The Impact of Vitamin D and L-Cysteine Co-Supplementation on Upregulating Glutathione and Vitamin D-Metabolizing Genes and in the Treatment of Circulating 25-Hydroxy Vitamin D Deficiency
Abstract
1. Introduction
2. VD Metabolism Genes and Blood 25(OH)VD Status in Humans
3. Bioavailable 25(OH)VD Is Linked with Better Health Outcomes
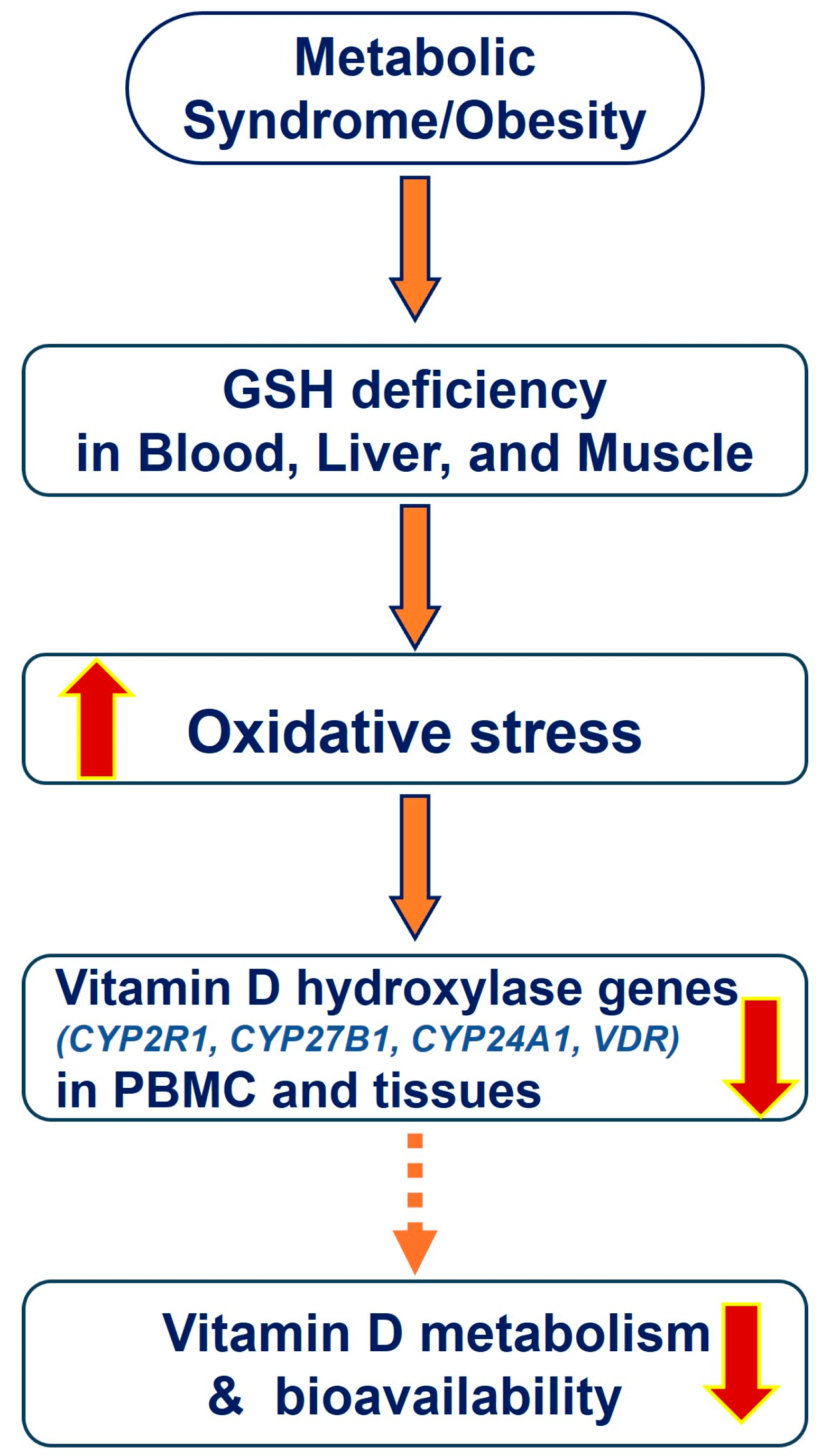
4. Impaired Vitamin D-Metabolizing Genes in Obesity/Population
5. Impaired Glutathione and Obesity
Link between GSH and 25(OH)VD
6. LC, GSH Biosynthesis, Oxidative Stress, and Inflammation
7. Testosterone and Vitamin D Metabolism
8. L-Cysteine, Nitric Oxide, Hydrogen Sulfide, and Vitamin D Metabolism
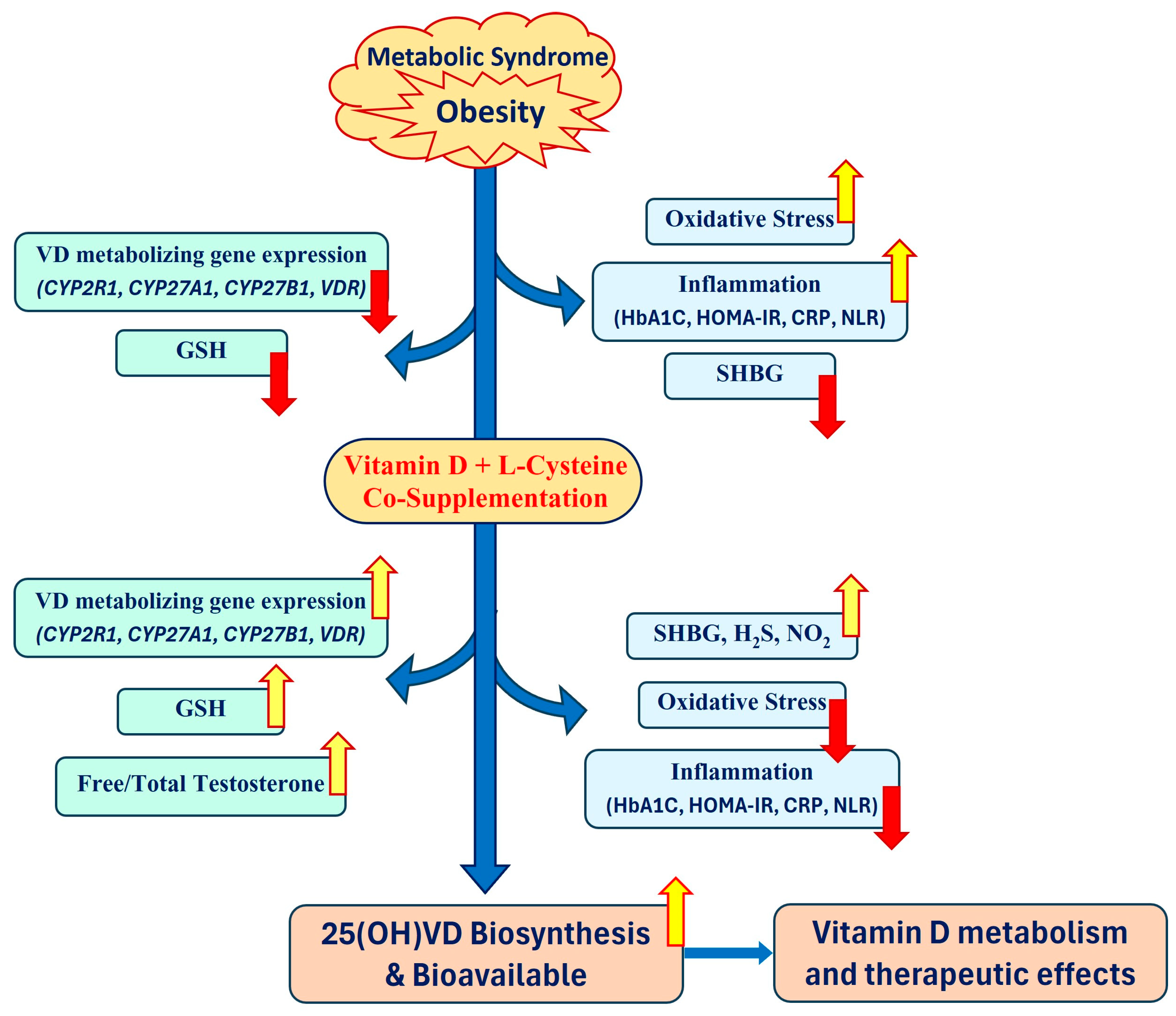
9. Justification for Combined Use of VD and LC
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Shapses, S.A.; Calvo, M.S. Health Benefits of Vitamin D Supplementation: Time to Move the Spotlight Away from Bone Health in Vitamin D-replete Individuals? Am. J. Clin. Nutr. 2023, 118, 489–490. [Google Scholar] [CrossRef]
- Vearing, R.M.; Hart, K.H.; Darling, A.L.; Probst, Y.; Olayinka, A.S.; Mendis, J.; Ribeiro, H.; Thakur, S.; Mendes, M.; Charlton, K.; et al. Global Perspective of the Vitamin D Status of African-Caribbean Populations: A Systematic Review and Meta-analysis. Eur. J. Clin. Nutr. 2022, 76, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Neme, A.; Seuter, S.; Malinen, M.; Nurmi, T.; Tuomainen, T.P.; Virtanen, J.K.; Carlberg, C. In vivo transcriptome changes of human white blood cells in response to vitamin D. J. Steroid Biochem. Mol. Biol. 2019, 188, 71–76. [Google Scholar] [CrossRef]
- Ginde, A.A.; Liu, M.C.; Camargo, C.A., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch. Intern. Med. 2009, 169, 626–632. [Google Scholar] [CrossRef]
- Maretzke, F.; Bechthold, A.; Egert, S.; Ernst, J.B.; Melo van Lent, D.; Pilz, S.; Reichrath, J.; Stangl, G.I.; Stehle, P.; Volkert, D.; et al. Role of Vitamin D in Preventing and Treating Selected Extraskeletal Diseases—An Umbrella Review. Nutrients 2020, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.J. Why do so many trials of vitamin D supplementation fail? Endocr. Connect. 2020, 9, R195–R206. [Google Scholar] [CrossRef] [PubMed]
- Wilger-Gahche, J.J.; Bailey, R.L.; Burt, V.L.; Radimer, K.; McDowell, M.; Picciano, M.F.; Dwyer, J.; Sempos, C. Mean daily intake of calcium, folate and vitamin D from dietary supplements and the proportion getting above certain Dietary Reference Intake (DRI) levels, in the US population ages 14 years and older: Third National Health and Nutrition Examination Survey (NHANES III) and NHANES, 1999–2006. FASEB J. 2009, 23, 341.7. [Google Scholar] [CrossRef]
- Elkhwanky, M.S.; Kummu, O.; Piltonen, T.T.; Laru, J.; Morin-Papunen, L.; Mutikainen, M.; Tavi, P.; Hakkola, J. Obesity Represses CYP2R1, the Vitamin D 25-Hydroxylase, in the Liver and Extrahepatic Tissues. JBMR Plus 2020, 4, e10397. [Google Scholar] [CrossRef]
- Roizen, J.D.; Long, C.; Casella, A.; O’Lear, L.; Caplan, I.; Lai, M.; Sasson, I.; Singh, R.; Makowski, A.J.; Simmons, R.; et al. Obesity Decreases Hepatic 25-Hydroxylase Activity Causing Low Serum 25-Hydroxyvitamin D. J. Bone Miner. Res. 2019, 34, 1068–1073. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [PubMed]
- Bikle, D.D. The Free Hormone Hypothesis: When, Why, and How to Measure the Free Hormone Levels to Assess Vitamin D, Thyroid, Sex Hormone, and Cortisol Status. JBMR Plus 2021, 5, e10418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.B.; Levine, M.A.; Bell, N.H.; Mangelsdorf, D.J.; Russell, D.W. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. USA 2004, 101, 7711–7715. [Google Scholar] [CrossRef] [PubMed]
- Engelman, C.D.; Meyers, K.J.; Iyengar, S.K.; Liu, Z.; Karki, C.K.; Igo, R.P., Jr.; Truitt, B.; Robinson, J.; Sarto, G.E.; Wallace, R.; et al. Vitamin D intake and season modify the effects of the GC and CYP2R1 genes on 25-hydroxyvitamin D concentrations. J. Nutr. 2013, 143, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nissen, J.; Vogel, U.; Ravn-Haren, G.; Andersen, E.W.; Madsen, K.H.; Nexo, B.A.; Andersen, R.; Mejborn, H.; Bjerrum, P.J.; Rasmussen, L.B.; et al. Common variants in CYP2R1 and GC genes are both determinants of serum 25-hydroxyvitamin D concentrations after UVB irradiation and after consumption of vitamin D(3)-fortified bread and milk during winter in Denmark. Am. J. Clin. Nutr. 2015, 101, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, M.; Huang, G.; Delanghe, J.R.; Taes, Y.E. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin. Chim. Acta 2006, 372, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef]
- Miller, W.L. Genetic disorders of Vitamin D biosynthesis and degradation. J. Steroid Biochem. Mol. Biol. 2017, 165, 101–108. [Google Scholar] [CrossRef]
- Safadi, F.F.; Thornton, P.; Magiera, H.; Hollis, B.W.; Gentile, M.; Haddad, J.G.; Liebhaber, S.A.; Cooke, N.E. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J. Clin. Investig. 1999, 103, 239–251. [Google Scholar] [CrossRef]
- Robien, K.; Butler, L.M.; Wang, R.; Beckman, K.B.; Walek, D.; Koh, W.P.; Yuan, J.M. Genetic and environmental predictors of serum 25-hydroxyvitamin D concentrations among middle-aged and elderly Chinese in Singapore. Br. J. Nutr. 2013, 109, 493–502. [Google Scholar] [CrossRef]
