Hand Grip Force–Time Curve Indicators Evaluated by Dynamometer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process, Data Collection Process, and Data Items
2.5. Study Risk of Bias Assessment
3. Results
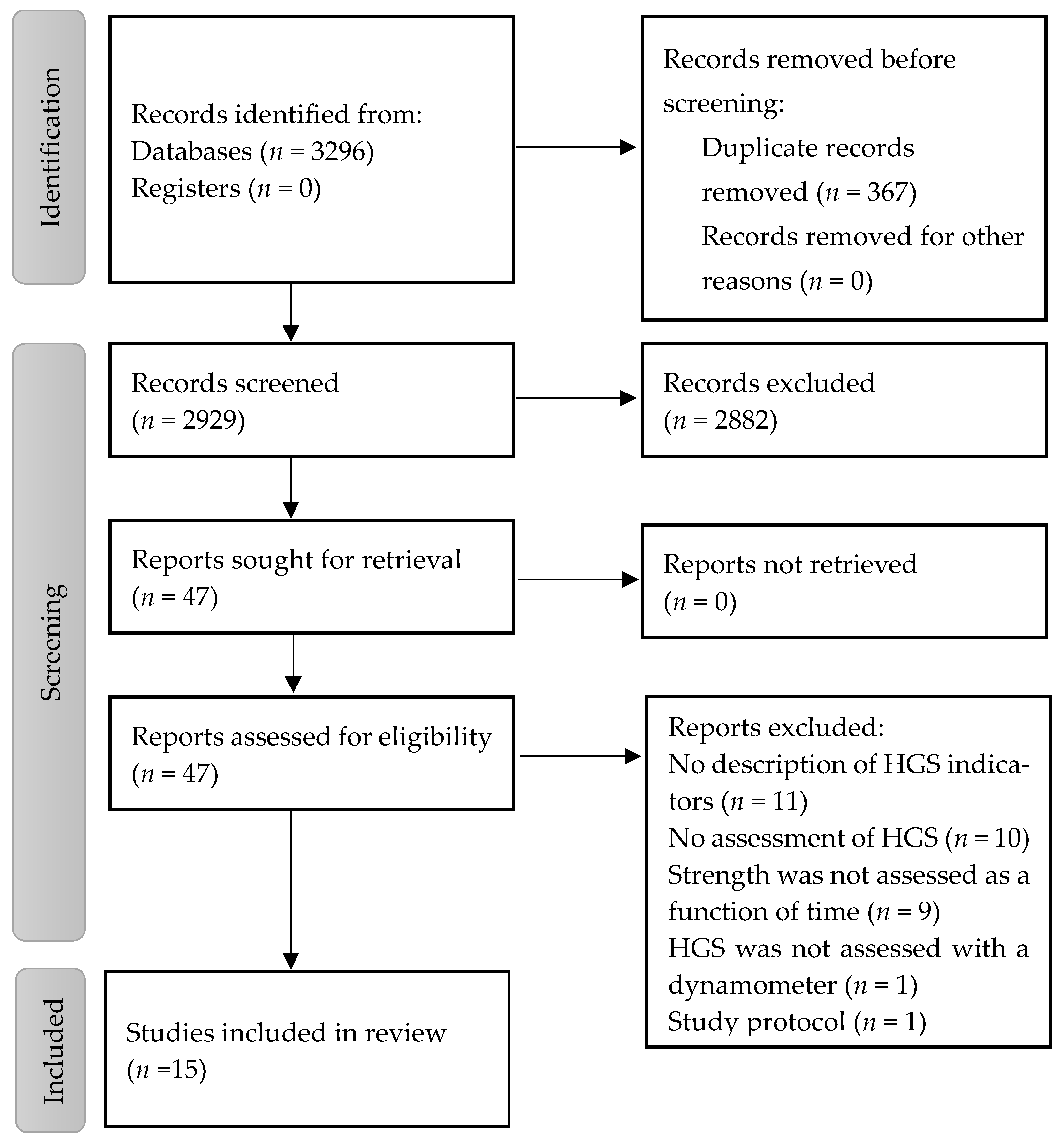
3.1. Study Selection
3.2. Study Characteristics
3.3. Participants Characteristics
| Study Author (year), Country | Participants | Health Condition | Study Design | Dynamometer Model and Type | Grip Duration | Number of Repeated Tests | Rest Time | Indicators | Study Quality |
|---|---|---|---|---|---|---|---|---|---|
| P Helliwell [39] (1987) UK | Group A: n = 20, most of whom were women #, aged 18–30 years Group B: n= 30 (n = 20 women, n = 10 men), 47–90 years Group C: n= 46 (n = 33 women, n = 13 men), 33–77 years | Healthy group and group of patients with rheumatoid arthritis | Cross-sectional | Extensometer torsion dynamometer | 4.4 s | 2 to 3 | NI | Time to the maximum value T-90% Fatigue rate Grip fatigue (%) Release rate Power factor | 3/10 ‡ |
| S N Chengalur [55] (1990) USA | n = 60 (n = 30 men, mean age 27.2 years, and n = 30 women, 28.5 years) | Upper extremity injuries | Quasi-experimental | Modified Jamar adjusted | 5 s | 3 | 1 min | Plateau coefficient of variation | Serious risk § |
| Y Ikemoto [54] (2006) Japan | n = 30 #, mean age of men 21.9 years and mean age of women 21.4 years | Healthy | Cross-sectional * | Digital hand dynamometer (EG-290, Sakai, Japan) | 5 s | 3 | 1 min | Time to the maximum value T-90% Inflection point Int0.25 s, Int0.5 s, Int1 s | 5/10 ‡ |
| S Demura [53] (2008) Japan | n = 10 men, 20–26 years | Healthy | Cross-sectional | Digital hand dynamometer (EG-290, Sakai, Japan) | NI | 12, 15, 20, and 30 grips per minute | 5, 4, 3, and 2 s | Time to 80% maximal voluntary contraction Average integrated area Final force value Inflection Time | 6/10 ‡ |
| I J Baguley [56] (2010) Australia | n = 5 (n = 2 men and n = 3 women), mean age 54 years | Healthy and post-stroke patients | Pilot | Computerized hand dynamometer (G100 Precision Dynamometer; Biometrics Pty Ltd.; Jamar configuration) | NA | Hand grasp and release during repetitive maximal force generation. 10 cycles | NI | Cycle time Time between cycles Maximum force velocity Minimum force velocity Grip work | Serious risk § |
| Z D Alkurdi [42] (2010) Jordan | n = 20 men students | Healthy | Cross-sectional | Grip force transducers attached to a power laboratory | As long as possible | 1 | 2 min | 50% fatigue | 4/9 ‡ |
| K Watanabe [52] (2011) Japan | n = 57 (n = 30 healthy young women, mean age 22.3 years, and n = 27 healthy older women, mean age 78.5 years) | Healthy | Cross-sectional | Digital hand dynamometer (EG-210, Smedley type; Sakai Co. Ltd., Chiyoda-ku, Tokyo) | 6 s | 3 | 3 min | Maximal rate of grip force development Rate of grip force development | 5/9 ‡ |
| H Barden [57] (2012) Australia | Patients: n= 36 #, mean age 50 years. Control participants: n = 27 #, mean age 40 years | Upper motor neuron syndrome following acquired brain injury | Quasi-experimental | Jamar-style Biometrics G100 Precision Dynamometer. The raw dynamometer signal was sampled at 400 Hz and amplified through a general-purpose amplifier to a Power-Lab 26 | NI | 3 | NI | Cycle duration Grip work Maximum force velocity Minimum force velocity | Serious risk § |
