Anabolic Strategies for ICU-Acquired Weakness. What Can We Learn from Bodybuilders?
Abstract
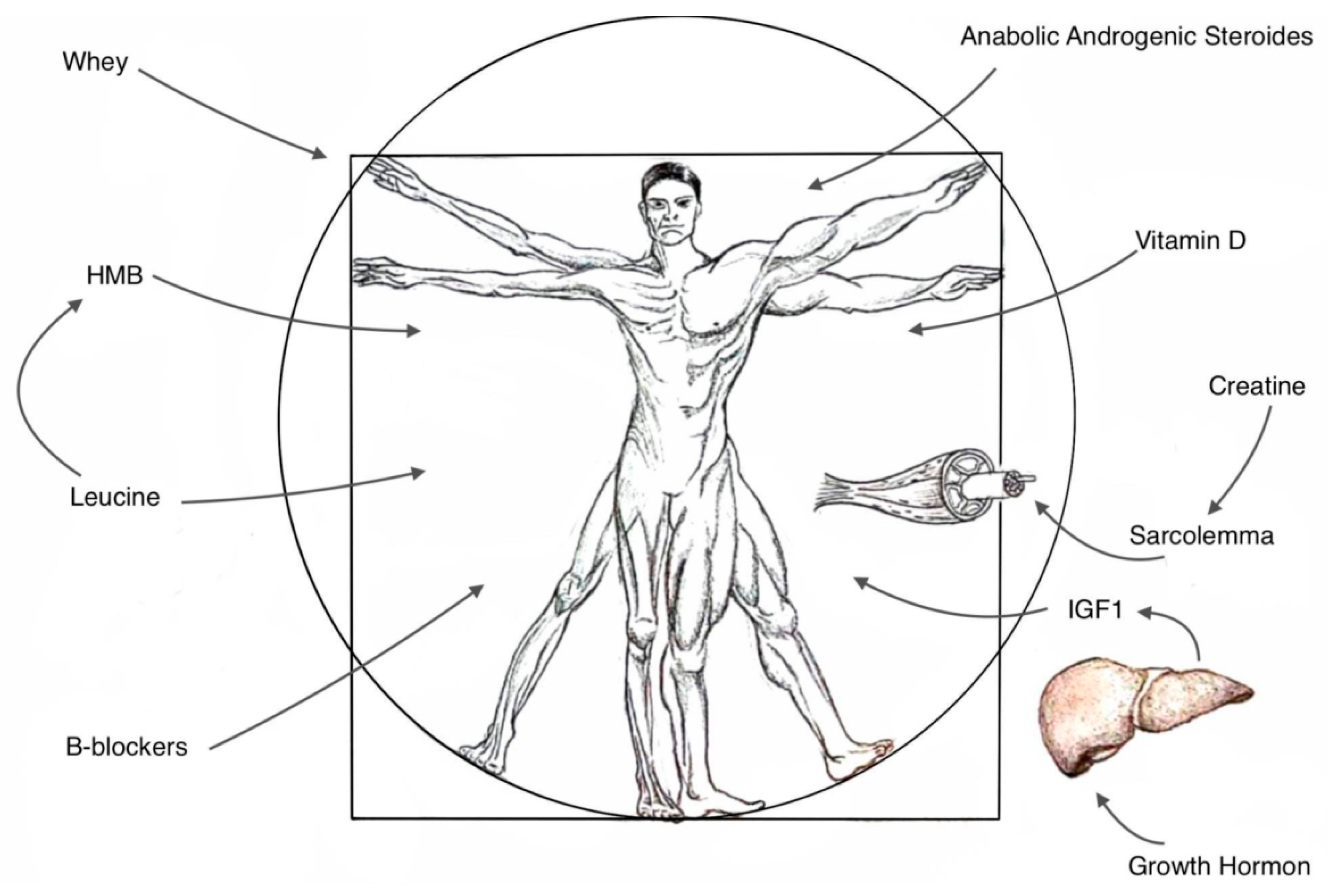
1. Introduction
Materials and Methods
2. In-Depth Review and Insights
2.1. Definition, Diagnosis, and Consequences
2.2. Pathomechanisms
2.3. Strategies and Recommendations
2.4. Creatine
2.5. Whey
2.6. β-Hydroxy-β-Methylbutyrate (HMB)
2.7. Vitamin D
2.8. Anabolic-Androgenic Steroids (AAS)
| Form of Testosteron Suplement | Typical Adult Dose | Myotrophic to Androgenic Activity Ratio | Individual Properties |
|---|---|---|---|
| Oxandrolone | 10 mg; twice daily; orally | 13:1 | - Primarily anabolic with minimal androgenic effects; - Minimal risk of liver enzyme elevation; - Effective in AIDS, COPD, and CKD patients; |
| Nandrolone | 100–200 mg male, 50–100 mg female; intramuscularly; weekly | 12:1 | - Primarily anabolic with minimal androgenic effect; - Possible reduction of endogenous testosterone production; |
| Testosterone cypionate | 200–400 mg; intramuscularly; every 2 weeks | 0.7–1.3:1 | - Commonly utilized outpatient testosterone replacement intervention; - More virilizing effects for women, potential aggression, and negative impact on cholesterol levels; |
2.9. Growth Hormone, Insulin Growth Factor 1
2.10. Beta-Blockers
3. Conclusions
- Athletes and bodybuilders provide valuable insights and nutritional possibilities into effective supplementation strategies for muscle growth and regeneration, which hold potential utility in the treatment of ICU-acquired weakness due to common mechanisms of work;
- Creatine and whey, extensively studied gym supplements, are known to promote muscle growth and potentially reduce muscle damage;
- HMB supplementation shows promise in preserving and enhancing lean body muscle mass;
- Vitamin D supplementation can improve outcomes, especially in individuals with critically low baseline levels;
- Consideration of AASs may be warranted in specific cases, particularly when addressing hormonal imbalances implicated in conditions like sarcopenia combined with B-blockers.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ICUAW | Intensive Care Unit-acquired weakness |
| CIP | critical illness polyneuropathy |
| CIM | critical illness myopathy |
| CIPNM | critical illness polyneuromyopathy |
| ICU | Intensive Care Unit |
| MRC | Medical Research Council |
| MMT | Manual muscle testing |
| NMES | neuromuscular electrical stimulation |
| UPS | Ubiquitin–proteasome system |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| EN | enteral nutrition |
| PN | parenteral nutrition |
| HMB | β-Hydroxy-β-methylbutyrate |
| BCAA | branched-chain amino acids |
| WP | whey protein |
| VDR | Vitamin D receptors |
| AAS | Anabolic-androgenic steroids |
| Low-T | low testosterone level |
| IGF-1 | Insulin Growth Factor 1 |
| GH | growth hormone |
References
- Appleton, R.T.D.; Kinsella, J.; Quasim, T. The Incidence of Intensive Care Unit-Acquired Weakness Syndromes: A Systematic Review. J. Intensive Care Soc. 2015, 16, 126–136. [Google Scholar] [CrossRef]
