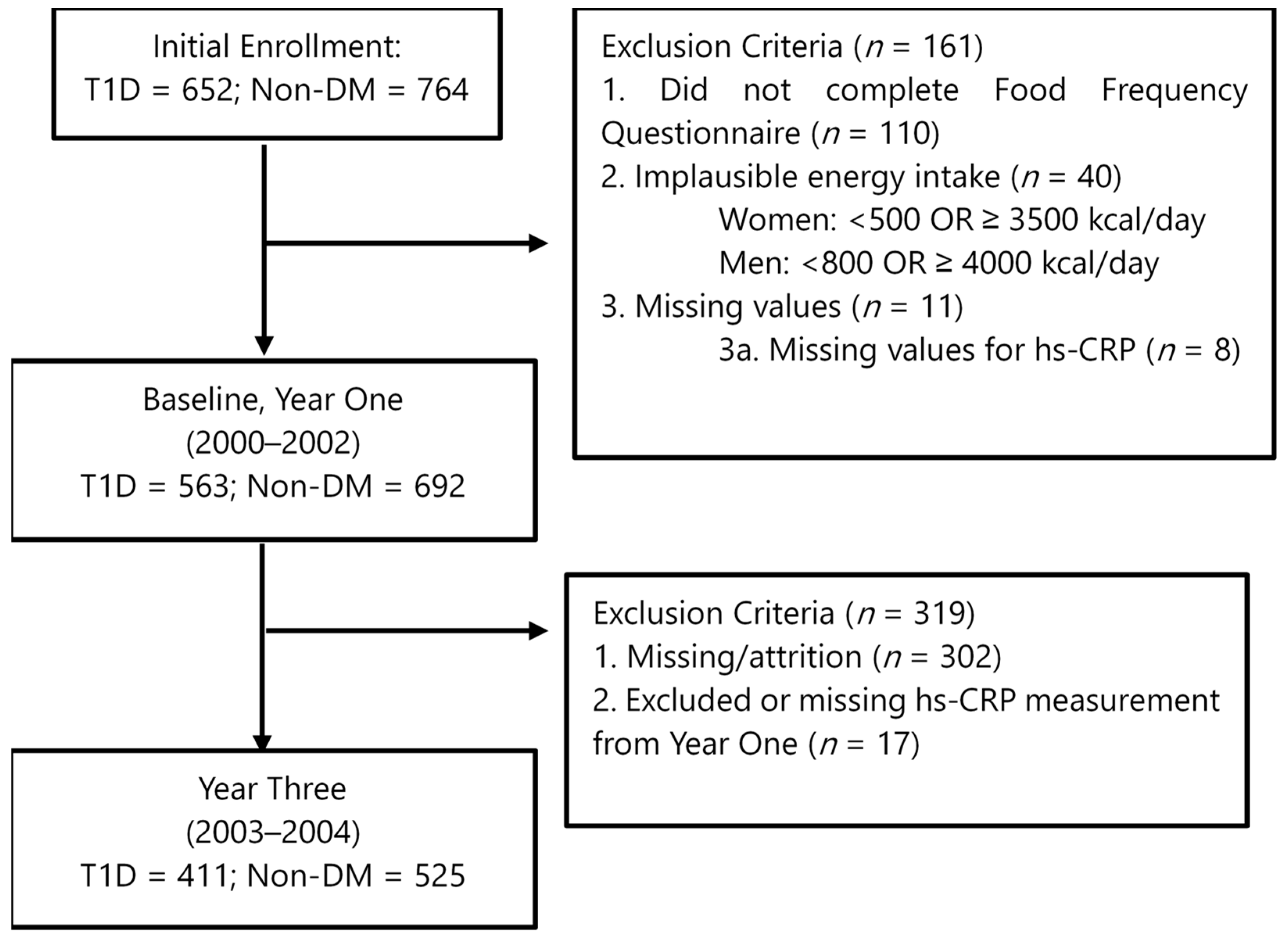
2.1. Participants
2.2. Dietary Intakes and Pattern Scores
2.3. Study Measurements
2.4. Measurement of hs-CRP
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 29 October 2023).