| Y Matsui [51] (2014) Japan | n = 347 patients (n = 205 women and n = 142 men), mean age 75 years | Patients who visited the memory disorders clinic for the first time | Cross-sectional | A newly developed dynamometer device for measuring grip strength (made by IMADA, Toyohashi, Japan) | NI | NI | NI | Time to the maximum value Response time | 5/9 ‡ |
| CE Plant [38] (2015) UK | n = 25 patients (n = 10 women and n = 15 men), mean age 40 years, | Healthy, and patients with a fracture of the distal radius | Quasi-experimental | Tracker Freedom wireless dynamometer (JTECH Medical, Salt Lake City, UT, USA) using the version 5 software | 10 s | 6 | 15 s | Grip fatigue | Serious risk § |
| M LI [37] (2018) USA | n= 19 subjects with muscular complaints (n = 5 women and n = 14 men), mean age 46.6 years; n = 18 subjects without muscular complaints (n = 4 women and n = 14 men), mean age 45.3 years, | Participants with muscular complaints and healthy | Quasi-experimental | Digital hand dynamometer | NI | 3 | NI | Endurance Fatigue level (%) | Serious risk § |
| L De Dobbeleer [44] (2019) Denmark | Group A: n = 175 men, mean age 57.9 years. Group B: n = 100 (n = 41 men, mean age 56.6 years; n = 59 women, 56.8 years) Group C: n = 687 (n = 319 men, mean age 50.1 years; n = 368 women, mean age 50.0 years) | Healthy | Cross-sectional | JD G100 system, consisting of an adjustable hand grip handle (standard JD configuration) equipped with an in-built compression load cell and connected via a strain gauge amplifier to a computer | Maintain maximum pressure for as long as possible | 3 | 30 s | Fatigue resistance Grip work | 6/9 ‡ |
| L Klawitter [40] (2020) USA | n = 13 (n = 7 women and n = 6 men), mean age 70.9 years | NI | Cross-sectional | Biopac hand grip dynamometer and Student Lab software (Biopac; Goleta, CA, USA) | 10 s; as long as possible | 2 | 30 s | Submaximal control 25% Fatigability index | 5/10 ‡ |
| S F Almashaqbeh [43] (2022) Jordan | n = 100 (n = 41 women, mean age 21.8 years, n = 59 men, mean age 22.1 years) | Healthy | Cross-sectional † | Grip force transducer connected to the Power Lab unit (AD Instruments corporation). | Maintain maximum pressure for as long as possible | 7 | 5 min | Fatigue resistance 25% Fatigue resistance 50% Fatigue resistance 75% | 5/10 ‡ |
| L Klawitter [41] (2023) USA | n = 31 (n = 8 women and n = 23 men), mean age 49.1 years | Healthy | Pilot | Biopac hand grip dynamometer and Student Lab software (Biopac; Goleta, CA, USA) | 1, 10 s; as long as possible | 2 | 1 min | Rate of hand grip force development Hand grip fatigability index Submaximal hand grip force control (coefficient of variation) | 4/9 ‡ |
3.4. Hand Grip Strength Measurement
3.5. Hand Grip Strength Indicators
3.6. Risk of Bias in Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Y.; Li, G.; Ferrari, P.; Freisling, H.; Qiao, Y.; Wu, L.; Shao, L.; Ke, C. Associations of handgrip strength with morbidity and all-cause mortality of cardiometabolic multimorbidity. BMC Med. 2022, 20, 191. [Google Scholar] [CrossRef]
- Innes, E. Handgrip strength testing: A review of the literature. Aust. Occup. Ther. J. 1999, 46, 120–140. [Google Scholar] [CrossRef]
- Shaughnessy, K.A.; Hackney, K.J.; Clark, B.C.; Kraemer, W.J.; Terbizan, D.J.; Bailey, R.R.; McGrath, R. A Narrative Review of Handgrip Strength and Cognitive Functioning: Bringing a New Characteristic to Muscle Memory. J. Alzheimers Dis. 2020, 73, 1265–1278. [Google Scholar] [CrossRef]