- Wollersheim, T.; Woehlecke, J.; Krebs, M.; Hamati, J.; Lodka, D.; Luther-Schroeder, A.; Langhans, C.; Haas, K.; Radtke, T.; Kleber, C.; et al. Dynamics of Myosin Degradation in Intensive Care Unit-Acquired Weakness during Severe Critical Illness. Intensive Care Med. 2014, 40, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Batt, J.; Dos Santos, C.C.; Cameron, J.I.; Herridge, M.S. Intensive Care Unit-Acquired Weakness Clinical Phenotypes and Molecular Mechanisms. Am. J. Respir. Crit. Care Med. 2013, 187, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, O.; Reid, M.B.; Van Den Berghe, G.; Vanhorebeek, I.; Hermans, G.; Rich, M.M.; Larsson, L. The Sick and the Weak: Neuropathies/ Myopathies in the Critically Ill. Physiol. Rev. 2015, 95, 1025–1109. [Google Scholar] [CrossRef]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid Disuse Atrophy of Diaphragm Fibers in Mechanically Ventilated Humans. New Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Batt, J.; Herridge, M.; dos Santos, C. Mechanism of ICU-Acquired Weakness: Skeletal Muscle Loss in Critical Illness. Intensive Care Med. 2017, 43, 1844–1846. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Cheek, F.; Chlan, L.; Gosselink, R.; Hart, N.; Herridge, M.S.; Hopkins, R.O.; Hough, C.L.; Kress, J.P.; Latronico, N.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Diagnosis of Intensive Care Unit-Acquired Weakness in Adults. Am. J. Respir. Crit. Care Med. 2014, 190, 1437–1446. [Google Scholar] [CrossRef]
- Jolley, S.E.; Bunnell, A.E.; Hough, C.L. ICU-Acquired Weakness. Chest 2016, 150, 1129–1140. [Google Scholar] [CrossRef]
- Kashani, K.B.; Frazee, E.N.; Kukrálová, L.; Sarvottam, K.; Herasevich, V.; Young, P.M.; Kashyap, R.; Lieske, J.C. Evaluating Muscle Mass by Using Markers of Kidney Function. Crit. Care Med. 2017, 45, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Vanhorebeek, I.; Latronico, N.; Van den Berghe, G. ICU-Acquired Weakness. Intensive Care Med. 2020, 46, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, P.; Moret, C.S.; Dziewas, R.; Schefold, J.C. Dysphagia in the Intensive Care Unit: Epidemiology, Mechanisms, and Clinical Management. Crit. Care 2019, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-H.; Nam, J.; Ko, M.G.; Chung, C.R.; Suh, G.Y.; Jeon, K. Impact of Limb Weakness on Extubation Failure after Planned Extubation in Medical Patients. Respirology 2018, 23, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Kelmenson, D.A.; Held, N.; Allen, R.R.; Quan, D.; Burnham, E.L.; Clark, B.J.; Ho, P.M.; Kiser, T.H.; Vandivier, R.W.; Moss, M. Outcomes of ICU Patients with a Discharge Diagnosis of Critical Illness Polyneuromyopathy: A Propensity-Matched Analysis. Crit. Care Med. 2017, 45, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Van Mechelen, H.; Clerckx, B.; Vanhullebusch, T.; Mesotten, D.; Wilmer, A.; Casaer, M.P.; Meersseman, P.; Debaveye, Y.; Van Cromphaut, S.; et al. Acute Outcomes and 1-Year Mortality of Intensive Care Unit-Acquired Weakness: A Cohort Study and Propensity-Matched Analysis. Am. J. Respir. Crit. Care Med. 2014, 190, 410–420. [Google Scholar] [CrossRef]
- Lad, H.; Saumur, T.M.; Herridge, M.S.; dos Santos, C.C.; Mathur, S.; Batt, J.; Gilbert, P.M. Intensive Care Unit-Acquired Weakness: Not Just Another Muscle Atrophying Condition. Int. J. Mol. Sci. 2020, 21, 7840. [Google Scholar] [CrossRef]