- Nimitphong, H.; Saetung, S.; Chanprasertyotin, S.; Chailurkit, L.O.; Ongphiphadhanakul, B. Changes in circulating 25-hydroxyvitamin D according to vitamin D binding protein genotypes after vitamin D(3) or D(2)supplementation. Nutr. J. 2013, 12, 39. [Google Scholar] [CrossRef]
- Fu, L.; Yun, F.; Oczak, M.; Wong, B.Y.; Vieth, R.; Cole, D.E. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin. Biochem. 2009, 42, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. Clinical practice. Vitamin D insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Preveraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv. Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef]
- Korn, S.; Hubner, M.; Jung, M.; Blettner, M.; Buhl, R. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respir. Res. 2013, 14, 25. [Google Scholar] [CrossRef]
- Muindi, J.R.; Adjei, A.A.; Wu, Z.R.; Olson, I.; Huang, H.; Groman, A.; Tian, L.; Singh, P.K.; Sucheston, L.E.; Johnson, C.S.; et al. Serum vitamin D metabolites in colorectal cancer patients receiving cholecalciferol supplementation: Correlation with polymorphisms in the vitamin D genes. Horm. Cancer 2013, 4, 242–250. [Google Scholar] [CrossRef]
- Johnson, M.A.; Davey, A.; Park, S.; Hausman, D.B.; Poon, L.W.; Georgia Centenarian, S. Age, race and season predict vitamin D status in African American and white octogenarians and centenarians. J. Nutr. Health Aging 2008, 12, 690–695. [Google Scholar] [CrossRef]
- Powe, C.E.; Evans, M.K.; Wenger, J.; Zonderman, A.B.; Berg, A.H.; Nalls, M.; Tamez, H.; Zhang, D.; Bhan, I.; Karumanchi, S.A.; et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013, 369, 1991–2000. [Google Scholar] [CrossRef]
- Looker, A.C.; Johnson, C.L.; Lacher, D.A.; Pfeiffer, C.M.; Schleicher, R.L.; Sempos, C.T. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011, 59, 1–8. [Google Scholar]
- Grant, W.B.; Peiris, A.N. Possible role of serum 25-hydroxyvitamin D in black-white health disparities in the United States. J. Am. Med. Dir. Assoc. 2010, 11, 617–628. [Google Scholar] [CrossRef]
- Williams, S.K.; Fiscella, K.; Winters, P.; Martins, D.; Ogedegbe, G. Association of racial disparities in the prevalence of insulin resistance with racial disparities in vitamin D levels: National Health and Nutrition Examination Survey (2001–2006). Nutr. Res. 2013, 33, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Malmstroem, S.; Schwartz, J. Current Controversies: Are Free Vitamin Metabolite Levels a More Accurate Assessment of Vitamin D Status than Total Levels? Endocrinol. Metab. Clin. N. Am. 2017, 46, 901–918. [Google Scholar] [CrossRef]
- Fang, A.P.; Long, J.A.; Zhang, Y.J.; Liu, Z.Y.; Li, Q.J.; Zhang, D.M.; Luo, Y.; Zhong, R.H.; Zhou, Z.G.; Xu, Y.J.; et al. Serum Bioavailable, Rather Than Total, 25-hydroxyvitamin D Levels Are Associated With Hepatocellular Carcinoma Survival. Hepatology 2020, 72, 169–182. [Google Scholar] [CrossRef]
- Zhu, A.; Kuznia, S.; Boakye, D.; Schottker, B.; Brenner, H. Vitamin D-Binding Protein, Bioavailable, and Free 25(OH)D, and Mortality: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3894. [Google Scholar] [CrossRef]
- AlSedairy, S.A.; Al-Harbi, L.N.; Binobead, M.A.; Athinarayanan, J.; Arzoo, S.; Al-Tamimi, D.S.; Shamlan, G.; Alshatwi, A.A.; Periasamy, V.S. Association of CYP2R1 and CYP27B1 genes with the risk of obesity and vitamin D metabolism in Saudi women. J. Genet. Eng. Biotechnol. 2023, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Bakos, B.; Szili, B.; Szabo, B.; Horvath, P.; Kirschner, G.; Kosa, J.P.; Toldy, E.; Lakatos, P.; Tabak, A.G.; Takacs, I. Genetic variants of VDR and CYP2R1 affect BMI independently of serum vitamin D concentrations. BMC Med. Genet. 2020, 21, 129. [Google Scholar] [CrossRef]
- Wamberg, L.; Christiansen, T.; Paulsen, S.K.; Fisker, S.; Rask, P.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Expression of vitamin D-metabolizing enzymes in human adipose tissue—The effect of obesity and diet-induced weight loss. Int. J. Obes. 2013, 37, 651–657. [Google Scholar] [CrossRef]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Rocca, M.S.; De Filippis, V.; Opocher, G.; Azzena, B.; Vettor, R.; Plebani, M.; Foresta, C. Impaired Release of Vitamin D in Dysfunctional Adipose Tissue: New Cues on Vitamin D Supplementation in Obesity. J. Clin. Endocrinol. Metab. 2017, 102, 2564–2574. [Google Scholar] [CrossRef]
- Yuzbashian, E.; Asghari, G.; Hedayati, M.; Zarkesh, M.; Mirmiran, P.; Khalaj, A. Determinants of vitamin D receptor gene expression in visceral and subcutaneous adipose tissue in non-obese, obese, and morbidly obese subjects. J. Steroid Biochem. Mol. Biol. 2019, 187, 82–87. [Google Scholar] [CrossRef]
- Araujo, E.; Lima, S.; Galdino, O.A.; Arrais, R.F.; de Souza, K.S.C.; de Rezende, A.A. Association of CYP2R1 and VDR Polymorphisms with Metabolic Syndrome Components in Non-Diabetic Brazilian Adolescents. Nutrients 2022, 14, 4612. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Park, C.Y.; Han, S.N. High fat diet-Induced obesity alters vitamin D metabolizing enzyme expression in mice. Biofactors 2015, 41, 175–182. [Google Scholar] [CrossRef]
