Effectiveness of Psychobiotic Bifidobacterium breve BB05 in Managing Psychosomatic Diarrhea in College Students by Regulating Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled Trial
Highlights
- Bifidobacterium breve BB05 effectively alleviated the symptoms of diarrhea and improved anxiety and depression levels in college students experiencing “psychosomatic” diarrhea.
- BB05 supplementation significantly restored the gut microbiota composition, particularly increasing the abundance of Bifidobacterium and Roseburia.
- Bifidobacterium breve BB05 shows promise as an adjunctive therapy for both gastrointestinal symptoms and mental health issues, highlighting its role as a psychobiotic agent.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Randomization and Masking
2.3. Intervention Procedure and Management
2.4. Questionnaires
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. DNA Extraction and 16S rRNA Sequencing
2.7. Sample Size
2.8. Statistical Analysis
3. Results
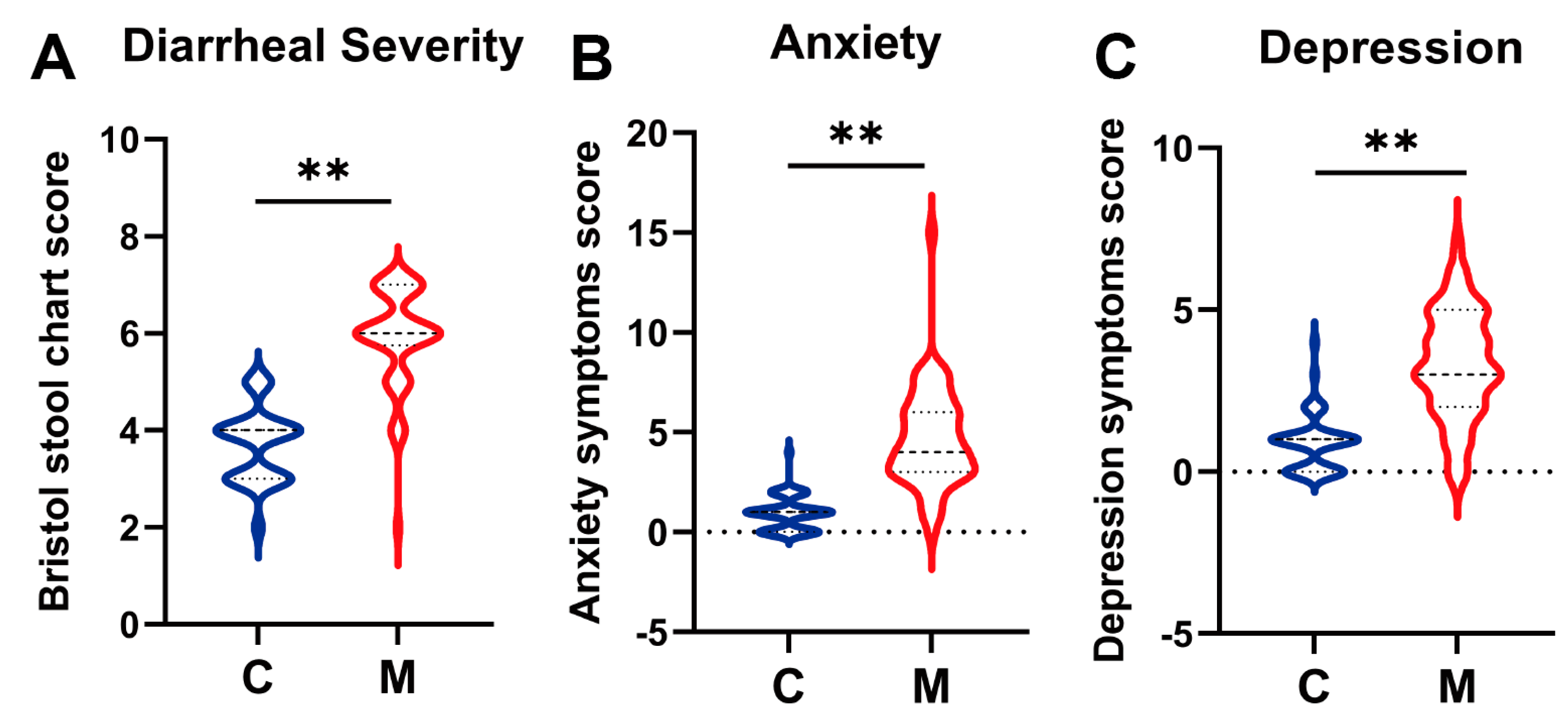
3.1. Diarrhea Affects Mental Health and Gut Microbiota on College Students (Observational Experiment)
3.1.1. Baseline Characteristics and Scales Results of Participants in the Observational Experiment
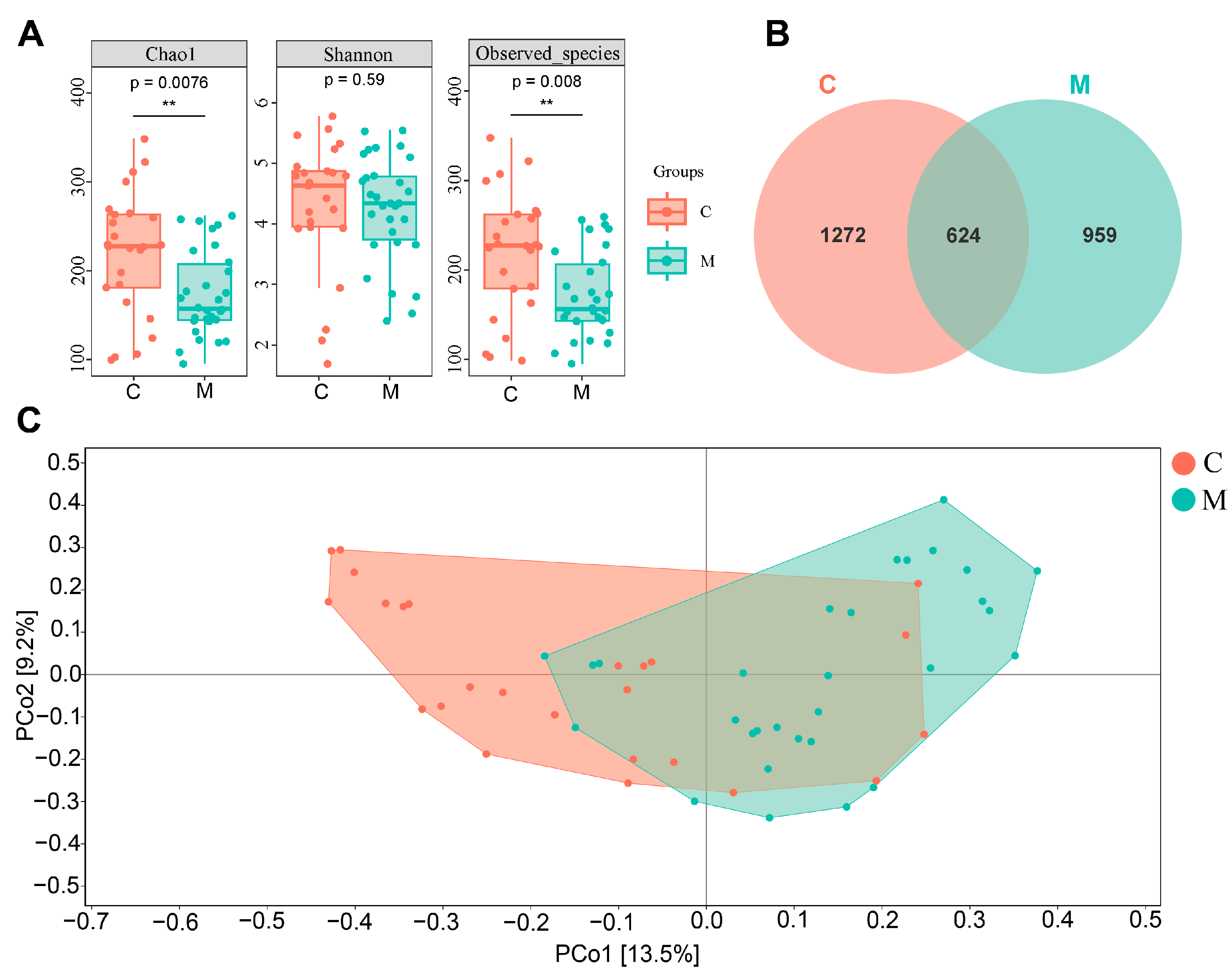
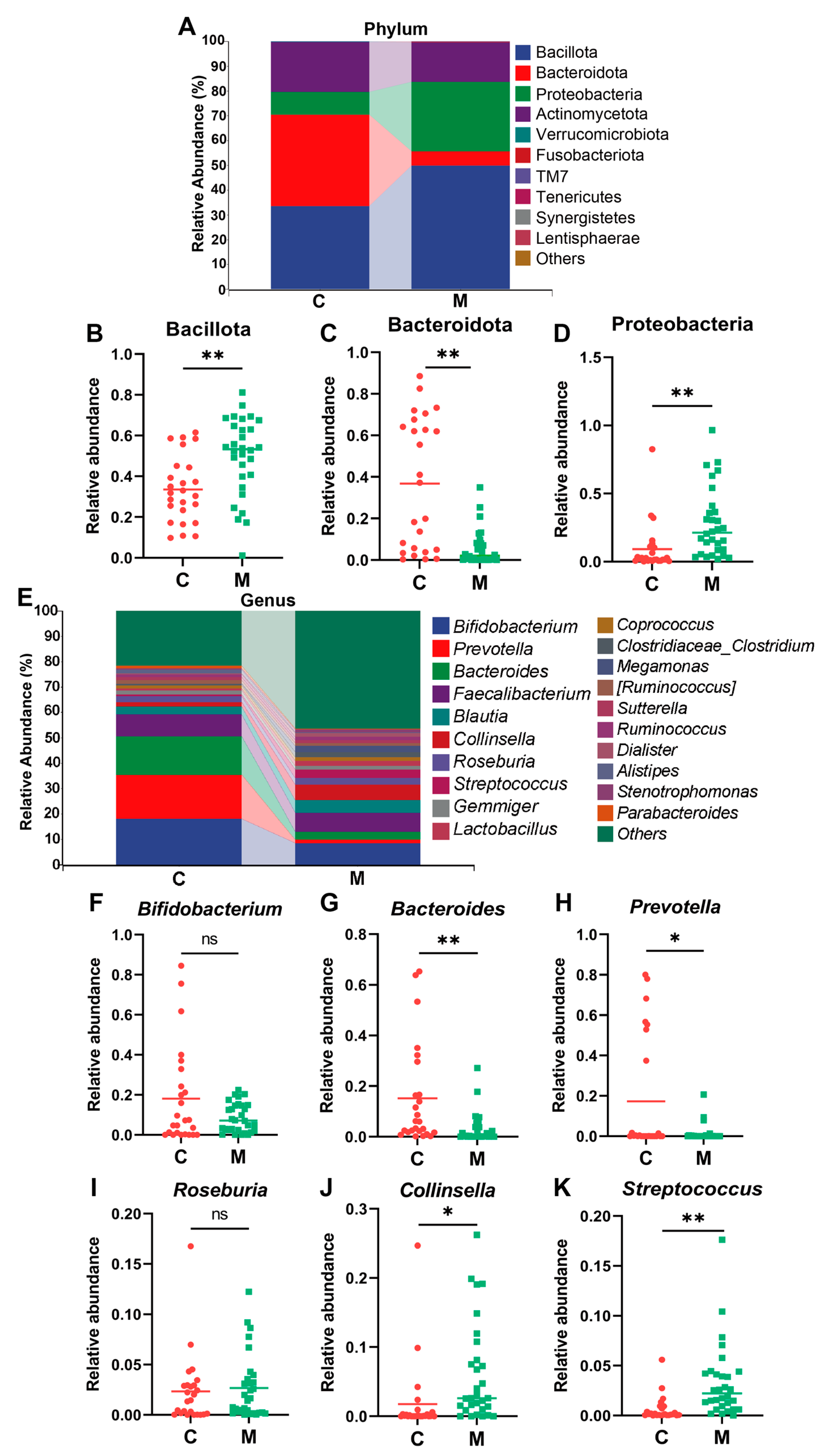
3.1.2. Diarrhea and Perturbance in Gut Microbial Diversity and Composition in College Students
3.2. B. breve BB05 Intervention Improves Gut Dysbiosis and Mental Health in Diarrheal College Students (Intervention Experiment)
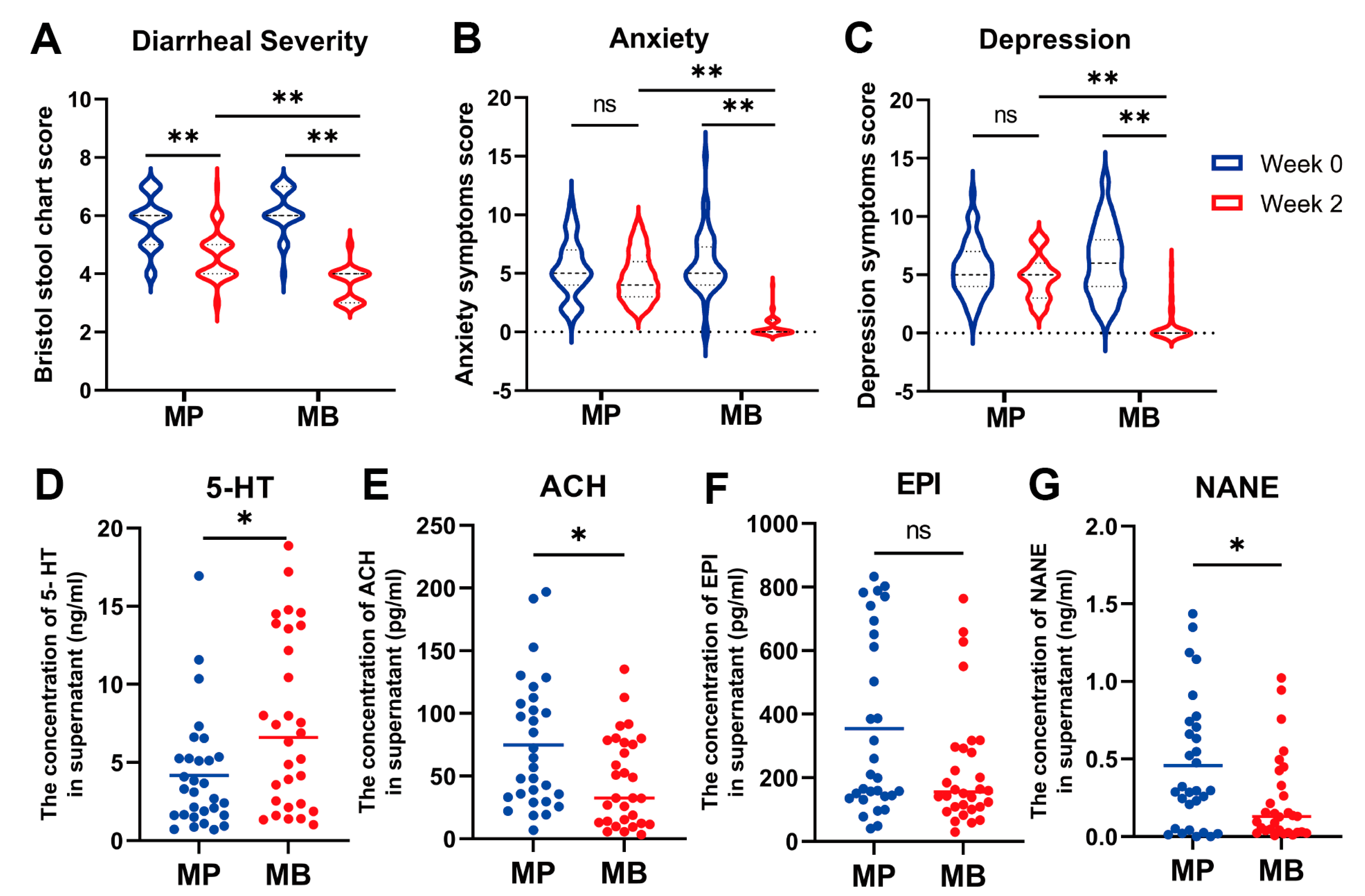
3.2.1. Baseline Characteristics and The impact of B. breve BB05 on Diarrhea Symptoms and Associated Anxiety and Depression