- Van Aerde, N.; Van den Berghe, G.; Wilmer, A.; Gosselink, R.; Hermans, G.; Meersseman, P.; Gunst, J.; Aerts, V.; Balthazar, T.; Barbé, A.; et al. Intensive Care Unit Acquired Muscle Weakness in COVID-19 Patients. Intensive Care Med. 2020, 46, 2083–2085. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, O. Critical Illness Myopathy: What Is Happening? Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 403–409. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe, B.; Sharshar, T.; Lefaucheur, J.P.; Authier, F.J.; Durand-Zaleski, I.; Boussarsar, M.; Cerf, C.; Renaud, E.; Mesrati, F.; Carlet, J.; et al. Paresis Acquired in the Intensive Care Unit: A Prospective Multicenter Study. JAMA 2002, 288, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Wieske, L.; Witteveen, E.; Petzold, A.; Verhamme, C.; Schultz, M.J.; van Schaik, I.N.; Horn, J. Neurofilaments as a Plasma Biomarker for ICU-Acquired Weakness: An Observational Pilot Study. Crit. Care 2014, 18, R18. [Google Scholar] [CrossRef]
- Singer, P.; Reintam Blaser, A.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- López-Baamonde, M.; Arguis, M.J.; Navarro-Ripoll, R.; Gimeno-Santos, E.; Romano-Andrioni, B.; Sisó, M.; Terès-Bellès, S.; López-Hernández, A.; Burniol-García, A.; Farrero, M.; et al. Multimodal Prehabilitation in Heart Transplant Recipients Improves Short-Term Post-Transplant Outcomes without Increasing Costs. J. Clin. Med. 2023, 12, 3724. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN Practical Guideline: Clinical Nutrition in Surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, A.K.; Lönn, K.; Gunningberg, L. Does Nutritional Intervention for Patients with Hip Fractures Reduce Postoperative Complications and Improve Rehabilitation? J. Clin. Nurs. 2009, 18, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H.; Lee, H.J.; Na, J.R.; Kim, W.G.; Han, D.S.; Park, S.H.; Hong, H.; Choi, Y.; Ahn, H.S.; Suh, Y.S.; et al. Effect of Perioperative Oral Nutritional Supplementation in Malnourished Patients Who Undergo Gastrectomy: A Prospective Randomized Trial. Surgery 2018, 164, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- van Dijkman, S.C.; de Jager, N.C.B.; Rauwé, W.M.; Danhof, M.; Della Pasqua, O. Effect of Age-Related Factors on the Pharmacokinetics of Lamotrigine and Potential Implications for Maintenance Dose Optimisation in Future Clinical Trials. Clin. Pharmacokinet. 2018, 57, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Wilmer, A.; Meersseman, W.; Milants, I.; Wouters, P.J.; Bobbaers, H.; Bruyninckx, F.; Van Den Berghe, G. Impact of Intensive Insulin Therapy on Neuromuscular Complications and Ventilator Dependency in the Medical Intensive Care Unit. Am. J. Respir. Crit. Care Med. 2007, 175, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive Insulin Therapy in Critically Ill Patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus Late Parenteral Nutrition in Critically Ill Adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Fivez, T.; Kerklaan, D.; Mesotten, D.; Verbruggen, S.; Wouters, P.J.; Vanhorebeek, I.; Debaveye, Y.; Vlasselaers, D.; Desmet, L.; Casaer, M.P.; et al. Early versus Late Parenteral Nutrition in Critically Ill Children. N. Engl. J. Med. 2016, 374, 1111–1122. [Google Scholar] [CrossRef]
- Hermans, G.; Casaer, M.P.; Clerckx, B.; Güiza, F.; Vanhullebusch, T.; Derde, S.; Meersseman, P.; Derese, I.; Mesotten, D.; Wouters, P.J.; et al. Effect of Tolerating Macronutrient Deficit on the Development of Intensive-Care Unit Acquired Weakness: A Subanalysis of the EPaNIC Trial. Lancet Respir. Med. 2013, 1, 621–629. [Google Scholar] [CrossRef]