- Jain, S.K.; Parsanathan, R.; Achari, A.E.; Kanikarla-Marie, P.; Bocchini, J.A., Jr. Glutathione Stimulates Vitamin D Regulatory and Glucose-Metabolism Genes, Lowers Oxidative Stress and Inflammation, and Increases 25-Hydroxy-Vitamin D Levels in Blood: A Novel Approach to Treat 25-Hydroxyvitamin D Deficiency. Antioxid. Redox Signal 2018, 29, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, L.; Hachemi, M.A.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Martin, J.C.; Tourniaire, F.; Landrier, J.F. Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice. J. Steroid Biochem. Mol. Biol. 2019, 185, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Aatsinki, S.M.; Elkhwanky, M.S.; Kummu, O.; Karpale, M.; Buler, M.; Viitala, P.; Rinne, V.; Mutikainen, M.; Tavi, P.; Franko, A.; et al. Fasting-Induced Transcription Factors Repress Vitamin D Bioactivation, a Mechanism for Vitamin D Deficiency in Diabetes. Diabetes 2019, 68, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019, 9, 14784. [Google Scholar] [CrossRef]
- Feng, M.; Wang, K.; Wei, H.; Zhang, S.; Chen, Y. Serum 25OHD3 of Obese Mice Is Affected by Liver Injury and Correlates with Testosterone Levels and Sperm Motility. Obes. Facts 2021, 14, 559–567. [Google Scholar] [CrossRef]
- Park, C.Y.; Shin, Y.; Kim, J.H.; Zhu, S.; Jung, Y.S.; Han, S.N. Effects of high fat diet-induced obesity on vitamin D metabolism and tissue distribution in vitamin D deficient or supplemented mice. Nutr. Metab. 2020, 17, 44. [Google Scholar] [CrossRef]
- Zhu, T.; Zhao, J.; Zhuo, S.; Hu, Z.; Ouyang, S.; Wunier; Yu, S.; Chen, Y.; Li, Y.; Le, Y. High Fat Diet and High Cholesterol Diet Reduce Hepatic Vitamin D-25-Hydroxylase Expression and Serum 25-Hydroxyvitamin D(3) Level through Elevating Circulating Cholesterol, Glucose, and Insulin Levels. Mol. Nutr. Food Res. 2021, 65, e2100220. [Google Scholar] [CrossRef]
- Bonnet, L.; Karkeni, E.; Couturier, C.; Astier, J.; Defoort, C.; Svilar, L.; Tourniaire, F.; Mounien, L.; Landrier, J.F. Four days high fat diet modulates vitamin D metabolite levels and enzymes in mice. J. Endocrinol. 2021, 248, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Yu, Y.H.; Shew, J.Y.; Lee, W.J.; Hwang, J.J.; Chen, Y.H.; Chen, Y.R.; Wei, P.C.; Chuang, L.M.; Lee, W.H. Deficiency of NPGPx, an oxidative stress sensor, leads to obesity in mice and human. EMBO Mol. Med. 2013, 5, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, M.; Siodmiak, J.; Borkowski, B.; Lorkowski, M.; Olszewska-Slonina, D.M. Lipid Peroxidation as a Possible Factor Affecting Bone Resorption in Obese Subjects-Preliminary Research. Int. J. Mol. Sci. 2023, 24, 11629. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.M.; Hahn, W.S.; Long, E.K.; Burrill, J.S.; Arriaga, E.A.; Bernlohr, D.A. Protein carbonylation and metabolic control systems. Trends Endocrinol. Metab. 2012, 23, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Aspects Med. 2009, 30, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Hussain, S.N. Protein carbonylation in skeletal muscles: Impact on function. Antioxid. Redox Signal 2010, 12, 417–429. [Google Scholar] [CrossRef]
- Evans, J.L.; Maddux, B.A.; Goldfine, I.D. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal 2005, 7, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Antoniades, C. Impaired Vascular Redox Signaling in the Vascular Complications of Obesity and Diabetes Mellitus. Antioxid. Redox Signal 2019, 30, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ren, W.; Yang, G.; Duan, J.; Huang, X.; Fang, R.; Li, C.; Li, T.; Yin, Y.; Hou, Y.; et al. L-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2016, 60, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Velusamy, T.; Croad, J.L.; Rains, J.L.; Bull, R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, and oxidative stress and inhibits NF-kappaB activation in the livers of Zucker diabetic rats. Free Radic. Biol. Med. 2009, 46, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.A.; Hardy, G. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.G.; Tang, W.; Hootman, K.C.; Brannon, P.M.; Houston, D.K.; Kritchevsky, S.B.; Harris, T.B.; Garcia, M.; Lohman, K.; Liu, Y.; et al. Genetic and environmental factors are associated with serum 25-hydroxyvitamin D concentrations in older African Americans. J. Nutr. 2015, 145, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Hanifa, Y.; Witt, K.D.; Venton, T.R.; Rowe, M.; Timms, P.M.; Hypponen, E.; Walton, R.T.; Griffiths, C.J.; Martineau, A.R. Environmental and genetic determinants of vitamin D status among older adults in London, UK. J. Steroid Biochem. Mol. Biol. 2016, 164, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.Z.; Abdel-Alnaby, M.; Atta, M.N.; Abdul-Aziz, A.A.; Megahed, F. The association between body mass index elevation and differentiation in vitamin D receptor gene expression, genetic polymorphism, and oxidative stress in adult Egyptian individuals. Sci. Rep. 2023, 13, 17696. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Kanikarla-Marie, P.; Warden, C.; Micinski, D. L-cysteine supplementation upregulates glutathione (GSH) and vitamin D binding protein (VDBP) in hepatocytes cultured in high glucose and in vivo in liver, and increases blood levels of GSH, VDBP, and 25-hydroxy-vitamin D in Zucker diabetic fatty rats. Mol. Nutr. Food Res. 2016, 60, 1090–1098. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D.; Huning, L.; Kahlon, G.; Bass, P.F.; Levine, S.N. Vitamin D and L-cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients. Eur. J. Clin. Nutr. 2014, 68, 1148–1153. [Google Scholar] [CrossRef]