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- GBD 2019 Chronic Respiratory Diseases Collaborators. Global Burden of Chronic Respiratory Diseases and Risk Factors, 1990–2019: An Update from the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 59, 101936. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Diabetes Collaborators. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, J.; Cai, W.; Chen, C.; Ma, J.; Xie, Z.; Dong, Y.; Liu, C.; Xue, R.; Zhao, J. Meta-Analysis of Type 1 Diabetes Mellitus and Risk of Cardiovascular Disease. J. Diabetes Complicat. 2021, 35, 107833. [Google Scholar] [CrossRef] [PubMed]
- Cano-Cano, F.; Gómez-Jaramillo, L.; Ramos-García, P.; Arroba, A.I.; Aguilar-Diosdado, M. IL-1β Implications in Type 1 Diabetes Mellitus Progression: Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Qiao, Y.-C.; Pan, Y.-H.; Xu, Y.; Huang, Y.-C.; Wang, Y.-H.; Geng, L.-J.; Zhao, H.-L.; Zhang, X.-X. Correlation between Serum Interleukin-6 Level and Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cytokine 2017, 94, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Chen, Y.; Pan, Y.; Tian, F.; Xu, Y.; Zhang, X.; Zhao, H. The Change of Serum Tumor Necrosis Factor Alpha in Patients with Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0176157. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The Role of Inflammation in Insulitis and Beta-Cell Loss in Type 1 Diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Pirot, P.; Eizirik, D.L.; Cardozo, A.K. Interferon-Gamma Potentiates Endoplasmic Reticulum Stress-Induced Death by Reducing Pancreatic Beta Cell Defence Mechanisms. Diabetologia 2006, 49, 1229–1236. [Google Scholar] [CrossRef]
- Navarro, J.F.; Mora, C. Role of Inflammation in Diabetic Complications. Nephrol. Dial. Transplant. 2005, 20, 2601–2604. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Poland, D.C.; van Dijk, W.; Kok, A.; Emeis, J.J.; Dräger, A.M.; Doni, A.; van Hinsbergh, V.W.; Stehouwer, C.D. Plasma Concentration of C-Reactive Protein Is Increased in Type I Diabetic Patients without Clinical Macroangiopathy and Correlates with Markers of Endothelial Dysfunction: Evidence for Chronic Inflammation. Diabetologia 1999, 42, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Akinboboye, O.; Williams, J.S.; Garacci, E.; Egede, L.E. The Relationship between C-Reactive Protein and Mortality in Adults with Diabetes: Influences of Demographic Characteristics, Lifestyle Behaviors, and Medications. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Tian, M.; Wang, L.; Qian, H.; Zhang, S.; Pang, H.; Liu, Z.; Fang, L.; Shen, Z. C-Reactive Protein for Predicting Cardiovascular and All-Cause Mortality in Type 2 Diabetic Patients: A Meta-Analysis. Cytokine 2019, 117, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cabrera, J.L.; Ankeny, J.; Fernández-Montero, A.; Kales, S.N.; Smith, D.L. A Systematic Review and Meta-Analysis of Advanced Biomarkers for Predicting Incident Cardiovascular Disease among Asymptomatic Middle-Aged Adults. Int. J. Mol. Sci. 2022, 23, 13540. [Google Scholar] [CrossRef]
- Best, L.G.; Zhang, Y.; Lee, E.T.; Yeh, J.-L.; Cowan, L.; Palmieri, V.; Roman, M.; Devereux, R.B.; Fabsitz, R.R.; Tracy, R.P.; et al. C-Reactive Protein as a Predictor of Cardiovascular Risk in a Population With a High Prevalence of Diabetes. Circulation 2005, 112, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, M.; Samols, D.; Kushner, I. STAT3 Participates in Transcriptional Activation of the C-Reactive Protein Gene by Interleukin-6 (∗). J. Biol. Chem. 1996, 271, 9503–9509. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Júnior, R.F.; de Souza, K.S.C.; Galdino, O.A.; da Silva Junior, A.A.; Arrais, R.F.; Machado, P.R.L.; Farias, K.J.S.; de Rezende, A.A. Chloroquine as a Promising Adjuvant Therapy for Type 1 Diabetes Mellitus. Sci. Rep. 2020, 10, 12098. [Google Scholar] [CrossRef] [PubMed]