- McGrath, R.P. Understanding the Feasibility and Validity of Muscle Strength Measurements in Aging Adults. J. Am. Med. Dir. Assoc. 2019, 20, 99–100. [Google Scholar] [CrossRef]
- Clark, B.C. Neuromuscular Changes with Aging and Sarcopenia. J. Frailty Aging 2019, 8, 7–9. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Kutty, V.R.; Lanas, F.; Chen, H.; Xiang, Q.; Qian, Z.; Tang, J.; Noorhassim, I.; et al. Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: A prospective urban rural epidemiologic (PURE) study. J. Cachexia Sarcopenia Muscle 2016, 7, 535–546. [Google Scholar] [CrossRef]
- Rantanen, T.; Masaki, K.; Foley, D.; Izmirlian, G.; White, L.; Guralnik, J.M. Grip strength changes over 27 yr in Japanese-American men. J. Appl. Physiol. 1998, 85, 2047–2053. [Google Scholar] [CrossRef]
- Blair, S.J.; McCormick, E.; Bear-Lehman, J.; Fess, E.E.; Rader, E. Evaluation of impairment of the upper extremity. Clin. Orthop. Relat. Res. 1987, 221, 42–58. [Google Scholar] [CrossRef]
- Swanson, A.B.; Hagert, C.G.; Swanson, G.d. Evaluation of impairment of hand function. J. Hand Surg. 1983, 8, 709–722. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr. Res. 2015, 35, 1031–1039. [Google Scholar] [CrossRef]
- Transon, C.S.; Nitschke, C.K.; McPherson, J.J.; Spaulding, S.J.; Rukamp, G.A.; Anderson, L.; Hecht, P. Grip strength and dexterity in adults with developmental delays. Occup. Ther. Health Care 1989, 6, 215–226. [Google Scholar] [CrossRef]
- Peterson, M.D.; Duchowny, K.; Meng, Q.; Wang, Y.; Chen, X.; Zhao, Y. Low Normalized Grip Strength is a Biomarker for Cardiometabolic Disease and Physical Disabilities Among U.S. and Chinese Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1525–1531. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Ntuk, U.E.; Celis-Morales, C.A.; Mackay, D.F.; Sattar, N.; Pell, J.P.; Gill, J.M.R. Association between grip strength and diabetes prevalence in black, South-Asian, and white European ethnic groups: A cross-sectional analysis of 418 656 participants in the UK Biobank study. Diabet. Med. 2017, 34, 1120–1128. [Google Scholar] [CrossRef]
- Li, D.; Guo, G.; Xia, L.; Yang, X.; Zhang, B.; Liu, F.; Ma, J.; Hu, Z.; Li, Y.; Li, W.; et al. Relative Handgrip Strength Is Inversely Associated with Metabolic Profile and Metabolic Disease in the General Population in China. Front. Physiol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Sayer, A.A.; Syddall, H.E.; Martin, H.J.; Dennison, E.M.; Anderson, F.H.; Cooper, C. Falls, sarcopenia, and growth in early life: Findings from the Hertfordshire cohort study. Am. J. Epidemiol. 2006, 164, 665–671. [Google Scholar] [CrossRef]
- Low Choy, N.L.; Brauer, S.G.; Nitz, J.C. Age-related changes in strength and somatosensation during midlife: Rationale for targeted preventive intervention programs. Ann. N. Y. Acad. Sci. 2007, 1114, 180–193. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Fox, K.M.; Gandra, S.R.; Delmonico, M.J.; Chiou, C.F.; Anthony, M.S.; Sewall, A.; Goodpaster, B.; Satterfield, S.; Cummings, S.R.; et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J. Am. Geriatr. Soc. 2009, 57, 1411–1419. [Google Scholar] [CrossRef]
- Gale, C.R.; Martyn, C.N.; Cooper, C.; Sayer, A.A. Grip strength, body composition, and mortality. Int. J. Epidemiol. 2007, 36, 228–235. [Google Scholar] [CrossRef]