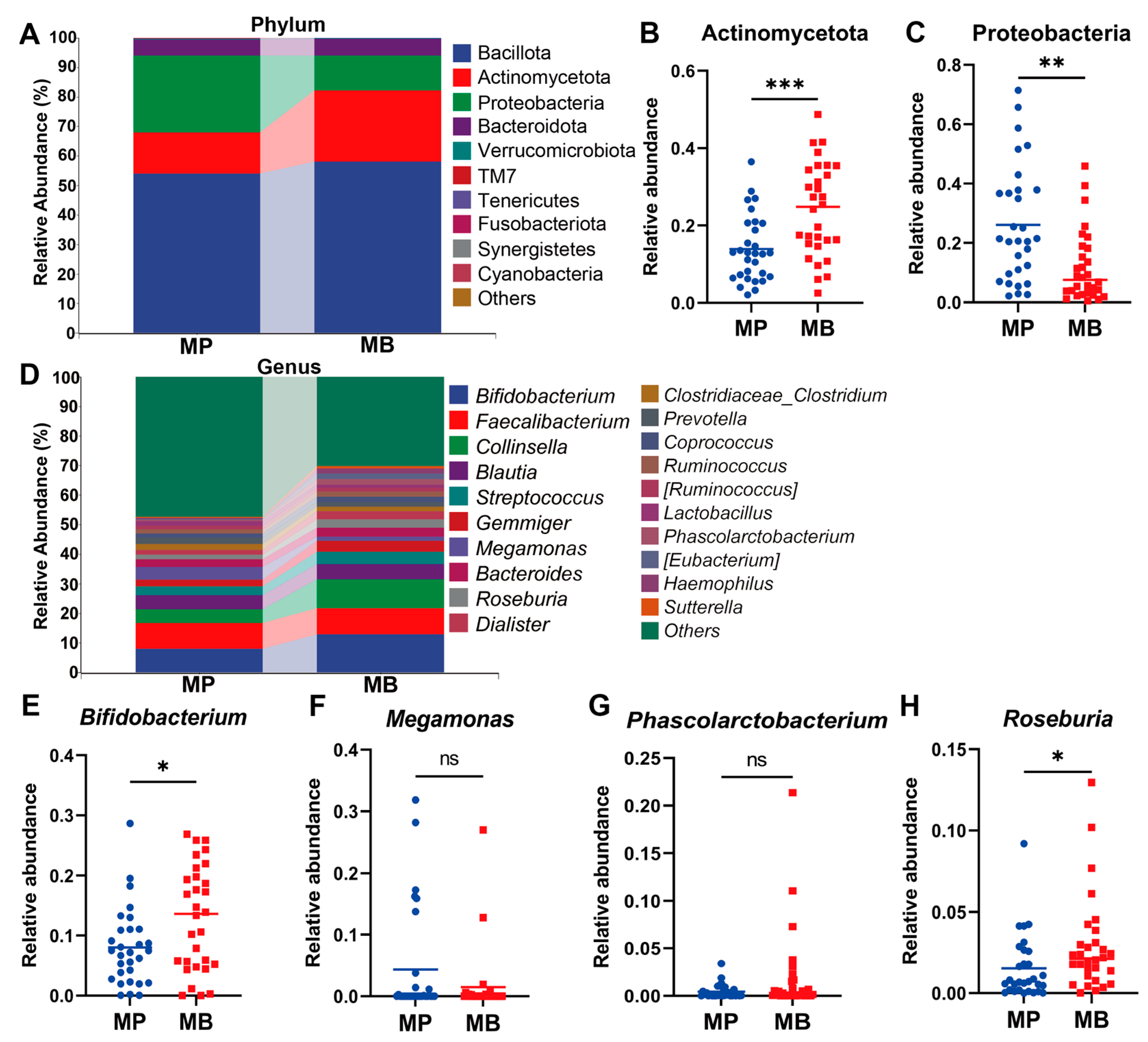
3.2.2. B. breve BB05 Supplement Enriches and Improves the Compromised Gut Microbiota in Diarrheal Students
3.3. Correlation Analysis among Phenotypes, Gut Microbiota, and Related Fecal Neurotransmitters
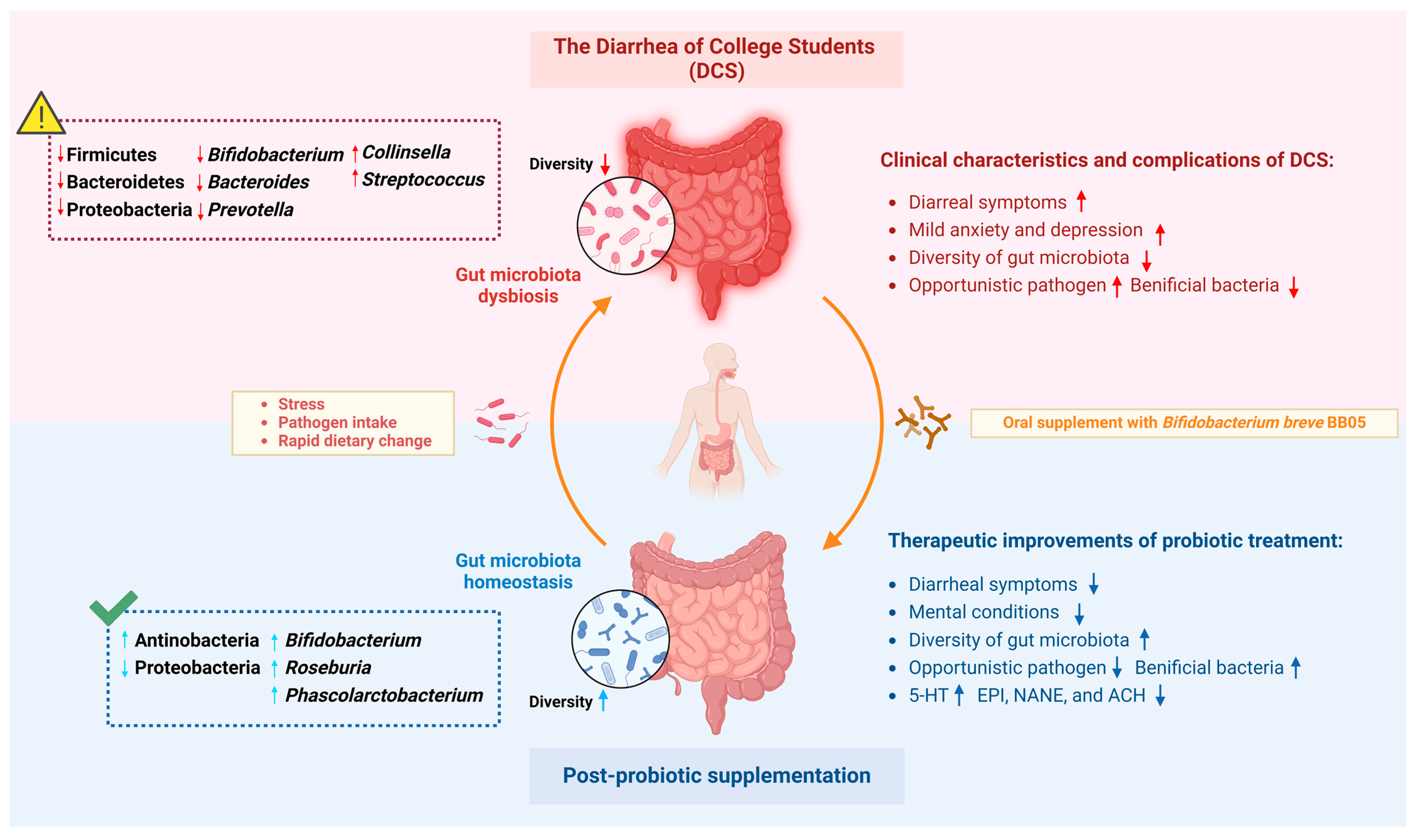
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut microbiota and diarrhea: An updated review. Front. Cell. Infect. Microbiol. 2021, 11, 625210. [Google Scholar] [CrossRef] [PubMed]
- Barr, W.; Smith, A. Acute diarrhea. Am. Fam. Physician 2014, 89, 180–189. [Google Scholar] [PubMed]
- Grindrod, K.A.; Houle, S.K.D.; Fernandes, H. Traveler’s diarrhea. Can. Fam. Physician Med. Fam. Can. 2019, 65, 483–486. [Google Scholar]
- Yates, J. Traveler’s diarrhea. Am. Fam. Physician 2005, 71, 2095–2100. [Google Scholar] [PubMed]
- Riddle, M.S.; DuPont, H.L.; Connor, B.A. ACG Clinical Guideline: Diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am. J. Gastroenterol. 2016, 111, 602–622. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef]
- Fujita, K.; Kaku, M.; Yanagase, Y.; Ezaki, T.; Furuse, K.; Ozawa, A.; Saidi, S.M.; Sang, W.K.; Waiyaki, P.G. Physicochemical characteristics and flora of diarrhoeal and recovery faeces in children with acute gastro-enteritis in Kenya. Ann. Trop. Paediatr. 1990, 10, 339–345. [Google Scholar] [CrossRef]
- Monira, S.; Nakamura, S.; Gotoh, K.; Izutsu, K.; Watanabe, H.; Alam, N.H.; Nakaya, T.; Horii, T.; Ali, S.I.; Iida, T.; et al. Metagenomic profile of gut microbiota in children during cholera and recovery. Gut Pathog. 2013, 5, 1. [Google Scholar] [CrossRef]