- Pollack, R.M.; Donath, M.Y.; LeRoith, D.; Leibowitz, G. Anti-Inflammatory Agents in the Treatment of Diabetes and Its Vascular Complications. Diabetes Care 2016, 39, S244–S252. [Google Scholar] [CrossRef]
- Maithili Karpaga Selvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R. Efficacy of Turmeric as Adjuvant Therapy in Type 2 Diabetic Patients. Indian. J. Clin. Biochem. 2015, 30, 180–186. [Google Scholar] [CrossRef]
- Moslemi, E.; Musazadeh, V.; Kavyani, Z.; Naghsh, N.; Shoura, S.M.S.; Dehghan, P. Efficacy of Vitamin D Supplementation as an Adjunct Therapy for Improving Inflammatory and Oxidative Stress Biomarkers: An Umbrella Meta-Analysis. Pharmacol. Res. 2022, 186, 106484. [Google Scholar] [CrossRef]
- Zwickey, H.; Horgan, A.; Hanes, D.; Schiffke, H.; Moore, A.; Wahbeh, H.; Jordan, J.; Ojeda, L.; McMurry, M.; Elmer, P.; et al. Effect of the Anti-Inflammatory Diet in People with Diabetes and Pre-Diabetes: A Randomized Controlled Feeding Study. J. Restor. Med. 2019, 8, e20190107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piccand, E.; Vollenweider, P.; Guessous, I.; Marques-Vidal, P. Association between Dietary Intake and Inflammatory Markers: Results from the CoLaus Study. Public Health Nutr. 2019, 22, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Bermudez, O.I.; Tucker, K.L. Plasma C-Reactive Protein and Homocysteine Concentrations Are Related to Frequent Fruit and Vegetable Intake in Hispanic and Non-Hispanic White Elders. J. Nutr. 2004, 134, 913–918. [Google Scholar] [CrossRef]
- Oliveira, A.; Rodríguez-Artalejo, F.; Lopes, C. The Association of Fruits, Vegetables, Antioxidant Vitamins and Fibre Intake with High-Sensitivity C-Reactive Protein: Sex and Body Mass Index Interactions. Eur. J. Clin. Nutr. 2009, 63, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Nanri, H.; Nakamura, K.; Hara, M.; Higaki, Y.; Imaizumi, T.; Taguchi, N.; Sakamoto, T.; Horita, M.; Shinchi, K.; Tanaka, K. Association between Dietary Pattern and Serum C-Reactive Protein in Japanese Men and Women. J. Epidemiol. 2011, 21, 122–131. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Bellissimo, N.; Totosy de Zepetnek, J.O.; Rouhani, M.H. Association of Vegetarian Diet with Inflammatory Biomarkers: A Systematic Review and Meta-Analysis of Observational Studies. Public Health Nutr. 2017, 20, 2713–2721. [Google Scholar] [CrossRef]
- Jacobs, S.; Boushey, C.J.; Franke, A.A.; Shvetsov, Y.B.; Monroe, K.R.; Haiman, C.A.; Kolonel, L.N.; Le Marchand, L.; Maskarinec, G. A Priori-Defined Diet Quality Indices, Biomarkers and Risk for Type 2 Diabetes in Five Ethnic Groups: The Multiethnic Cohort. Br. J. Nutr. 2017, 118, 312–320. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Tucker, K.L. Greater Variety in Fruit and Vegetable Intake Is Associated with Lower Inflammation in Puerto Rican Adults. Am. J. Clin. Nutr. 2011, 93, 37–46. [Google Scholar] [CrossRef]
- Julia, C.; Meunier, N.; Touvier, M.; Ahluwalia, N.; Sapin, V.; Papet, I.; Cano, N.; Hercberg, S.; Galan, P.; Kesse-Guyot, E. Dietary Patterns and Risk of Elevated C-Reactive Protein Concentrations 12 Years Later. Br. J. Nutr. 2013, 110, 747–754. [Google Scholar] [CrossRef]
- Nouri, F.; Sadeghi, M.; Mohammadifard, N.; Roohafza, H.; Feizi, A.; Sarrafzadegan, N. Longitudinal Association between an Overall Diet Quality Index and Latent Profiles of Cardiovascular Risk Factors: Results from a Population Based 13-Year Follow up Cohort Study. Nutr. Metab. 2021, 18, 28. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.B.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Inflammatory Biomarkers and Immune Cell Populations: A Systematic Literature Review and Meta-Analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Sánchez-Rosales, A.I.; Guadarrama-López, A.L.; Gaona-Valle, L.S.; Martínez-Carrillo, B.E.; Valdés-Ramos, R. The Effect of Dietary Patterns on Inflammatory Biomarkers in Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 4577. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Egea Rodrigues, C.; Aleksandrova, K. Effects of Dietary Patterns on Biomarkers of Inflammation and Immune Responses: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 101–115. [Google Scholar] [CrossRef]