- Cooper, R.; Kuh, D.; Hardy, R. Objectively measured physical capability levels and mortality: Systematic review and meta-analysis. Bmj 2010, 341, c4467. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Barre, L.K.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 2014, 68, 1001–1007. [Google Scholar] [CrossRef]
- Syddall, H.E.; Martin, H.J.; Harwood, R.H.; Cooper, C.; Aihie Sayer, A. The SF-36: A simple, effective measure of mobility-disability for epidemiological studies. J. Nutr. Health Aging 2009, 13, 57–62. [Google Scholar] [CrossRef]
- Sun, S.; Lee, H.; Yim, H.W.; Won, H.S.; Ko, Y.H. The impact of sarcopenia on health-related quality of life in elderly people: Korean National Health and Nutrition Examination Survey. Korean J. Intern. Med. 2019, 34, 877–884. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- McGrath, R.; Tomkinson, G.R.; Clark, B.C.; Cawthon, P.M.; Cesari, M.; Al Snih, S.; Jurivich, D.A.; Hackney, K.J. Assessing Additional Characteristics of Muscle Function With Digital Handgrip Dynamometry and Accelerometry: Framework for a Novel Handgrip Strength Protocol. J. Am. Med. Dir. Assoc. 2021, 22, 2313–2318. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J. Parenter. Enteral Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef]
- Duchateau, J.; Semmler, J.G.; Enoka, R.M. Training adaptations in the behavior of human motor units. J. Appl. Physiol. 2006, 101, 1766–1775. [Google Scholar] [CrossRef]
- Enoka, R.M.; Farina, D. Force Steadiness: From Motor Units to Voluntary Actions. Physiology 2021, 36, 114–130. [Google Scholar] [CrossRef]
- Kim, C.R.; Jeon, Y.J.; Kim, M.C.; Jeong, T.; Koo, W.R. Reference values for hand grip strength in the South Korean population. PLoS ONE 2018, 13, e0195485. [Google Scholar] [CrossRef]
- Sousa-Santos, A.R.; Amaral, T.F. Differences in handgrip strength protocols to identify sarcopenia and frailty—A systematic review. BMC Geriatr. 2017, 17, 238. [Google Scholar] [CrossRef]
- Bohannon, R.W. Muscle strength: Clinical and prognostic value of hand-grip dynamometry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 465–470. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, J. Association between handgrip strength and cognitive impairment in elderly Koreans: A population-based cross-sectional study. J. Phys. Ther. Sci. 2015, 27, 3911–3915. [Google Scholar] [CrossRef]
- McGrath, R.; Johnson, N.; Klawitter, L.; Mahoney, S.; Trautman, K.; Carlson, C.; Rockstad, E.; Hackney, K.J. What are the association patterns between handgrip strength and adverse health conditions? A topical review. SAGE Open Med. 2020, 8, 2050312120910358. [Google Scholar] [CrossRef]
- Gilbert, J.C.; Knowlton, R.G. Simple method to determine sincerity of effort during a maximal isometric test of grip strength. Am. J. Phys. Med. Rehabil. 1983, 62, 135–144. [Google Scholar]
- Kamimura, T.; Ikuta, Y. Evaluation of grip strength with a sustained maximal isometric contraction for 6 and 10 s. J. Rehabil. Med. 2001, 33, 225–229. [Google Scholar]
- Carson, R.G. Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol. Aging 2018, 71, 189–222. [Google Scholar] [CrossRef]
- Li, M.; Yao, W.; Sundahl, C. Motor Unit Number Estimate and Isometric Hand Grip Strength in Military Veterans with or Without Muscular Complaints: Reference Values for Longitudinal Follow-up. Mil. Med. 2018, 183, e399–e404. [Google Scholar] [CrossRef]
- Plant, C.E.; Parsons, N.R.; Edwards, A.T.; Rice, H.; Denninson, K.; Costa, M.L. A comparison of electronic and manual dynamometry and goniometry in patients with fracture of the distal radius and healthy participants. J. Hand Ther. 2016, 29, 73–80, quiz 80. [Google Scholar] [CrossRef]