- Kress, J.P.; Pohlman, A.S.; O’Connor, M.F.; Hall, J.B. Daily Interruption of Sedative Infusions in Critically Ill Patients Undergoing Mechanical Ventilation. N. Engl. J. Med. 2000, 342, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, E.; Felicetti, G.; Maini, M.; Fracchia, C. Peripheral Muscle Strength Training in Bed-Bound Patients with COPD Receiving Mechanical Ventilation: Effect of Electrical Stimulation. Chest 2003, 124, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Kim, J.; Kim, H.B.; Choi, H.; Kim, K.; Lee, D.; Na, S. The Effect of Electrical Muscle Stimulation and In-Bed Cycling on Muscle Strength and Mass of Mechanically Ventilated Patients: A Pilot Study. Acute Crit. Care 2018, 33, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Morton, J.P.; van Loon, L.J.C. Strategies to Maintain Skeletal Muscle Mass in the Injured Athlete: Nutritional Considerations and Exercise Mimetics. Eur. J. Sport. Sci. 2015, 15, 53–62. [Google Scholar] [CrossRef]
- Gala, K.; Desai, V.; Liu, N.; Omer, E.M.; McClave, S.A. How to Increase Muscle Mass in Critically Ill Patients: Lessons Learned from Athletes and Bodybuilders. Curr. Nutr. Rep. 2020, 9, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common Questions and Misconceptions about Creatine Supplementation: What Does the Scientific Evidence Really Show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- Persky AM, B.G. Clinical Pharmacology of the Dietary Supplement Creatine Monohydrate—PubMed. Pharmacol. Rev. 2001, 53, 161–176. [Google Scholar] [PubMed]
- Burke, D.G.; Candow, D.G.; Chilibeck, P.D.; MacNeil, L.G.; Roy, B.D.; Tarnopolsky, M.A.; Ziegenfuss, T. Effect of Creatine Supplementation and Resistance-Exercise Training on Muscle Insulin-like Growth Factor in Young Adults. Int. J. Sport. Nutr. Exerc. Metab. 2008, 18, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Kreider, R.B.; Stout, J.R.; Greenwood, M.; Campbell, B.; Spano, M.; Ziegenfuss, T.; Lopez, H.; Landis, J.; Antonio, J. International Society of Sports Nutrition Position Stand: Creatine Supplementation and Exercise. J. Int. Soc. Sports Nutr. 2007, 4, 6. [Google Scholar] [CrossRef]
- Volek, J.S.; Rawson, E.S. Scientific Basis and Practical Aspects of Creatine Supplementation for Athletes. Nutrition 2004, 20, 609–614. [Google Scholar] [CrossRef]
- Branch, J.D. Effect of Creatine Supplementation on Body Composition and Performance: A Meta-Analysis. Int. J. Sport. Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.C.; Oosterlaar, A.M.; Hartgens, F.; Hesselink, M.K.C.; Snow, R.J.; Wagenmakers, A.J.M. Effects of Creatine Loading and Prolonged Creatine Supplementation on Body Composition, Fuel Selection, Sprint and Endurance Performance in Humans. Clin. Sci. 2003, 104, 153–162. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition Position Stand: Safety and Efficacy of Creatine Supplementation in Exercise, Sport, and Medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
- Rawson, E.S.; Venezia, A.C. Use of Creatine in the Elderly and Evidence for Effects on Cognitive Function in Young and Old. Amino Acids 2011, 40, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Saks, V.A.; Strumia, E. Phosphocreatine: Molecular and Cellular Aspects of the Mechanism of Cardioprotective Action. Curr. Ther. Res. 1993, 53, 565–598. [Google Scholar] [CrossRef]