- Neale, E.P.; Batterham, M.J.; Tapsell, L.C. Consumption of a Healthy Dietary Pattern Results in Significant Reductions in C-Reactive Protein Levels in Adults: A Meta-Analysis. Nutr. Res. 2016, 36, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Sarkhosh-Khorasani, S.; Hosseinzadeh, M. The Effect of Grape Products Containing Polyphenols on C-Reactive Protein Levels: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2021, 125, 1230–1245. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Pereira, L. Association between Berries Intake and Cardiovascular Diseases Risk Factors: A Systematic Review with Meta-Analysis and Trial Sequential Analysis of Randomized Controlled Trials. Food Funct. 2018, 9, 740–757. [Google Scholar] [CrossRef]
- Schell, J.; Betts, N.M.; Lyons, T.J.; Basu, A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann. Nutr. Metab. 2019, 74, 165–174. [Google Scholar] [CrossRef]
- Soltani, S.; Chitsazi, M.J.; Salehi-Abargouei, A. The Effect of Dietary Approaches to Stop Hypertension (DASH) on Serum Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Trials. Clin. Nutr. 2018, 37, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary Patterns and Associations with Biomarkers of Inflammation in Adults: A Systematic Review of Observational Studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef]
- Keys, A.; Mienotti, A.; Karvonen, M.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.; Dontas, A.; Fidanza, F.; Keys, M.; et al. The Diet and 15-Year Death Rate in the Seven Countries Study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- George, S.M.; Ballard-Barbash, R.; Manson, J.E.; Reedy, J.; Shikany, J.M.; Subar, A.F.; Tinker, L.F.; Vitolins, M.; Neuhouser, M.L. Comparing Indices of Diet Quality With Chronic Disease Mortality Risk in Postmenopausal Women in the Women’s Health Initiative Observational Study: Evidence to Inform National Dietary Guidance. Am. J. Epidemiol. 2014, 180, 616–625. [Google Scholar] [CrossRef] [PubMed]
- van Bussel, B.C.T.; Soedamah-Muthu, S.S.; Henry, R.M.A.; Schalkwijk, C.G.; Ferreira, I.; Chaturvedi, N.; Toeller, M.; Fuller, J.H.; Stehouwer, C.D.A. EURODIAB Prospective Complications Study Group Unhealthy Dietary Patterns Associated with Inflammation and Endothelial Dysfunction in Type 1 Diabetes: The EURODIAB Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Saraheimo, M.; Freese, R.; Forsblom, C.; Mäkimattila, S.; Groop, P.-H. Association between Adherence to Dietary Recommendations and High-Sensitivity C-Reactive Protein Level in Type 1 Diabetes. Diabetes Res. Clin. Pract. 2017, 126, 122–128. [Google Scholar] [CrossRef][Green Version]
- Ebrahimi, Z.; Shojaeian, Z.; Amiri, F.; Esmaillzadeh, A.; Sadeghi, O.; Esteghamati, A.; Jahed, S.A.; Sedaghat, S. Association of Major Dietary Patterns with Advanced Glycation End Products and High-Sensitivity C-Reactive Protein in People with Type 1 Diabetes Mellitus. Nutr. J. 2023, 22, 37. [Google Scholar] [CrossRef]
- Dabelea, D.; Kinney, G.; Snell-Bergeon, J.K.; Hokanson, J.E.; Eckel, R.H.; Ehrlich, J.; Garg, S.; Hamman, R.F.; Rewers, M. Effect of Type 1 Diabetes on the Gender Difference in Coronary Artery Calcification: A Role for Insulin Resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 2003, 52, 2833–2839. [Google Scholar] [CrossRef]
- Basu, A.; Alman, A.C.; Snell-Bergeon, J.K. Dietary Fiber Intake and Glycemic Control: Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Nutr. J. 2019, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sampson, L.; Browne, M.L.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Speizer, F.E. The Use of a Self-Administered Questionnaire to Assess Diet Four Years in the Past. Am. J. Epidemiol. 1988, 127, 188–199. [Google Scholar] [CrossRef]
- Willett, W.C.; Sampson, L.; Stampfer, M.J.; Rosner, B.; Bain, C.; Witschi, J.; Hennekens, C.H.; Speizer, F.E. Reproducibility and Validity of a Semiquantitative Food Frequency Questionnaire. Am. J. Epidemiol. 1985, 122, 51–65. [Google Scholar] [CrossRef]