- Helliwell, P.; Howe, A.; Wright, V. Functional assessment of the hand: Reproducibility, acceptability, and utility of a new system for measuring strength. Ann. Rheum. Dis. 1987, 46, 203–208. [Google Scholar] [CrossRef]
- Klawitter, L.; Mahoney, S.J.; Dahl, L.; Hackney, K.J.; Herrmann, S.D.; Edwards, B.; McGrath, R. Evaluating Additional Aspects of Muscle Function with a Digital Handgrip Dynamometer and Accelerometer for Cognitive Functioning in Older Adults: A Pilot Study. J. Alzheimers Dis. Rep. 2020, 4, 495–499. [Google Scholar] [CrossRef]
- Klawitter, L.A.; Hackney, K.J.; Christensen, B.K.; Hamm, J.M.; Hanson, M.; McGrath, R. Using Electronic Handgrip Dynamometry and Accelerometry to Examine Multiple Aspects of Handgrip Function in Master Endurance Athletes: A Pilot Study. J. Strength. Cond. Res. 2023, 37, 1777–1782. [Google Scholar] [CrossRef]
- Alkurdi, Z.D.; Dweiri, Y.M. A biomechanical assessment of isometric handgrip force and fatigue at different anatomical positions. J. Appl. Biomech. 2010, 26, 123–133. [Google Scholar] [CrossRef]
- Almashaqbeh, S.F.; Al-Momani, S.; Khader, A.; Qananwah, Q.; Marabeh, S.; Maabreh, R.; Al Badarneh, A.; Abdullah, K. The Effect of Gender and Arm Anatomical Position on the Hand Grip Strength and Fatigue Resistance during Sustained Maximal Handgrip Effort. J. Biomed. Phys. Eng. 2022, 12, 171–180. [Google Scholar] [CrossRef]
- De Dobbeleer, L.; Beyer, I.; Hansen, Å.M.; Molbo, D.; Mortensen, E.L.; Lund, R.; Bautmans, I. Grip Work Measurement with the Jamar Dynamometer: Validation of a Simple Equation for Clinical Use. J. Nutr. Health Aging 2019, 23, 221–224. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Su, C.Y.; Lin, J.H.; Chien, T.H.; Cheng, K.F.; Sung, Y.T. Grip Strength in Different Positions of Elbow and Shoulder. Arch. Phys. Med. Rehab. 1994, 75, 812–815. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Bmj 2016, 355, i4919. [Google Scholar] [CrossRef]
- Modesti, P.A.; Reboldi, G.; Cappuccio, F.P.; Agyemang, C.; Remuzzi, G.; Rapi, S.; Perruolo, E.; Parati, G. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0147601. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 August 2023).
- Matsui, Y.; Fujita, R.; Harada, A.; Sakurai, T.; Nemoto, T.; Noda, N.; Toba, K. Association of grip strength and related indices with independence of activities of daily living in older adults, investigated by a newly-developed grip strength measuring device. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S2), 77–86. [Google Scholar] [CrossRef]
- Watanabe, K.; Tsubota, S.; Chin, G.; Aoki, M. Differences in parameters of the explosive grip force test between young and older women. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 554–558. [Google Scholar] [CrossRef][Green Version]
- Demura, S.; Nakada, M.; Yamaji, S.; Nagasawa, Y. Relationships between force-time parameters and muscle oxygenation kinetics during maximal sustained isometric grip and maximal repeated rhythmic grip with different contraction frequencies. J. Physiol. Anthropol. 2008, 27, 161–168. [Google Scholar] [CrossRef]
- Ikemoto, Y.; Demura, S.; Yamaji, S.; Yamada, T. Comparison of force-time parameters and EMG in static explosive gripping by various exertion conditions: Muscle fatigue state and submaximal exertion. J. Sports Med. Phys. Fitness 2006, 46, 381–387. [Google Scholar]