- Jain, S.K.; Kahlon, G.; Bass, P.; Levine, S.N.; Warden, C. Can L-Cysteine and Vitamin D Rescue Vitamin D and Vitamin D Binding Protein Levels in Blood Plasma of African American Type 2 Diabetic Patients? Antioxid. Redox Signal 2015, 23, 688–693. [Google Scholar] [CrossRef]
- Jain, S.K.; McVie, R. Effect of glycemic control, race (white versus black), and duration of diabetes on reduced glutathione content in erythrocytes of diabetic patients. Metabolism 1994, 43, 306–309. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D.; Parsanathan, R. l-Cysteine Stimulates the Effect of Vitamin D on Inhibition of Oxidative Stress, IL-8, and MCP-1 Secretion in High Glucose Treated Monocytes. J. Am. Coll. Nutr. 2021, 40, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Chowdhury, R.; Jones, D.P.; Martin, G.S.; Brigham, K.L.; Binongo, J.N.; Ziegler, T.R.; Tangpricha, V. Vitamin D status is independently associated with plasma glutathione and cysteine thiol/disulphide redox status in adults. Clin. Endocrinol. 2014, 81, 458–466. [Google Scholar] [CrossRef]
- Mokhaneli, M.C.; Fourie, C.M.; Botha, S.; Mels, C.M. The association of oxidative stress with arterial compliance and vascular resistance in a bi-ethnic population: The SABPA study. Free Radic. Res. 2016, 50, 920–928. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Weber, D.; Davies, M.J.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Grimsrud, P.A.; Xie, H.; Griffin, T.J.; Bernlohr, D.A. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008, 283, 21837–21841. [Google Scholar] [CrossRef]
- Szanton, S.L.; Rifkind, J.M.; Mohanty, J.G.; Miller, E.R., 3rd; Thorpe, R.J.; Nagababu, E.; Epel, E.S.; Zonderman, A.B.; Evans, M.K. Racial discrimination is associated with a measure of red blood cell oxidative stress: A potential pathway for racial health disparities. Int. J. Behav. Med. 2012, 19, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Vicente-Salar, N.; Herranz, M.; Micol, V.; Walther, G.; Perez-Martin, A.; Vinet, A.; Roche, E. Glutathione-dependent enzyme activities of peripheral blood mononuclear cells decrease during the winter season compared with the summer in normal-weight and severely obese adolescents. J. Physiol. Biochem. 2019, 75, 321–327. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M.; Najafi, M. Dietary total antioxidant capacity (TAC) among candidates for coronary artery bypass grafting (CABG) surgery: Emphasis to possible beneficial role of TAC on serum vitamin D. PLoS ONE 2018, 13, e0208806. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Achari, A.E.; Manna, P.; Jain, S.K. l-Cysteine and Vitamin D Co-Supplementation Alleviates Markers of Musculoskeletal Disorders in Vitamin D-Deficient High-Fat Diet-Fed Mice. Nutrients 2020, 12, 3406. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; Wu, Y.; Burris, T.P.; Chin, W.W.; Suen, C.S. PGC-1 functions as a transcriptional coactivator for the retinoid X receptors. J. Biol. Chem. 2002, 277, 3913–3917. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Umesono, K.; Mangelsdorf, D.J.; Evans, R.M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 1992, 355, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Jusu, S.; Presley, J.F.; Kremer, R. Phosphorylation of Human Retinoid X Receptor alpha at Serine 260 Impairs Its Subcellular Localization, Receptor Interaction, Nuclear Mobility, and 1alpha,25-Dihydroxyvitamin D3-dependent DNA Binding in Ras-transformed Keratinocytes. J. Biol. Chem. 2017, 292, 1490–1509. [Google Scholar] [CrossRef]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, R.V. GlyNAC Supplementation Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Aging Hallmarks, Metabolic Defects, Muscle Strength, Cognitive Decline, and Body Composition: Implications for Healthy Aging. J. Nutr. 2021, 151, 3606–3616. [Google Scholar] [CrossRef] [PubMed]
- Borges-Santos, M.D.; Moreto, F.; Pereira, P.C.; Ming-Yu, Y.; Burini, R.C. Plasma glutathione of HIV(+) patients responded positively and differently to dietary supplementation with cysteine or glutamine. Nutrition 2012, 28, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hsu, J.W.; Jahoor, F.; Sekhar, R.V. Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 2014, 99, 169–177. [Google Scholar] [CrossRef]
- Vidal, K.; Breuille, D.; Serrant, P.; Denis, P.; Glomot, F.; Bechereau, F.; Papet, I. Long-term cysteine fortification impacts cysteine/glutathione homeostasis and food intake in ageing rats. Eur. J. Nutr. 2014, 53, 963–971. [Google Scholar] [CrossRef]
- Sacco, S.M.; Horcajada, M.N.; Offord, E. Phytonutrients for bone health during ageing. Br. J. Clin. Pharmacol. 2013, 75, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, E.; Olerod, G.; Konar, J.; Petzold, M.; Hammarsten, O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 2015, 49, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; De Ross, B.; Gianoudis, J.; Tan, S.Y. Dose-Response Effect of Consuming Commercially Available Eggs on Wintertime Serum 25-Hydroxyvitamin D Concentrations in Young Australian Adults: A 12-Week Randomized Controlled Trial. J. Nutr. 2022, 152, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Clemente Plaza, N.; Reig Garcia-Galbis, M.; Martinez-Espinosa, R.M. Effects of the Usage of l-Cysteine (l-Cys) on Human Health. Molecules 2018, 23, 575. [Google Scholar] [CrossRef]
- Chitapanarux, T.; Tienboon, P.; Pojchamarnwiputh, S.; Leelarungrayub, D. Open-labeled pilot study of cysteine-rich whey protein isolate supplementation for nonalcoholic steatohepatitis patients. J. Gastroenterol. Hepatol. 2009, 24, 1045–1050. [Google Scholar] [CrossRef]