- Hu, F.B.; Rimm, E.; Smith-Warner, S.A.; Feskanich, D.; Stampfer, M.J.; Ascherio, A.; Sampson, L.; Willett, W.C. Reproducibility and Validity of Dietary Patterns Assessed with a Food-Frequency Questionnaire. Am. J. Clin. Nutr. 1999, 69, 243–249. [Google Scholar] [CrossRef]
- Wirfält, A.K.; Jeffery, R.W.; Elmer, P.J. Comparison of Food Frequency Questionnaires: The Reduced Block and Willett Questionnaires Differ in Ranking on Nutrient Intakes. Am. J. Epidemiol. 1998, 148, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Rumawas, M.E.; Dwyer, J.T.; Mckeown, N.M.; Meigs, J.B.; Rogers, G.; Jacques, P.F. The Development of the Mediterranean-Style Dietary Pattern Score and Its Application to the American Diet in the Framingham Offspring Cohort. J. Nutr. 2009, 139, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Chiuve, S.E.; McCullough, M.L.; Rexrode, K.M.; Logroscino, G.; Hu, F.B. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch. Intern. Med. 2008, 168, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Pyle, L.; Kinney, G.L.; Rewers, M.; Johnson, R.J.; Maahs, D.M.; Snell-Bergeon, J.K. Adiponectin Is Associated with Early Diabetic Kidney Disease in Adults with Type 1 Diabetes: A Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. J. Diabetes Complicat. 2017, 31, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. C-Reactive Protein, Inflammation, and Cardiovascular Disease: Clinical Update. Tex. Heart Inst. J. 2005, 32, 384–386. [Google Scholar] [PubMed]
- Falsey, A.R.; Walsh, E.E.; Francis, C.W.; Looney, R.J.; Kolassa, J.E.; Hall, W.J.; Abraham, G.N. Response of C-Reactive Protein and Serum Amyloid A to Influenza A Infection in Older Adults. J. Infect. Dis. 2001, 183, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.R.; Navarro, P.; Harrington, J.M.; Shivappa, N.; Hébert, J.R.; Perry, I.J.; Phillips, C.M. Dietary Score Associations with Markers of Chronic Low-Grade Inflammation: A Cross-Sectional Comparative Analysis of a Middle- to Older-Aged Population. Eur. J. Nutr. 2022, 61, 3377–3390. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Harrington, J.M.; Perry, I.J. Relationship between Dietary Quality, Determined by DASH Score, and Cardiometabolic Health Biomarkers: A Cross-Sectional Analysis in Adults. Clin. Nutr. 2019, 38, 1620–1628. [Google Scholar] [CrossRef]
- Kearney, P.M.; Harrington, J.M.; Mc Carthy, V.J.; Fitzgerald, A.P.; Perry, I.J. Cohort Profile: The Cork and Kerry Diabetes and Heart Disease Study. Int. J. Epidemiol. 2013, 42, 1253–1262. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Peter, P.; Davis, C.R.; Crowell, J.A.; Mantzoros, C.S. Diet Quality Is Associated with Circulating C-Reactive Protein but Not Irisin Levels in Humans. Metabolism 2014, 63, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Chung, S.-J.; Claycombe, K.J.; Song, W.O. Serum C-Reactive Protein Concentrations Are Inversely Associated with Dietary Flavonoid Intake in U.S. Adults. J. Nutr. 2008, 138, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Xu, B. Anti-Inflammatory Effects of Phytochemicals from Fruits, Vegetables, and Food Legumes: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.L.; Moreau, R. Functional Properties of Spinach (Spinacia Oleracea L.) Phytochemicals and Bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Jung, J.I.; Cho, H.J.; Choi, M.-S.; Sung, M.-K.; Yu, R.; Kang, Y.-H.; Park, J.H.Y. Berteroin Present in Cruciferous Vegetables Exerts Potent Anti-Inflammatory Properties in Murine Macrophages and Mouse Skin. Int. J. Mol. Sci. 2014, 15, 20686–20705. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Lutein Decreases Oxidative Stress and Inflammation in Liver and Eyes of Guinea Pigs Fed a Hypercholesterolemic Diet. Nutr. Res. Pract. 2012, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Hadad, N.; Levy, R. The Synergistic Anti-Inflammatory Effects of Lycopene, Lutein, β-Carotene, and Carnosic Acid Combinations via Redox-Based Inhibition of NF-κB Signaling. Free Radic. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of Naturally Occurring Flavonoids on Nitric Oxide Production in the Macrophage Cell Line RAW 264.7 and Their Structure–Activity Relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Hassan, N.A.; El Bassossy, H.M.; Fahmy, A. Quercetin Protects against Diabetes-Induced Exaggerated Vasoconstriction in Rats: Effect on Low Grade Inflammation. PLoS ONE 2013, 8, e63784. [Google Scholar] [CrossRef]