- Chengalur, S.N.; Smith, G.A.; Nelson, R.C.; Sadoff, A.M. Assessing sincerity of effort in maximal grip strength tests. Am. J. Phys. Med. Rehabil. 1990, 69, 148–153. [Google Scholar] [CrossRef]
- Baguley, I.J.; Nott, M.T.; Barder, H.L. The use of computerised dynamometry to quantify functional grip and release in people post stroke: A pilot study. Open Rehabil. J. 2010, 3, 75–82. [Google Scholar] [CrossRef]
- Barden, H.L.; Nott, M.T.; Baguley, I.J.; Heard, R.; Chapparo, C. Test-retest reliability of computerised hand dynamometry in adults with acquired brain injury. Aust. Occup. Ther. J. 2012, 59, 319–327. [Google Scholar] [CrossRef]
- Porta, M. A Dictionary of Epidemiology; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- MacDermid, J.; Solomon, G.; Valdes, K. Clinical Assessment Recommendations: American Society of Hand Therapists; American Society of Hand Therapists: Mount Laurel, NJ, USA, 2015. [Google Scholar]
- Lou, J.S. Techniques in Assessing Fatigue in Neuromuscular Diseases. Phys. Med. Reh Clin. 2012, 23, 11–22. [Google Scholar] [CrossRef]
- Lagerström, C.; Nordgren, B. Methods for measuring maximal isometric grip strength during short and sustained contractions, including intra-rater reliability. Ups. J. Med. Sci. 1996, 101, 273–285. [Google Scholar] [CrossRef]
- Desrosiers, J.; Bravo, G.; Hebert, R. Isometric grip endurance of healthy elderly men and women. Arch. Gerontol. Geriatr. 1997, 24, 75–85. [Google Scholar] [CrossRef]
- Bautmans, I.; Gorus, E.; Njemini, R.; Mets, T. Handgrip performance in relation to self-perceived fatigue, physical functioning and circulating IL-6 in elderly persons without inflammation. BMC Geriatr. 2007, 7, 5. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Mirkov, D.M.; Nedeljkovic, A.; Milanovic, S.; Jaric, S. Muscle strength testing: Evaluation of tests of explosive force production. Eur. J. Appl. Physiol. 2004, 91, 147–154. [Google Scholar] [CrossRef]
- Abe, T.; Loenneke, J.P.; Thiebaud, R.S.; Loftin, M. The Bigger the Hand, the Bigger the Difference? Implications for Testing Strength With 2 Popular Handgrip Dynamometers. J. Sport. Rehabil. 2019, 28, 278–282. [Google Scholar] [CrossRef]
- McGrath, R. Maximal Handgrip Strength Alone Could Be an Incomplete Measure of Muscle Function. J. Am. Med. Dir. Assoc. 2021, 22, 882–883. [Google Scholar] [CrossRef]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of force development: Physiological and methodological considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [Google Scholar] [CrossRef]
- Hamilton, L.D.; Mazzo, M.R.; Petrigna, L.; Ahmed, A.A.; Enoka, R.M. Poor estimates of motor variability are associated with longer grooved pegboard times for middle-aged and older adults. J. Neurophysiol. 2019, 121, 588–601. [Google Scholar] [CrossRef]
- El-Sais, W.M.; Mohammad, W.S. Influence of different testing postures on hand grip strength. Eur. Sci. J. 2014, 10, 290–301. [Google Scholar]
- Parvatikar, V.; Mukkannavar, P. Comparative study of grip strength in different positions of shoulder and elbow with wrist in neutral and extension positions. J. Exerc. Sci. Physiother. 2009, 5, 67–75. [Google Scholar]
| Indicator | Assessment Method |
|---|---|
| Grip fatigue (%) [37,38,39] | Percentage of loss of grip from the maximum grip strength to the point of release of the grip. |
| Fatigability index [40,41] | |