- Walrand, S.; Guillet, C.; Salles, J.; Cano, N.; Boirie, Y. Physiopathological Mechanism of Sarcopenia. Clin. Geriatr. Med. 2011, 27, 365–385. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor-Alpha with Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Baumgartner, R.N.; Atkinson, H.H.; Penninx, B.W.; Lenchik, L.; Palla, S.L.; Ambrosius, W.T.; Tracy, R.P.; Pahor, M. Sarcopenia, Obesity, and Inflammation--Results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors Study. Am. J. Clin. Nutr. 2005, 82, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kritchevsky, S.B.; Nicklas, B.J.; Penninx, B.W.H.J.; Holvoet, P.; Koh-Banerjee, P.; Cummings, S.R.; Harris, T.B.; Newman, A.B.; Pahor, M. Lipoprotein Peroxidation and Mobility Limitation: Results from the Health, Aging, and Body Composition Study. Arch. Intern. Med. 2005, 165, 2148–2154. [Google Scholar] [CrossRef][Green Version]
- Roubenoff, R.; Harris, T.B.; Abad, L.W.; Wilson, P.W.E.; Dallal, G.E.; Dinarello, C.A. Monocyte Cytokine Production in an Elderly Population: Effect of Age and Inflammation. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, M20–M26. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D.; Cornish, S.M.; Antonio, J.; Kreider, R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.T.; Yang, L.; Jenkins, B.G.; Ferrante, R.J.; Rosen, B.R.; Kaddurah-Daouk, R.; Beal, M.F. Neuroprotective Effects of Creatine and Cyclocreatine in Animal Models of Huntington’s Disease. J. Neurosci. 1998, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Martinelli, C.; Bravi, G.; Piccoli, G.; Curci, R.; Battistelli, M.; Falcieri, E.; Agostini, D.; Gioacchini, A.M.; Stocchi, V. Creatine Supplementation Affords Cytoprotection in Oxidatively Injured Cultured Mammalian Cells via Direct Antioxidant Activity. Free Radic. Biol. Med. 2006, 40, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Trojian, T.H. Creatine Supplementation. Curr. Sports Med. Rep. 2013, 12, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Benvenuti, L.; De Santis, A.; Cinti, S.; Rossi, L. Sportsmen’s Attitude towards Dietary Supplements and Nutrition Knowledge: An Investigation in Selected Roman Area Gyms. Nutrients 2022, 14, 945. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise & Sports Nutrition Review Update: Research & Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.M.; Stapel, S.N.; De Groot, S.D.W.; Driessen, R.H.; De Jong, E.; Girbes, A.R.J.; Strack Van Schijndel, R.J.M.; Beishuizen, A. Optimal Protein and Energy Nutrition Decreases Mortality in Mechanically Ventilated, Critically Ill Patients. J. Parenter. Enter. Nutr. 2012, 36, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Van Zanten, A.R.H.; De Waele, E.; Wischmeyer, P.E. Nutrition Therapy and Critical Illness: Practical Guidance for the ICU, Post-ICU, and Long-Term Convalescence Phases. Crit. Care 2019, 23, 368. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.J.; Bistrian, B.R.; Martindale, B.; Dickerson, R. Nutrition in Critical Illness: A Current Conundrum. F1000Research 2016, 5, 2531. [Google Scholar] [CrossRef]
- Heyland, D.K.; Dhaliwal, R.; Wang, M.; Day, A.G. The Prevalence of Iatrogenic Underfeeding in the Nutritionally ‘at-Risk’ Critically Ill Patient: Results of an International, Multicenter, Prospective Study. Clin. Nutr. 2015, 34, 659–666. [Google Scholar] [CrossRef]