- Goralczyk-Binkowska, A.; Szmajda-Krygier, D.; Kozlowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- Huang, F.; Wu, X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021, 9, 649103. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Guevarra, R.B.; Kim, Y.T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Lo Vecchio, A.; Shamir, R.; Szajewska, H. European society for pediatric gastroenterology, hepatology, and nutrition/European society for pediatric infectious diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: Update 2014. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jin, S.; Ma, Y.; Cai, L.; Xu, P.; Nie, Y.; Luo, L.; Yu, Q.; Shen, Y.; Ma, W.; et al. Adjunctive efficacy of Lactis XLTG11 for Acute diarrhea in children: A randomized, blinded, placebo-controlled study. Nutrition 2023, 111, 112052. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Tanabe, Y.; Sakai, H.; Matsumoto, K.; Shimomura, A.; Doi, M.; Miyoshi, Y.; Takahashi, M.; Sagara, Y.; Tokunaga, S.; et al. Efficacy of probiotics and trimebutine maleate for abemaciclib-induced diarrhea: A randomized, open-label phase II trial (MERMAID, WJOG11318B). Breast 2023, 71, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Rau, S.; Gregg, A.; Yaceczko, S.; Limketkai, B. Prebiotics and Probiotics for Gastrointestinal Disorders. Nutrients 2024, 16, 778. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, M.; Zheng, L.; Cen, Q.; Wang, F.; Zhu, L.; Pang, R.; Zhang, A. Bifidobacterium: A probiotic for the prevention and treatment of depression. Front. Microbiol. 2023, 14, 1174800. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Vork, L.; Wilms, E.; Penders, J.; Jonkers, D. Stool consistency: Looking beyond the bristol stool form scale. J. Neurogastroenterol. Motil. 2019, 25, 625. [Google Scholar] [CrossRef]