- Zheng, Z.; Yin, Y.; Lu, R.; Jiang, Z. Lycopene Ameliorated Oxidative Stress and Inflammation in Type 2 Diabetic Rats. J. Food Sci. 2019, 84, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Degeneve, A.; Mullen, W.; Crozier, A. Identification of Flavonoid and Phenolic Antioxidants in Black Currants, Blueberries, Raspberries, Red Currants, and Cranberries. J. Agric. Food Chem. 2010, 58, 3901–3909. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet–Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Elks, C.M.; Reed, S.D.; Mariappan, N.; Shukitt-Hale, B.; Joseph, J.A.; Ingram, D.K.; Francis, J. A Blueberry-Enriched Diet Attenuates Nephropathy in a Rat Model of Hypertension via Reduction in Oxidative Stress. PLoS ONE 2011, 6, e24028. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Daugherty, A.; Kristo, A.S.; Riso, P.; Klimis-Zacas, D. Wild Blueberry (Vaccinium Angustifolium) Consumption Improves Inflammatory Status in the Obese Zucker Rat Model of the Metabolic Syndrome. J. Nutr. Biochem. 2013, 24, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of Anthocyanins and Flavones Are Associated with Biomarkers of Insulin Resistance and Inflammation in Women1, 2. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Qin, L.-Q.; Arafa, A.; Eshak, E.S.; Dong, J.-Y. Effects of Strawberry Intervention on Cardiovascular Risk Factors: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2020, 124, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, H.; Liu, W.; Li, C. Survey of Antioxidant Capacity and Phenolic Composition of Blueberry, Blackberry, and Strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Mainous, A.G.; Buchanan, T.A.; Pearson, W.S. C-Reactive Protein and Glycemic Control in Adults with Diabetes. Diabetes Care 2003, 26, 1535–1539. [Google Scholar] [CrossRef]
- Gautam, D.; Adhikari, S.; Thapa, R.; Kharel, L. Study to Determine between HbA1C and C-Reactive Protein in Diabetes Mellitus. J. Pathol. Nepal. 2023, 13, 1979–1982. [Google Scholar] [CrossRef]
- Khosrowbeygi, A.; Gholami, M.; Zarei, P.; Sedeh, B.S.; Rezvanfar, M.R. Correlations between Biomarkers of Oxidative Stress, Glycemic Control and Insulin Resistance in Women with Type 2 Diabetes. Clin. Diabetol. 2019, 8, 277–283. [Google Scholar] [CrossRef]
- Bernaud, F.S.R.; Beretta, M.V.; do Nascimento, C.; Escobar, F.; Gross, J.L.; Azevedo, M.J.; Rodrigues, T.C. Fiber Intake and Inflammation in Type 1 Diabetes. Diabetol. Metab. Syndr. 2014, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Alman, A.C.; Snell-Bergeon, J.K. Associations of Dietary Antioxidants with Glycated Hemoglobin and Insulin Sensitivity in Adults with and without Type 1 Diabetes. J. Diabetes Res. 2022, 2022, 4747573. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.A.; Basu, A.; Chien, L.-C.; Pang, T.; Alman, A.C.; Snell-Bergeon, J.K. Longitudinal Associations of the Alternative Healthy Eating Index with Coronary Artery Calcification and Pericardial Adiposity in US Adults with and without Type 1 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1741–1750. [Google Scholar] [CrossRef]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet Quality and Major Chronic Disease Risk in Men and Women: Moving toward Improved Dietary Guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef]
| Non-DM (n = 692) | Non-DM (n = 532) | Difference between Visits | T1D (n = 563) | T1D (n = 421) | Difference between Visits | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Year Three (Visit 2) | Baseline | Year Three (Visit 2) | |||||||