- Rittig, N.; Bach, E.; Thomsen, H.H.; Møller, A.B.; Hansen, J.; Johannsen, M.; Jensen, E.; Serena, A.; Jørgensen, J.O.; Richelsen, B.; et al. Anabolic Effects of Leucine-Rich Whey Protein, Carbohydrate, and Soy Protein with and without β-Hydroxy-β-Methylbutyrate (HMB) during Fasting-Induced Catabolism: A Human Randomized Crossover Trial. Clin. Nutr. 2017, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Bukhsh, A.; Rehman, H.; Waqas, M.K.; Shahid, N.; Khaliel, A.M.; Elhanish, A.; Karoud, M.; Telb, A.; Khan, T.M. Efficacy and Safety of Whey Protein Supplements on Vital Sign and Physical Performance Among Athletes: A Network Meta-Analysis. Front. Pharmacol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Mikušová, L.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; Mets, T.; Wijers, S.L.J.; Garthoff, J.A.; et al. Safety and Tolerability of 6-Month Supplementation with a Vitamin D, Calcium and Leucine-Enriched Whey Protein Medical Nutrition Drink in Sarcopenic Older Adults. Aging Clin. Exp. Res. 2020, 32, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, Q.D.J.S.; Bachur, T.P.R.; Aragão, G.F. Whey Protein Supplementation and Its Potentially Adverse Effects on Health: A Systematic Review. Appl. Physiol. Nutr. Metab. 2021, 46, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Wilund, K.; Fitschen, P.J.; Jeejeebhoy, K.; Agarwala, R.; Drover, J.W.; Mourtzakis, M. Elderly Persons with ICU-Acquired Weakness: The Potential Role for β-Hydroxy-β-Methylbutyrate (HMB) Supplementation? J. Parenter. Enter. Nutr. 2014, 38, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Wilson, J.M.; Manninen, A.H. Effects of Beta-Hydroxy-Beta-Methylbutyrate (HMB) on Exercise Performance and Body Composition across Varying Levels of Age, Sex, and Training Experience: A Review. Nutr. Metab. 2008, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.P.; Pereira, S.L.; Hays, N.P.; Oliver, J.S.; Edens, N.K.; Evans, C.M.; Wolfe, R.R. Effect of β-Hydroxy-β-Methylbutyrate (HMB) on Lean Body Mass during 10 Days of Bed Rest in Older Adults. Clin. Nutr. 2013, 32, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Malafarina, V.; Uriz-Otano, F.; Malafarina, C.; Martinez, J.A.; Zulet, M.A. Effectiveness of Nutritional Supplementation on Sarcopenia and Recovery in Hip Fracture Patients. A Multi-Centre Randomized Trial. Maturitas 2017, 101, 42–50. [Google Scholar] [CrossRef]
- Viana, M.V.; Becce, F.; Pantet, O.; Schmidt, S.; Bagnoud, G.; Thaden, J.J.; Ten Have, G.A.M.; Engelen, M.P.K.J.; Voidey, A.; Deutz, N.E.P.; et al. Impact of β-Hydroxy-β-Methylbutyrate (HMB) on Muscle Loss and Protein Metabolism in Critically Ill Patients: A RCT. Clin. Nutr. 2021, 40, 4878–4887. [Google Scholar] [CrossRef]
- Bear, D.E.; Wandrag, L.; Merriweather, J.L.; Connolly, B.; Hart, N.; Grocott, M.P.W. The Role of Nutritional Support in the Physical and Functional Recovery of Critically Ill Patients: A Narrative Review. Crit. Care 2017, 21, 226. [Google Scholar] [CrossRef]
- Wiciński, M.; Adamkiewicz, D.; Adamkiewicz, M.; Śniegocki, M.; Podhorecka, M.; Szychta, P.; Malinowski, B. Impact of Vitamin D on Physical Efficiency and Exercise Performance-A Review. Nutrients 2019, 11, 2826. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of Vitamin D on Skeletal Muscle Function: Oxidative Stress, Energy Metabolism and Anabolic State. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.M.; Ismaeel, A.; Griffis, R.B.; Weems, S. Effects of Vitamin D Supplementation on Muscle Strength in Athletes: A Systematic Review. J. Strength. Cond. Res. 2017, 31, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.M.; Verlaan, S.; Bautmans, I.; Brandt, K.; Donini, L.M.; Maggio, M.; McMurdo, M.E.T.; Mets, T.; Seal, C.; Wijers, S.L.; et al. Effects of a Vitamin D and Leucine-Enriched Whey Protein Nutritional Supplement on Measures of Sarcopenia in Older Adults, the PROVIDE Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2015, 16, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef] [PubMed]