- Ren, W.; Qiu, H.; Yang, Y.; Zhu, X.; Zhu, C.; Mao, G.; Mao, S.; Lin, Y.; Shen, S.; Li, C.; et al. Randomized controlled trial of cognitive behavioural therapy for depressive and anxiety symptoms in Chinese women with breast cancer. Psychiatry Res. 2019, 271, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.S.; Watt, K.A.; Albery, G.F.; Morgan, E.R.; Dungait, J.A.J. Lactoferrin quantification in cattle faeces by ELISA. PeerJ 2020, 8, e8631. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.H.; Chiu, C.H.; Kong, M.S.; Chang, C.J.; Chen, C.C. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients 2019, 11, 1150. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lim, M.H. Psychological factors to predict chronic diarrhea and constipation in Korean high school students. Medicine 2021, 100, e26442. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Nee, J. Role of diet in diarrhea-predominant irritable bowel syndrome. J. Clin. Gastroenterol. 2021, 55, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Werner-Seidler, A.; Perry, Y.; Calear, A.L.; Newby, J.M.; Christensen, H. School-based depression and anxiety prevention programs for young people: A systematic review and meta-analysis. Clin. Psychol. Rev. 2017, 51, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The gut-brain axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Sharp, R. The Hamilton rating scale for depression. Occup. Med. 2015, 65, 340. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Y.; Wu, Y.; Yang, H.; Zhu, P.; Yan, F.; Zhao, R.; Tian, P.; Wang, T.; Fan, Q.; et al. Medicinal herbs for the treatment of anxiety: A systematic review and network meta-analysis. Pharmacol. Res. 2022, 179, 106204. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Passaro, G.; Gasbarrini, A.; Landolfi, R.; Montalto, M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J. Gastroenterol. 2016, 22, 7186–7202. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Shin, S.H.; Shin, H.S. Immunomodulatory effects of Bifidobacterium spp. and Use of Bifidobacterium breve and Bifidobacterium longum on acute diarrhea in children. J. Microbiol. Biotechnol. 2022, 32, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Q.; Zhao, Y.; Zou, Y.; Chen, M.; Zhou, S.; Wang, Z. The relationship of Megamonas species with nonalcoholic fatty liver disease in children and adolescents revealed by metagenomics of gut microbiota. Sci. Rep. 2022, 12, 22001. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. Gut microbiome and modulation of CNS function. Compr. Physiol. 2019, 10, 57–72. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor-Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sanmarco, L.M.; Wheeler, M.A.; Gutiérrez-Vázquez, C.; Polonio, C.M.; Linnerbauer, M.; Pinho-Ribeiro, F.A.; Li, Z.; Giovannoni, F.; Batterman, K.V.; Scalisi, G.; et al. Gut-licensed IFNγ(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature 2021, 590, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, Z.; Frazer, G.; Ferro, A.; Clare, S.; Bouladoux, N.; Ferdinand, J.; Tuong, Z.K.; Negro-Demontel, M.L.; Kumar, N.; Suchanek, O.; et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature 2020, 587, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e1617. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Dooling, S.W.; Sgritta, M.; Noecker, C.; Murillo, O.D.; Felice, D.F.; Turnbaugh, P.J.; Costa-Mattioli, M. Dissecting the contribution of host genetics and the microbiome in complex behaviors. Cell 2021, 184, 1740–1756.e1716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Q.; Liu, X. The microbiota-gut-brain axis and neurodevelopmental disorders. Protein Cell 2023, 14, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, J.J.; Stednitz, S.J.; Grice, M.Z.; Zaidan, D.; Massaquoi, M.S.; Larsch, J.; Tallafuss, A.; Guillemin, K.; Washbourne, P.; Eisen, J.S. The microbiota promotes social behavior by modulating microglial remodeling of forebrain neurons. PLoS Biol. 2022, 20, e3001838. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Turco, F.; Iannotta, M.; De Gregorio, D.; Palumbo, I.; Sarnelli, G.; Furiano, A.; Napolitano, F.; Boccella, S.; Luongo, L.; et al. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018, 67, 230–245. [Google Scholar] [CrossRef]
- Sushma, G.; Vaidya, B.; Sharma, S.; Devabattula, G.; Bishnoi, M.; Kondepudi, K.K.; Sharma, S.S. Bifidobacterium breve Bif11 supplementation improves depression-related neurobehavioural and neuroinflammatory changes in the mouse. Neuropharmacology 2023, 229, 109480. [Google Scholar] [CrossRef]
- Baldwin, D.; Rudge, S. The role of serotonin in depression and anxiety. Int. Clin. Psychopharmacol. 1995, 9 (Suppl. S4), 41–45. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Picciotto, M.R. The role of acetylcholine in negative encoding bias: Too much of a good thing? Eur. J. Neurosci. 2021, 53, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhou, Q.; Cao, Y.; Shi, H.; Wu, H.; Zhang, B.; Huang, F.; Wu, X. P2Y(12) deficiency in mouse impairs noradrenergic system in brain, and alters anxiety-like neurobehavior and memory. Genes. Brain Behav. 2019, 18, e12458. [Google Scholar] [CrossRef] [PubMed]