- Sekhar, R.V.; Patel, S.G.; Guthikonda, A.P.; Reid, M.; Balasubramanyam, A.; Taffet, G.E.; Jahoor, F. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am. J. Clin. Nutr. 2011, 94, 847–853. [Google Scholar] [CrossRef]
- Bizzozero, O.A.; Reyes, S.; Ziegler, J.; Smerjac, S. Lipid peroxidation scavengers prevent the carbonylation of cytoskeletal brain proteins induced by glutathione depletion. Neurochem. Res. 2007, 32, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Jersin, R.A.; Jonassen, L.R.; Dankel, S.N. The neutral amino acid transporter SLC7A10 in adipose tissue, obesity and insulin resistance. Front. Cell Dev. Biol. 2022, 10, 974338. [Google Scholar] [CrossRef]
- Steele, M.L.; Fuller, S.; Patel, M.; Kersaitis, C.; Ooi, L.; Munch, G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 2013, 1, 441–445. [Google Scholar] [CrossRef]
- Morales Pantoja, I.E.; Hu, C.L.; Perrone-Bizzozero, N.I.; Zheng, J.; Bizzozero, O.A. Nrf2-dysregulation correlates with reduced synthesis and low glutathione levels in experimental autoimmune encephalomyelitis. J. Neurochem. 2016, 139, 640–650. [Google Scholar] [CrossRef]
- Leiser, S.F.; Miller, R.A. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol. Cell Biol. 2010, 30, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef] [PubMed]
- Leick, L.; Fentz, J.; Bienso, R.S.; Knudsen, J.G.; Jeppesen, J.; Kiens, B.; Wojtaszewski, J.F.; Pilegaard, H. PGC-1alpha is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E456–E465. [Google Scholar] [CrossRef] [PubMed]
- Gannon, N.P.; Schnuck, J.K.; Mermier, C.M.; Conn, C.A.; Vaughan, R.A. trans-Cinnamaldehyde stimulates mitochondrial biogenesis through PGC-1alpha and PPARbeta/delta leading to enhanced GLUT4 expression. Biochimie 2015, 119, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sczelecki, S.; Besse-Patin, A.; Abboud, A.; Kleiner, S.; Laznik-Bogoslavski, D.; Wrann, C.D.; Ruas, J.L.; Haibe-Kains, B.; Estall, J.L. Loss of Pgc-1alpha expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E157–E167. [Google Scholar] [CrossRef] [PubMed]
- Eisele, P.S.; Furrer, R.; Beer, M.; Handschin, C. The PGC-1 coactivators promote an anti-inflammatory environment in skeletal muscle in vivo. Biochem. Biophys. Res. Commun. 2015, 464, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.M.; Peng, F.; Liu, Y.P.; Chai, D.J.; Ning, R.B.; Xu, C.S.; Lin, J.X. Coadministration of VDR and RXR agonists synergistically alleviates atherosclerosis through inhibition of oxidative stress: An in vivo and in vitro study. Atherosclerosis 2016, 251, 273–281. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Mermier, C.M.; Bisoffi, M.; Trujillo, K.A.; Conn, C.A. Dietary stimulators of the PGC-1 superfamily and mitochondrial biosynthesis in skeletal muscle. A mini-review. J. Physiol. Biochem. 2014, 70, 271–284. [Google Scholar] [CrossRef]
- Smith, C.V.; Jones, D.P.; Guenthner, T.M.; Lash, L.H.; Lauterburg, B.H. Compartmentation of glutathione: Implications for the study of toxicity and disease. Toxicol. Appl. Pharmacol. 1996, 140, 1–12. [Google Scholar] [CrossRef]
- Jain, S.K.; Justin Margret, J.; Zachary, A., Jr.; Lally, M.M.; Vanchiere, J.A.; Mhanna, M.J.; Shi, R.; Levine, S.N. The effects of Vitamin D and L-cysteine co-supplementation on circulating bioavailable and total 25-hydroxy-vitamin D, the free/total testosterone ratio, and inflammatory biomarkers in healthy vitamin D-deficient African Americans: A placebo-controlled double-blind clinical trial. BMJ Nutr. Prev. Health 2024, in press. [Google Scholar]
- Foresta, C.; Strapazzon, G.; De Toni, L.; Perilli, L.; Di Mambro, A.; Muciaccia, B.; Sartori, L.; Selice, R. Bone mineral density and testicular failure: Evidence for a role of vitamin D 25-hydroxylase in human testis. J. Clin. Endocrinol. Metab. 2011, 96, E646–E652. [Google Scholar] [CrossRef]
- Blomberg Jensen, M.; Nielsen, J.E.; Jorgensen, A.; Rajpert-De Meyts, E.; Kristensen, D.M.; Jorgensen, N.; Skakkebaek, N.E.; Juul, A.; Leffers, H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 2010, 25, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, P.R.; Suarez, S.M.; Kozak, A.E.; Knoblovits, P. Seasonal Variations in Sex Steroids in a Young Male Population and Their Relationship with Plasma Levels of Vitamin D. World J. Mens Health 2022, 40, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.B.; Almeida, O.P.; Hyde, Z.; Chubb, S.A.; Hankey, G.J.; Jamrozik, K.; Flicker, L. Higher serum free testosterone is associated with better cognitive function in older men, while total testosterone is not. The Health in Men Study. Clin. Endocrinol. 2008, 68, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Cigolini, M.; Deurenberg, P.; Oosterlee, A.; Doornbos, G. Fat distribution, androgens, and metabolism in nonobese women. Am. J. Clin. Nutr. 1989, 50, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Kravchick, S.; Peled, R.; Dorfman, D.; Agulansky, L.; Ben-Dor, D.; Cytron, S. Predictive criteria for prostate cancer detection in men with serum PSA concentration of 2.0 to 4.0 ng/mL. Urology 2005, 66, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Narinx, N.; David, K.; Walravens, J.; Vermeersch, P.; Claessens, F.; Fiers, T.; Lapauw, B.; Antonio, L.; Vanderschueren, D. Role of sex hormone-binding globulin in the free hormone hypothesis and the relevance of free testosterone in androgen physiology. Cell Mol. Life Sci. 2022, 79, 543. [Google Scholar] [CrossRef] [PubMed]