| Fatigue rate [39] | Rate of loss of grip from maximum value to the point of release of grip, expressed in newtons per second. |
| Fatigue resistance 25% [43] Fatigue resistance 50% [42,43,44] Fatigue resistance 75% [43] Time to 80% maximal voluntary contraction [53] | Time required for hand grip strength to decrease to 25%, 50%, 75%, or 80% of its maximum during maximum sustained hand grip effort. |
| Plateau coefficient of variation [55] | Plateau region of the force–time curve from the occurrence of 90% of peak to end of the contraction. |
| Time to maximum value [39,51,54] | The time it takes to reach the maximum. |
| T-90% [39,54] | Time until reaching 90% of the maximum peak value. |
| Release rate [39] | Rate of loss of grip from the point of release to the baseline value, expressed in newtons per second. |
| Power factor [39] | The integral of the grip/time curve, expressed in newton seconds. |
| Grip work [44,56,57] | The area under the curve is calculated by integrating the actual grip force at each time interval (i.e., 0.01 s) based on measurements recorded during the drop in grip force to 50% of its maximum value. t—time interval (at 100 Hz = 0.01 s) |
| Average integrated area [53] | Average of all strength values during sustained isometric grip and repeated rhythmic grip during the stipulated minutes. |
| Endurance [37] | Time that participants maintain the grip on the dynamometer for as long as possible in the range of 70% to 90% of maximum HGS. |
| Cycle duration [56,57] | The duration of time of the cycle from the minimum grip strength until the minimum grip strength is reached again. |
| Time between cycles [56] | Time that passes between repeated cycles of maximum contraction and relaxation. |
| Maximum force velocity [56,57] Minimum force velocity [56,57] | The force–velocity curve (force/time) is the speed at which a person changes their isometric force production during the grip and release of the dynamometer. The area above the horizontal zero line represents the gripping phase, which starts at zero and increases until the maximum force–velocity curve, defined as the point at which peak velocity occurs. The release phase is represented below the horizontal zero line, also starting at zero and decreasing to the minimum level, the point at which release is being generated most quickly. |
| Rate of grip force development (RGFD) | Defined as the slope of the force–time curve at intervals of 10 ms up to 250 ms, with each measurement using point 0 as the baseline [52]. The rate of force development is calculated as peak force (kgs) normalized to time (s) [41]. |
| Maximal rate of grip force development (Max RGFD) [52] | Maximum value of the slope of the force–time curve in each 10 ms interval. |
| Maximal rate of grip force development normalized (Max RGFD) [52] | |
| Final force value [53] | The final force value is the force in a defined time (e.g., 360 s). |
| Inflection point [53,54] | The inflection point of decreasing velocity in the force–time curve distinguishes between a sharp decreasing phase and a nearly stationary phase during repeated rhythmic grips. The time-series-sustained force data were divided into the former and later phases at all combinations and the respective regression lines were calculated. The inflection point was determined at the time from the best-fitting regression lines in the combination of time series data. |