- Sobko, T.; Liang, S.; Cheng, W.H.G.; Tun, H.M. Impact of outdoor nature-related activities on gut microbiota, fecal serotonin, and perceived stress in preschool children: The Play&Grow randomized controlled trial. Sci. Rep. 2020, 10, 21993. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khalil, A.A.; Rahman, U.U.; Khalid, A.; Naz, S.; Shariati, M.A.; Rebezov, M.; Urtecho, E.Z.; de Albuquerque, R.; Anwar, S.; et al. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6034–6054. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Z.; Wang, Y.; Zhang, K.; Li, J.; Zhou, L.; Li, B. Milk-derived Lactobacillus with high production of short-chain fatty acids relieves antibiotic-induced diarrhea in mice. Food Funct. 2024, 15, 5329–5342. [Google Scholar] [CrossRef]
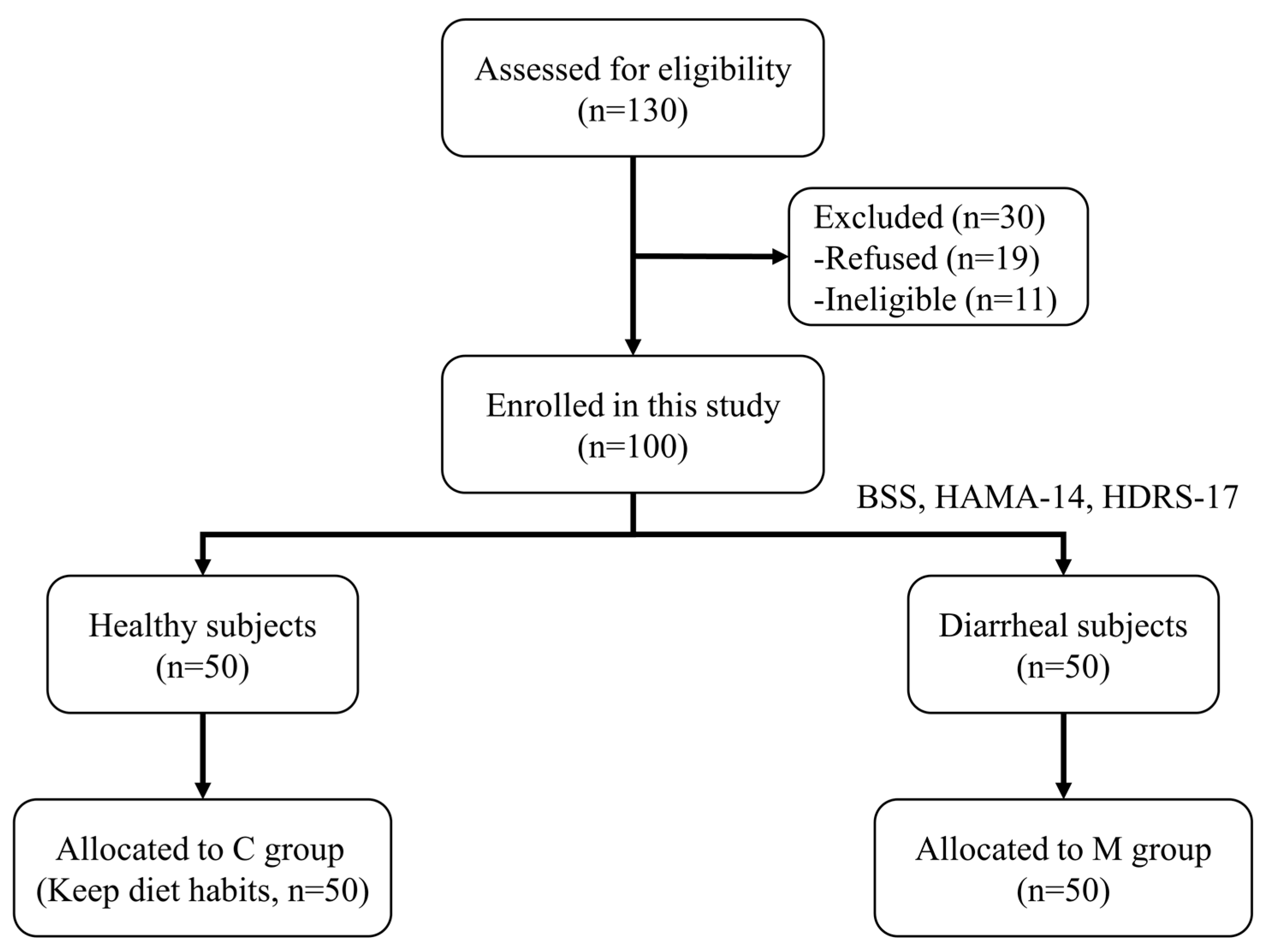



| Characteristics | C Group (n = 50) | M Group (n = 50) | p Value |
|---|---|---|---|
| Age | 19.70 ± 0.92 | 19.4 ± 0.86 | / |
| BMI | 22.34 ± 1.97 | 22.58 ± 1.19 | / |
| Female:Male (n:n) | 1:1 (25.00:25.00) | 1:1 (25.00:25.00) | / |
| HAMA-14 a | 1.00 ± 0.91 | 4.60 ± 3.03 ** | <0.01 |
| HDRS-17 b | 0.86 ± 0.93 | 3.33 ± 1.88 ** | <0.01 |
| BSS c | 3.70 ± 0.76 | 5.90 ± 1.03 ** | <0.01 |
| Characteristics | MP Group (n = 50) | p Value | MB Group (n = 50) | p Value | ||
|---|---|---|---|---|---|---|
| Week 0 | Week 2 | Week 0 | Week 2 | |||
| Age | 19.43 ± 1.04 | / | 19.63 ± 0.81 | / | ||
| BMI | 22.40 ± 1.72 | / | 21.77 ± 1.64 | / | ||
| Female:Male (n:n) | 1:1 (25.00:25.00) | / | 1:1 (25.00:25.00) | / | ||