| Variables | Count | % | Count | % | p-Value | Count | % | Count | % | p-Value |
| Sex (Female) | 349 | 50 | 265 | 51 | 0.9480 | 319 | 57 | 243 | 56 | 0.6180 |
| Hispanic | 59 | 9 | 41 | 8 | 0.6040 | 15 | 3 | 7 | 2 | 0.2931 |
| Non-Hispanic White | 582 | 84 | 461 | 88 | 0.2128 | 536 | 95 | 407 | 94 | 0.2535 |
| Never Smoker | 464 | 67 | 361 | 69 | 0.8407 | 383 | 68 | 296 | 68 | 0.4348 |
| Current Smoker | 59 | 9 | 46 | 9 | 0.9570 | 65 | 12 | 29 | 7 | 0.0140 |
| Former Smoker | 165 | 24 | 124 | 24 | 0.7975 | 114 | 20 | 95 | 22 | 0.3766 |
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| Hs-CRP (mg/dL) | 1.2 | (0.9–2.0) | 1.3 | (0.6–3.2) | 0.9269 ¶ | 1.2 | (0.9–2.2) | 1.5 | (0.7–3.8) | 0.1630 ¶ |
| Physical Activity | 84 | (0–300) | 60 | (0–270) | 0.1404 ¶ | 45 | (0–300) | 40 | (0–263) | 0.3410 ¶ |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (year) | 39 | 9 | 43 | 9 | <0.0001 | 37 | 9 | 40 | 9 | <0.0001 |
| BMI (kg/m2) | 26.2 | 5 | 26.6 | 5 | <0.0001 | 26.2 | 4 | 26.3 | 4 | 0.0052 |
| Calories (kcal/day) | 1821 | 619 | 1758 | 622 | 0.0178 | 1768 | 613 | 1732 | 598 | 0.8247 |
| HbA1c (%) | 5.5 | 0.4 | 5.3 | 0.5 | <0.0001 | 7.9 | 1.2 | 7.6 | 1.1 | <0.0001 |
| SBP (mm Hg) | 114 | 12 | 110 | 12 | <0.0001 | 117 | 14 | 112 | 13 | <0.0001 |
| Total Berries † | 0.24 | 0.96 | 0.25 | 0.82 | 0.4681 | 0.32 | 1.08 | 0.24 | 0.84 | 0.6002 |
| Strawberries † | 0.22 | 0.68 | 0.20 | 0.47 | 0.8723 | 0.29 | 0.89 | 0.22 | 0.69 | 0.5839 |
| Blueberries † | 0.24 | 0.84 | 0.23 | 0.67 | 0.6246 | 0.28 | 0.89 | 0.21 | 0.66 | 0.2901 |
| Total Fruit *† | 2.16 | 3.62 | 2.42 | 5.76 | 0.1289 | 2.61 | 4.18 | 2.26 | 4.66 | 0.4051 |
| Total Vegetables *† | 2.97 | 5.47 | 3.29 | 7.45 | 0.0415 | 3.84 | 8.97 | 3.07 | 2.98 | 0.1595 |
| Dietary Fiber Intake | 16.79 | 8.13 | 16.42 | 8.04 | 0.2457 | 16.72 | 7.80 | 15.75 | 7.88 | 0.0352 |
| MSDPS Fruit Score | 4.50 | 2.83 | 4.43 | 2.83 | 0.4830 | 4.56 | 2.94 | 4.50 | 2.82 | 0.2335 |
| MSDPS Vegetable Score | 3.83 | 2.27 | 3.88 | 2.72 | 0.3249 | 3.93 | 2.42 | 3.85 | 2.31 | 0.7111 |
| DASH Fruit Score | 2.89 | 1.41 | 2.98 | 1.40 | 0.0648 | 3.08 | 1.40 | 2.95 | 1.38 | 0.1274 |
| DASH Vegetable Score | 2.94 | 1.39 | 2.97 | 1.39 | 0.0971 | 3.07 | 1.43 | 2.99 | 1.42 | 0.8788 |
| AHEI Fruit Score | 3.08 | 2.65 | 3.27 | 2.68 | 0.0719 | 3.33 | 2.72 | 3.14 | 2.55 | 0.3902 |
| AHEI Vegetable Score | 4.79 | 2.86 | 4.85 | 2.79 | 0.0685 | 5.12 | 3.00 | 5.00 | 2.89 | 0.7806 |
| Variables | Pooled | Non-DM | T1D | ||||
|---|---|---|---|---|---|---|---|
| % Change (95% CI) | p-Value | % Change (95% CI) | p-Value | % Change (95% CI) | p-Value | ||
| Total Berries | Model 1 | −1.56 (−4.53, 1.50) | 0.3137 | −2.61 (−6.64, 1.58) | 0.2185 | −0.44 (−4.75, 4.07) | 0.8465 |
| Model 2 | −1.48 (−4.39, 1.51) | 0.3281 | −3.43 (−7.16, 0.45) | 0.0825 | 0.78 (−3.73, 5.51) | 0.7385 | |
| Strawberry | Model 1 | −1.67 (−6.28, 3.16) | 0.4906 | −2.29 (−8.84, 4.74) | 0.5134 | −1.18 (−7.62, 5.71) | 0.7291 |
| Model 2 | −1.66 (−6.31, 3.22) | 0.4976 | −4.73 (−10.83, 1.78) | 0.1506 | 0.86 (−6.22, 8.47) | 0.8173 | |
| Blueberry | Model 1 | −5.09 (−10.51, 0.65) | 0.0809 | −6.50 (−13.38, 0.92) | 0.0844 | −3.28 (−11.7, 5.93) | 0.4710 |
| Model 2 | −4.80 (−9.95, 0.65) | 0.0830 | −8.29 (−14.50, −1.64) | 0.0155 | −0.15 (−8.68, 9.18) | 0.9733 | |
| Variables | Pooled | Non-DM | T1D | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Total Berries | Model 1 | 0.91 (0.75, 1.06) | 0.2518 | 0.87 (0.59, 1.10) | 0.3039 | 0.94 (0.72, 1.12) | 0.5281 |
| Model 2 | 0.95 (0.84, 1.09) | 0.4758 | 0.92 (0.76, 1.12) | 0.4042 | 1.01 (0.83, 1.23) | 0.9487 | |
| Strawberry | Model 1 | 0.88 (0.61, 1.10) | 0.3160 | 0.82 (0.23, 1.21) | 0.4159 | 0.91 (0.59, 1.16) | 0.5371 |
| Model 2 | 0.93 (0.76, 1.15) | 0.5027 | 0.86 (0.62, 1.19) | 0.3622 | 1.01 (0.76, 1.33) | 0.9732 | |
| Blueberry | Model 1 | 0.89 (0.55, 1.14) | 0.4161 | 0.83 (0.25, 1.22) | 0.4370 | 0.93 (0.45, 1.26) | 0.7166 |