- Laurent, M.R.; Hammond, G.L.; Blokland, M.; Jardi, F.; Antonio, L.; Dubois, V.; Khalil, R.; Sterk, S.S.; Gielen, E.; Decallonne, B.; et al. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: Validation of the free hormone hypothesis. Sci. Rep. 2016, 6, 35539. [Google Scholar] [CrossRef] [PubMed]
- Simo, R.; Saez-Lopez, C.; Barbosa-Desongles, A.; Hernandez, C.; Selva, D.M. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol. Metab. 2015, 26, 376–383. [Google Scholar] [CrossRef]
- Li, H.; Pham, T.; McWhinney, B.C.; Ungerer, J.P.; Pretorius, C.J.; Richard, D.J.; Mortimer, R.H.; d’Emden, M.C.; Richard, K. Sex Hormone Binding Globulin Modifies Testosterone Action and Metabolism in Prostate Cancer Cells. Int. J. Endocrinol. 2016, 2016, 6437585. [Google Scholar] [CrossRef]
- Saez-Lopez, C.; Villena, J.A.; Simo, R.; Selva, D.M. Sex hormone-binding globulin overexpression protects against high-fat diet-induced obesity in transgenic male mice. J. Nutr. Biochem. 2020, 85, 108480. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, N.; Ngo, T.; Smieszek, A.; Bourebaba, L.; Marycz, K. Sex hormone binding globulin as a potential drug candidate for liver-related metabolic disorders treatment. Biomed. Pharmacother. 2022, 153, 113261. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Kushiyama, A.; Sakoda, H.; Fujishiro, M.; Yamamotoya, T.; Nakatsu, Y.; Kikuchi, T.; Kaneko, S.; Tanaka, H.; Asano, T. Protective Effect of Sex Hormone-Binding Globulin against Metabolic Syndrome: In Vitro Evidence Showing Anti-Inflammatory and Lipolytic Effects on Adipocytes and Macrophages. Mediat. Inflamm. 2018, 2018, 3062319. [Google Scholar] [CrossRef] [PubMed]
- Jana, K.; Dutta, A.; Chakraborty, P.; Manna, I.; Firdaus, S.B.; Bandyopadhyay, D.; Chattopadhyay, R.; Chakravarty, B. Alpha-lipoic acid and N-acetylcysteine protects intensive swimming exercise-mediated germ-cell depletion, pro-oxidant generation, and alteration of steroidogenesis in rat testis. Mol. Reprod. Dev. 2014, 81, 833–850. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, M.J.; Park, N.C.; Park, H.J. Effect of N-acetyl-L-cysteine on Testicular Tissue in Busulfan-Induced Dysfunction in the Male Reproductive System. World J. Mens Health 2023, 41, 882–891. [Google Scholar] [CrossRef]
- Boşgelmez, İ.İ.; Güvendik, G. Beneficial Effects of N-Acetyl-L-cysteine or Taurine Pre- or Post-treatments in the Heart, Spleen, Lung, and Testis of Hexavalent Chromium-Exposed Mice. Biol. Trace Elem. Res. 2019, 190, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Huang, H.; Yang, Y.; Yan, T.; Jin, Y.; Cheng, X.; Cui, L. Ameliorative effects of N-acetylcysteine on fluoride-induced oxidative stress and DNA damage in male rats’ testis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 792, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, T.; Shao, J.; Sheng, C.; Hong, Y.; Ying, W.; Xia, W. Antioxidant protects blood-testis barrier against synchrotron radiation X-ray-induced disruption. Spermatogenesis 2015, 5, e1009313. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, G.M.; Nassar, A.Y.; Bakr, M.H.; Mohamed, S.; Nassar, G.A.; Kamel, A.A. Acetylated Oligopeptide and N-acetyl cysteine Protected Against Oxidative Stress, Inflammation, Testicular-Blood Barrier Damage, and Testicular Cell Death in Iron-Overload Rat Model. Appl. Biochem. Biotechnol. 2023, 195, 5053–5071. [Google Scholar] [CrossRef]
- Kemahli, E.; Uyeturk, U.; Cetinkaya, A.; Erimsah, S.; Uyeturk, U.; Gucuk, A. Protective Effects of N-Acetyl Cysteine on Undescended Testis after Orchiopexy: A Rat-model Study. J. Coll. Physicians Surg. Pak. 2023, 33, 319–324. [Google Scholar] [CrossRef]
- Abedi, B.; Tayefi-Nasrabadi, H.; Kianifard, D.; Basaki, M.; Shahbazfar, A.A.; Piri, A.; Dolatyarieslami, M. The effect of co-administration of artemisinin and N-acetyl cysteine on antioxidant status, spermatological parameters and histopathology of testis in adult male mice. Horm. Mol. Biol. Clin. Investig. 2023, 44, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Acer-Demir, T.; Mammadov, M.; Ocbe, P.; Coruhlu, A.; Coskun, D.; Nazik, Y.; Tufekci, I.; Guney, L.H.; Hicsonmez, A. The long term effects of intrascrotal low dose and high dose N-acetylcysteine on testis damage in rat model of testicular torsion. J. Pediatr. Surg. 2020, 55, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Bodur, A.; Alver, A.; Kahraman, C.; Altay, D.U.; Ince, I. Investigation of N-acetylcysteine on contralateral testis tissue injury by experimental testicular torsion: Long-term effect. Am. J. Emerg. Med. 2016, 34, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Liddle, L.; Monaghan, C.; Burleigh, M.C.; Baczynska, K.A.; Muggeridge, D.J.; Easton, C. Reduced nitric oxide synthesis in winter: A potential contributing factor to increased cardiovascular risk. Nitric Oxide 2022, 127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Hussin, A.M.; Calella, P.; Ashor, A.; Shannon, O.M.; Mendes, I.; Stephan, B.C.; Zheng, D.; Hill, T.; Mathers, J.C. Associations between Aging and Vitamin D Status with Whole-Body Nitric Oxide Production and Markers of Endothelial Function. J. Nutr. 2024, 154, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Jain, S. L-cysteine Supplementation Increases Blood Levels of Hydrogen Sulfide and Nitrite, and Decreases Insulin Resistance and Vascular Inflammation in Zucker Diabetic Rats. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), 405. [Google Scholar] [CrossRef]