| HAMA-14 a | 5.42 ± 2.41 | 4.74 ± 1.99 ns | 0.1314 | 5.86 ± 2.65 | 0.38 ± 0.75 ** | <0.01 |
| HDRS-17 b | 5.50 ± 2.32 | 4.70 ± 1.81 ns | 0.0865 | 6.12 ± 2.98 | 0.58 ± 1.37 ** | <0.01 |
| BSS c | 5.78 ± 0.91 | 4.62 ± 0.83 *** | <0.001 | 6.10 ± 0.76 | 3.66 ± 0.59 ** | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Ding, K.; Liu, Y.; Liu, D.; Chen, W.; Zhang, X.; Luo, C.; Zhang, H.; Xu, T.; et al. Effectiveness of Psychobiotic Bifidobacterium breve BB05 in Managing Psychosomatic Diarrhea in College Students by Regulating Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 1989. https://doi.org/10.3390/nu16131989
Wang Y, Wang Y, Ding K, Liu Y, Liu D, Chen W, Zhang X, Luo C, Zhang H, Xu T, et al. Effectiveness of Psychobiotic Bifidobacterium breve BB05 in Managing Psychosomatic Diarrhea in College Students by Regulating Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024; 16(13):1989. https://doi.org/10.3390/nu16131989
Chicago/Turabian StyleWang, Yufan, Yufei Wang, Kunpeng Ding, Yuhan Liu, Dingming Liu, Weijun Chen, Xinyi Zhang, Chuanlin Luo, Hongyan Zhang, Tangchang Xu, and et al. 2024. "Effectiveness of Psychobiotic Bifidobacterium breve BB05 in Managing Psychosomatic Diarrhea in College Students by Regulating Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 16, no. 13: 1989. https://doi.org/10.3390/nu16131989
APA StyleWang, Y., Wang, Y., Ding, K., Liu, Y., Liu, D., Chen, W., Zhang, X., Luo, C., Zhang, H., Xu, T., & Chen, T. (2024). Effectiveness of Psychobiotic Bifidobacterium breve BB05 in Managing Psychosomatic Diarrhea in College Students by Regulating Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients, 16(13), 1989. https://doi.org/10.3390/nu16131989