| Model 2 | 0.94 (0.73, 1.20) | 0.5998 | 0.88 (0.62, 1.24) | 0.4632 | 1.02 (0.66, 1.57) | 0.9426 | |
| Dietary Pattern Scores | Variables | Models | Pooled | Non-DM | T1D | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| %Change | 95% CI | p-Value | %Change | 95% CI | p-Value | %Change | 95% CI | p-Value | |||
| MSDPS | Fruit Score (including berries) | Model 1 | −0.68 | (−1.75, 0.41) | 0.2232 | −1.23 | (−2.62, 0.18) | 0.0867 | 0.12 | (−1.55, 1.83) | 0.8862 |
| Model 2 | 0.13 | (−0.95, 1.22) | 0.8180 | 0.08 | (−1.27, 1.45) | 0.9057 | 0.27 | (−1.45, 2.02) | 0.7630 | ||
| Fruit Score (excluding berries) | Model 1 | −0.78 | (−1.91, 0.36) | 0.1770 | −1.11 | (−2.56, 0.36) | 0.1374 | −0.38 | (−2.14, 1.41) | 0.6778 | |
| Model 2 | −0.01 | (−1.13, 1.13) | 0.9854 | 0.11 | (−1.29, 1.54) | 0.8737 | −0.22 | (−2.01, 1.60) | 0.8139 | ||
| Vegetable Score | Model 1 | −0.63 | (−2.04, 0.80) | 0.3846 | −1.78 | (−3.64, 0.12) | 0.0657 | 0.45 | (−1.65, 2.59) | 0.6756 | |
| Model 2 | −0.59 | (−1.97, 0.81) | 0.4067 | −1.29 | (−3.07, 0.52) | 0.1623 | 0.03 | (−2.08, 2.19) | 0.9756 | ||
| DASH | Fruit Score (including berries) | Model 1 | −1.59 | (−4.00, 0.88) | 0.2050 | −2.07 | (−5.18, 1.15) | 0.2052 | −1.38 | (−5.08, 2.48) | 0.4780 |
| Model 2 | −0.77 | (−3.15, 1.67) | 0.5335 | −0.62 | (−3.62, 2.48) | 0.6911 | −1.10 | (−4.83, 2.77) | 0.5716 | ||
| Fruit Score (excluding berries) | Model 1 | −1.67 | (−4.07, 0.79) | 0.1813 | −1.56 | (−4.66, 1.64) | 0.3340 | −2.21 | (−5.89, 1.62) | 0.2546 | |
| Model 2 | −0.86 | (−3.23, 1.57) | 0.4833 | −0.14 | (−3.14, 2.95) | 0.9287 | −1.92 | (−5.61, 1.92) | 0.3220 | ||
| Vegetable Score | Model 1 | −1.99 | (−4.35, 0.43) | 0.1057 | −3.69 | (−6.69, −0.59) | 0.0202 | 0.08 | (−3.64, 3.95) | 0.9655 | |
| Model 2 | −2.05 | (−4.35, 0.31) | 0.0879 | −3.22 | (−6.08, −0.28) | 0.0321 | −0.47 | (−4.18, 3.39) | 0.8090 | ||
| AHEI | Fruit Score (including berries) | Model 1 | −0.64 | (−1.89, 0.62) | 0.3192 | −0.95 | (−2.60, 0.72) | 0.2612 | −0.39 | (−2.28, 1.54) | 0.6896 |
| Model 2 | −0.24 | (−1.47, 1.00) | 0.7002 | −0.47 | (−2.04, 1.14) | 0.5669 | −0.12 | (−2.03, 1.81) | 0.8985 | ||
| Fruit Score (excluding berries) | Model 1 | −0.66 | (−2.00, 0.71) | 0.3426 | −0.91 | (−2.66, 0.88) | 0.3189 | −0.50 | (−2.55, 1.60) | 0.6391 | |
| Model 2 | −0.25 | (−1.57, 1.08) | 0.7107 | −0.36 | (−2.05, 1.35) | 0.6746 | −0.27 | (−2.31, 1.82) | 0.7989 | ||
| Vegetable Score | Model 1 | −0.83 | (−1.99, 0.34) | 0.1630 | −1.53 | (−3.05, 0.02) | 0.0525 | −0.14 | (−1.91, 1.66) | 0.8794 | |
| Model 2 | −0.99 | (−2.12, 0.15) | 0.0897 | −1.45 | (−2.88, 0.00) | 0.0504 | −0.50 | (−2.27, 1.31) | 0.5857 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helm, M.M.; Basu, A.; Richardson, L.A.; Chien, L.-C.; Izuora, K.; Alman, A.C.; Snell-Bergeon, J.K. Longitudinal Three-Year Associations of Dietary Fruit and Vegetable Intake with Serum hs-C-Reactive Protein in Adults with and without Type 1 Diabetes. Nutrients 2024, 16, 2058. https://doi.org/10.3390/nu16132058
Helm MM, Basu A, Richardson LA, Chien L-C, Izuora K, Alman AC, Snell-Bergeon JK. Longitudinal Three-Year Associations of Dietary Fruit and Vegetable Intake with Serum hs-C-Reactive Protein in Adults with and without Type 1 Diabetes. Nutrients. 2024; 16(13):2058. https://doi.org/10.3390/nu16132058
Chicago/Turabian StyleHelm, Macy M., Arpita Basu, Leigh Ann Richardson, Lung-Chang Chien, Kenneth Izuora, Amy C. Alman, and Janet K. Snell-Bergeon. 2024. "Longitudinal Three-Year Associations of Dietary Fruit and Vegetable Intake with Serum hs-C-Reactive Protein in Adults with and without Type 1 Diabetes" Nutrients 16, no. 13: 2058. https://doi.org/10.3390/nu16132058
APA StyleHelm, M. M., Basu, A., Richardson, L. A., Chien, L.-C., Izuora, K., Alman, A. C., & Snell-Bergeon, J. K. (2024). Longitudinal Three-Year Associations of Dietary Fruit and Vegetable Intake with Serum hs-C-Reactive Protein in Adults with and without Type 1 Diabetes. Nutrients, 16(13), 2058. https://doi.org/10.3390/nu16132058