- Golden, S.H.; Selvin, E.; Cunningham, K.E. Glycaemic status and cardiovascular disease in type 2 diabetes mellitus: Re-visiting glycated haemoglobin targets for cardiovascular disease prevention. Diabetes Obes. Metab. 2007, 9, 792–798. [Google Scholar] [CrossRef]
- Shah, S.; Iqbal, M.; Karam, J.; Salifu, M.; McFarlane, S.I. Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: Pathophysiological insights. Antioxid. Redox Signal 2007, 9, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Oxidative stress and ageing: Is ageing a cysteine deficiency syndrome? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 2355–2372. [Google Scholar] [CrossRef]
- Abu El Maaty, M.A.; Hanafi, R.S.; El-Badawy, S.; Gad, M.Z. Interplay of vitamin D and nitric oxide in post-menopausal knee osteoarthritis. Aging Clin. Exp. Res. 2014, 26, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Szczurek, M.; Hassan, C.; Masrur, M.; Gangemi, A.; Phillips, S.A. Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients. Nutrients 2019, 11, 2521. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Slavic, S.; Zeitz, U.; Riesen, S.C.; Heppelmann, M.S.; Ambrisko, T.D.; Markovic, M.; Kuebler, W.M.; Erben, R.G. Vitamin D is a regulator of endothelial nitric oxide synthase and arterial stiffness in mice. Mol. Endocrinol. 2014, 28, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.T.; Jablonski, N.G.; Ferguson, S.B.; Alexander, L.M.; Kenney, W.L. Four weeks of vitamin D supplementation improves nitric oxide-mediated microvascular function in college-aged African Americans. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H906–H914. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Bukhari, I.; Yakout, S.M.; Sabico, S.; Khattak, M.N.K.; Aziz, I.; Alokail, M.S. Associations of Serum Nitric Oxide with Vitamin D and Other Metabolic Factors in Apparently Healthy Adolescents. Biomed. Res. Int. 2018, 2018, 1489132. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Cordova, A.; Caballero-Garcia, A.; Noriega-Gonzalez, D.; Bello, H.J.; Pons, A.; Roche, E. Nitric-Oxide-Inducing Factors on Vitamin D Changes in Older People Susceptible to Suffer from Sarcopenia. Int. J. Environ. Res. Public Health 2022, 19, 5938. [Google Scholar] [CrossRef]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Jia-Ling, S.; Wong, B.S.; Cheung, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004, 90, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Ping, C.Y.; Mok, Y.Y.; Ling, L.; Whiteman, M.; Bhatia, M.; Moore, P.K. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006, 149, 625–634. [Google Scholar] [CrossRef]
- Caballero-Garcia, A.; Pascual-Fernandez, J.; Noriega-Gonzalez, D.C.; Bello, H.J.; Pons-Biescas, A.; Roche, E.; Cordova-Martinez, A. L-Citrulline Supplementation and Exercise in the Management of Sarcopenia. Nutrients 2021, 13, 3133. [Google Scholar] [CrossRef] [PubMed]
- Justin Margret, J.; Jain, S.K. Regulatory Effect of L-Cysteine on Testosterone Biosynthesis Genes in Leydig Cells and THP-1 Monocytes. Physiology 2024, 39, 292. [Google Scholar] [CrossRef]
- Lasram, M.M.; Dhouib, I.B.; Annabi, A.; El Fazaa, S.; Gharbi, N. A review on the possible molecular mechanism of action of N-acetylcysteine against insulin resistance and type-2 diabetes development. Clin. Biochem. 2015, 48, 1200–1208. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Jain, S.K. L-Cysteine supplementation reduces high-glucose and ketone-induced adhesion of monocytes to endothelial cells by inhibiting ROS. Mol. Cell Biochem. 2014, 391, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, K.; Braakhuis, A. Performance and Side Effects of Supplementation with N-Acetylcysteine: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Marcinowska-Suchowierska, E.; Kupisz-Urbanska, M.; Lukaszkiewicz, J.; Pludowski, P.; Jones, G. Vitamin D Toxicity-A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]

| Subject Subjects | Sample Size (n) | Purpose/Hypothesis | Outcome | Reference |
|---|---|---|---|---|
| Obese and lean adults | 63 20 obese and 20 lean women 17 obese adults 6 lean women | VD-metabolizing enzymes were expressed differently in AT of lean and obese individuals and visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), and their expression was influenced by weight loss. | VD-metabolizing enzyme expression differed within different ATs. CYP27B1 ↓ in SAT of the obese. CYP27A1 ↑ after weight loss. | [39] |
| Obese Italian men | 121 54—non-obese 67—obese | To determine whether the trafficking of VD is altered in dysfunctional AT. | Dysfunctional AT shows a reduced catecholamine-induced release of D3 and 25(OH)D3 and altered activity of VD-metabolizing enzymes. | [40] |
| Obese Iranian patients | 91 35—non-obese 33—morbidly obese 23—obese | To illustrate the determinants of VDR gene expression in visceral and subcutaneous adipose tissue among individuals without diabetes. | VDR ↓ in obese subjects and is negatively associated with 25(OH)D; positively associated with HOMA-IR. | [41] |