- Almoosa, K.F.; Gupta, A.; Pedroza, C.; Watts, N.B. Low Testosterone Levels Are Frequent in Patients with Acute Respiratory Failure and Are Associated with Poor Outcomes. Endocr. Pract. 2014, 20, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Vena, W.; Pizzocaro, A.; Pallotti, F.; Paoli, D.; Rastrelli, G.; Baldi, E.; Cilloni, N.; Gacci, M.; Semeraro, F.; et al. Andrological Effects of SARS-Cov-2 Infection: A Systematic Review and Meta-Analysis. J. Endocrinol. Invest. 2022, 45, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Suman, O.E.; Kozar, R.; Wolf, S.E.; Molinger, J.; Pastva, A.M. Role of Anabolic Testosterone Agents and Structured Exercise to Promote Recovery in ICU Survivors. Curr. Opin. Crit. Care 2020, 26, 508–515. [Google Scholar] [CrossRef]
- Kicman, A.T. Pharmacology of Anabolic Steroids. Br. J. Pharmacol. 2008, 154, 502–521. [Google Scholar] [CrossRef]
- Falqueto, H.; dos Santos, M.R.; Manfredi, L.H. Anabolic-Androgenic Steroids and Exercise Training: Breaking the Myths and Dealing with Better Outcome in Sarcopenia. Front. Physiol. 2022, 13, 838526. [Google Scholar] [CrossRef]
- Skinner, J.W.; Otzel, D.M.; Bowser, A.; Nargi, D.; Agarwal, S.; Peterson, M.D.; Zou, B.; Borst, S.E.; Yarrow, J.F. Muscular Responses to Testosterone Replacement Vary by Administration Route: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2018, 9, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.F.; Christensen, J.C.; Stevens-Lapsley, J.; Bartley, J.; Berry, S.D.; Dobs, A.S.; Fortinsky, R.H.; Hildreth, K.L.; Kiel, D.P.; Kuchel, G.A.; et al. A Multi-Center Trial of Exercise and Testosterone Therapy in Women after Hip Fracture: Design, Methods and Impact of the COVID-19 Pandemic. Contemp. Clin. Trials 2021, 104, 106356. [Google Scholar] [CrossRef] [PubMed]
- Anstey, M.; Desai, S.; Torre, L.; Wibrow, B.; Seet, J.; Osnain, E. Anabolic Steroid Use for Weight and Strength Gain in Critically Ill Patients: A Case Series and Review of the Literature. Case Rep. Crit. Care 2018, 2018, 4545623. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro de Oliveira Longo Schweizer, J.; Ribeiro-Oliveira Jr, A.; Bidlingmaier, M. Growth Hormone: Isoforms, Clinical Aspects and Assays Interference. Clin. Diabetes Endocrinol. 2018, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A.M.J.L. Advantages and Disadvantages of GH/IGF-I Combination Treatment. Rev. Endocr. Metab. Disord. 2009, 10, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.E.; Buchman, T.G. Is There a Role for Growth Hormone Therapy in Refractory Critical Illness? Curr. Opin. Crit. Care 2008, 14, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Park, J.; Thorner, M.O. Growth Hormone Supplementation in the Elderly. Endocrinol. Metab. Clin. North. Am. 2007, 36, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.C.; Carroll, P.V.; Russell-Jones, D.L.; Sönksen, P.H.; Treacher, D.F.; Umpleby, A.M. Effects of Glutamine Supplementation, GH, and IGF-I on Glutamine Metabolism in Critically Ill Patients. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E226–E233. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Merriam, G.R. Growth Hormone Therapy in Adults. Annu. Rev. Med. 2003, 54, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Hatton, J.; Kryscio, R.; Ryan, M.; Linda, O.; Young, B. Systemic Metabolic Effects of Combined Insulin-like Growth Factor–I and Growth Hormone Therapy in Patients Who Have Sustained Acute Traumatic Brain Injury. J. Neurosurg. 2006, 105, 843–852. [Google Scholar] [CrossRef]
- Thiele, R.I.; Jakob, H.; Hund, E.; Tantzky, S.; Keller, S.; Kamler, M.; Herold, U.; Hagl, S. Sepsis and Catecholamine Support Are the Major Risk Factors for Critical Illness Polyneuropathy after Open Heart Surgery’. Thorac. Cardiovasc. Surg. 2000, 48, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W.; Long, J.M.; Mason, A.D.; Skreen, R.W.; Pruitt, B.A. Catecholamines: Mediator of the Hypermetabolic Response to Thermal Injury. Ann. Surg. 1974, 180, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Wang, V.; Auger, C.; Patsouris, D.; Amini-Nik, S.; Jeschke, M.G. Catecholamines Induce Endoplasmic Reticulum Stress Via Both Alpha and Beta Receptors. Shock. 2020, 53, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Baron, P.W.; Barrow, R.E.; Pierre, E.J.; Herndon, D.N. Prolonged Use of Propranolol Safely Decreases Cardiac Work in Burned Children. J. Burn. Care Rehabil. 1997, 18, 223–227. [Google Scholar] [CrossRef]
- Herndon, D.N.; Dasu, M.R.K.; Wolfe, R.R.; Barrow, R.E. Gene Expression Profiles and Protein Balance in Skeletal Muscle of Burned Children after Beta-Adrenergic Blockade. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E783–E789. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Hart, D.W.; Wolf, S.E.; Chinkes, D.L.; Wolfe, R.R. Reversal of Catabolism by Beta-Blockade after Severe Burns. N. Engl. J. Med. 2001, 345, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhi, V.G.M. Propranolol. StatPearls Publishing LLC: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Brown, D.A.; Gibbons, J.; Honari, S.; Klein, M.B.; Pham, T.N.; Gibran, N.S. Propranolol Dosing Practices in Adult Burn Patients: Implications for Safety and Efficacy. J. Burn. Care Res. 2016, 37, e218–e226. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.N.; Herndon, D.N.; Kulp, G.A.; Jeschke, M.G. Propranolol Decreases Cardiac Work in a Dose-Dependent Manner in Severely Burned Children. Surgery 2011, 149, 231–239. [Google Scholar] [CrossRef]
- Pereira, C.; Murphy, K.; Jeschke, M.; Herndon, D.N. Post Burn Muscle Wasting and the Effects of Treatments. Int. J. Biochem. Cell Biol. 2005, 37, 1948–1961. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarnawski, J.; Czub, M.; Dymecki, M.; Sunil, M.; Folwarski, M. Anabolic Strategies for ICU-Acquired Weakness. What Can We Learn from Bodybuilders? Nutrients 2024, 16, 2011. https://doi.org/10.3390/nu16132011
Tarnawski J, Czub M, Dymecki M, Sunil M, Folwarski M. Anabolic Strategies for ICU-Acquired Weakness. What Can We Learn from Bodybuilders? Nutrients. 2024; 16(13):2011. https://doi.org/10.3390/nu16132011
Chicago/Turabian StyleTarnawski, Jakub, Maja Czub, Marta Dymecki, Medha Sunil, and Marcin Folwarski. 2024. "Anabolic Strategies for ICU-Acquired Weakness. What Can We Learn from Bodybuilders?" Nutrients 16, no. 13: 2011. https://doi.org/10.3390/nu16132011
APA StyleTarnawski, J., Czub, M., Dymecki, M., Sunil, M., & Folwarski, M. (2024). Anabolic Strategies for ICU-Acquired Weakness. What Can We Learn from Bodybuilders? Nutrients, 16(13), 2011. https://doi.org/10.3390/nu16132011