| Obese female patients and HFD mice | Human—4 women Mice—23 (M, 11; F, 12) | To explore the relationship between obesity and CYP2R1 gene expression in human and mouse tissues. | CYP2R1 expression is regulated by energy homeostasis in both humans and mice. CYP2R1 ↓. | [10] |
| Hungarian adults | 462 (M, 228; F, 234) | To investigate the relationship between BMI and genetic polymorphism of VD metabolizing genes. | Two SNPs in CYP2R1 and VDR showed significant association with BMI. | [38] |
| Non-diabetic obese/overweight Brazilian adolescents | 174 (MS, 48; non-MS, 126) | To investigate the associations of CYP2R1 and VDR variants with MS and MS components in non-diabetic Brazilian adolescents. | SNPs are associated with increased risks of diabetes and hypertension in overweight/obese subjects. rs12794714 in CYP2R1 is associated with MS and could be a possible new marker for predicting the risk of MS. | [42] |
| Obese Saudi women | 100 (31 non-obese; 69 obese) | Testing the associations and the mechanisms involved in the silencing of the CYP2R1 gene in normal and obese Saudi female patients. | Hypermethylation of specific sites in CYP2R1 and CYP27B1 regulates gene expression and is linked to obesity and VD metabolism. | [37] |
| Mice/Treatment | Sample Size (n) | Purpose/Hypothesis | Outcome | Reference |
|---|---|---|---|---|
| HFD-induced obese mice and control mice | 28 (14 per group) | To investigate the effects of HFD-induced obesity on VD metabolizing enzyme expression. | HFD-induced obesity influences VD-metabolizing enzyme expression, leading to abnormal regulation of serum 1,25(OH)2D. Cyp2r1, Cyp27a1, Cyp2j3 ↓ in liver; Cyp27b1 ↑, Cyp24 ↓ in kidney. | [43] |
| HFD VD-deficient mice and control mice | 25 (control, 7; 3 treatment groups, 6 each) | Glutathione stimulates VD regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25(OH)VD levels. | HFD downregulates VD metabolism genes, VD+LC supplementation upregulates the gene expression and is a novel and better strategy to increase VD levels. | [44] |
| Female HFD and control mice | 14 (5 per group) | To investigate the alternative mechanism that reduced the capacity to convert parent VD to 25(OH)D due to decreased expression of Cyp2r1. | Cyp2r1 ↓ VD supplementation is less effective in obese subjects. | [11] |
| HFD and control mice | 20 (10 per group) | Obesity disrupts VD homeostasis in key organs of VD metabolism. | Adipose tissue plays a vital role in the modulation of VD metabolism during obesity. Cyp2r1 induction is associated with low VD levels in adipose tissue. | [45] |
| HFD and control mice | 19 (control, 10; HFD, 9) | Nutritional deprivation-responsive mechanisms regulate VD metabolism. | Both fasting and diabetes suppressed hepatic cytochrome P450 Cyp2r1. | [46] |
| HFD and control mice | 4 per group | GSH deficiency induces epigenetic alterations of VD metabolizing genes, thereby reducing the circulating 25(OH)VD3 levels in obesity. | Cyp2r1 ↓ in the mice liver. GSH is a potential adjuvant therapeutic target for normalizing 25(OH)VD3 status in vulnerable populations. | [47] |
| Obese and control mice | 80 (20 per group) | To study the correlation of 25(OH)D3, physiological and pathological changes caused by obesity, and the motility of sperm. | Cyp2r1 ↓ reduces the levels of 25(OH)VD, which interferes with regulating reproductive hormones. | [48] |
| HFD and control mice | 56 (6 groups) Control and HFD with either LVd, CVd, or HVd | Low VD status in obesity decreases the bioavailability of VD to sequestration in adipose tissue. | Excess of body adiposity contributes to lower serum 25(OH)D levels. | [49] |
| High fat and high cholesterol diet mice and control mice | 30 (10 per group) | Diet could impair VD metabolism. | HFD and HCD reduce serum 25(OH)D3 by suppressing hepatic Cyp2r1 ↓. | [50] |
| HFD and control mice | 20 (10 per group) | To investigate the impact of a short-term HFD on VD metabolism. | HFD-induced obesity decreases 25(OH)D and modulates gene expression in VD metabolism. Cyp2r1, Cyp3a11 ↓ in the liver, Cyp24a1, and Cyp27b1↑ in the kidney of obese mice. | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, S.K.; Justin Margret, J.; Abrams, S.A.; Levine, S.N.; Bhusal, K. The Impact of Vitamin D and L-Cysteine Co-Supplementation on Upregulating Glutathione and Vitamin D-Metabolizing Genes and in the Treatment of Circulating 25-Hydroxy Vitamin D Deficiency. Nutrients 2024, 16, 2004. https://doi.org/10.3390/nu16132004
Jain SK, Justin Margret J, Abrams SA, Levine SN, Bhusal K. The Impact of Vitamin D and L-Cysteine Co-Supplementation on Upregulating Glutathione and Vitamin D-Metabolizing Genes and in the Treatment of Circulating 25-Hydroxy Vitamin D Deficiency. Nutrients. 2024; 16(13):2004. https://doi.org/10.3390/nu16132004
Chicago/Turabian StyleJain, Sushil K., Jeffrey Justin Margret, Steven A. Abrams, Steven N. Levine, and Kamal Bhusal. 2024. "The Impact of Vitamin D and L-Cysteine Co-Supplementation on Upregulating Glutathione and Vitamin D-Metabolizing Genes and in the Treatment of Circulating 25-Hydroxy Vitamin D Deficiency" Nutrients 16, no. 13: 2004. https://doi.org/10.3390/nu16132004
APA StyleJain, S. K., Justin Margret, J., Abrams, S. A., Levine, S. N., & Bhusal, K. (2024). The Impact of Vitamin D and L-Cysteine Co-Supplementation on Upregulating Glutathione and Vitamin D-Metabolizing Genes and in the Treatment of Circulating 25-Hydroxy Vitamin D Deficiency. Nutrients, 16(13), 2004. https://doi.org/10.3390/nu16132004