| Int0.25 s Int0.5 s Int1 s [54] | Integrated area during 0.25, 0.5, and 1 s. |
| Submaximal control 25% [40,41] | This is calculated as 25% of the maximum HGS. Participants were asked to squeeze the dynamometer and maintain a submaximal target grip strength of 25%. The coefficient of variation was calculated during the intermediate period of 8 s. |
| Response time [51] | Time from time zero until grip strength begins. |
| Indicator | Main Findings |
|---|---|
| Fatigue Grip fatigue (%) [37,38,39] Fatigability index [40,41] Fatigue rate [39] Fatigue resistance 25% [43] Fatigue resistance 50% [42,43,44] Fatigue resistance 75% [43] Time to 80% maximal voluntary contraction [53] | The level of fatigue (%) in a group with carpal tunnel syndrome was similar to that in healthy individuals and there were no significant correlations with the estimated number of motor units [37]. Grip fatigue in patients with distal radius fracture was related to hand and wrist impairment [38]. Individuals with rheumatoid arthritis showed considerably more fatigue than healthy individuals. In this group of patients, fatigue (%) was independent of maximum HGS; however, the fatigue rate in the healthy group and the individuals with rheumatoid arthritis was closely related to HGS [39]. Assessment of fatigability may be an alternative clinical tool, providing a more accurate identifier of older people at risk of a rapid decline in physical function [44]. |
| Plateau coefficient of variation [55] | In upper extremity injuries, the plateau coefficient of variation distinguished sincere effort from fake effort. The sincere trials typically showed a rapid increase in force, reaching a plateau at near-peak force levels where the force remained relatively constant. In contrast, the faking trials also exhibited a rapid initial increase in force but often included an initial “spike” where the subject overshot the intended force application, resulting in an early peak force [55]. |
| Time to maximum value [39,51,54] | The time to maximum grip strength in a group of patients with rheumatoid arthritis was significantly prolonged compared to the healthy group [39]. The time to the maximum value in patients who visited a memory disorders clinic for the first time was not significant in either gender or in the maintenance of elderly patients’ independence in activities of daily living (assessed by the Barthel Index) [51]. |
| T-90% [39,54] | No information. |
| Release rate [39] | The release rate in the healthy group and group of patients with rheumatoid arthritis was closely related to maximum grip strength and probably did not provide any additional information [39]. |
| Power factor [39] | No information. |
| Grip work [44,56,57] | In post-stroke patients, the degree of grip work performed by the affected upper limb of the post-stroke groups was related to less than the grip work performed by the unaffected upper limb. These findings appear to be typical of adults with negative upper motor neuron features such as weakness, reduced motor control, and fatigue [56]. |
| Average integrated area [53] | The average integrated area was closely related to the final force values [53]. |
| Endurance [37] | Endurance was slightly affected by carpal tunnel syndrome, suggesting that it is a potentially important addition to motor unit number estimation for the longitudinal follow-up of motor neuron function among veterans with carpal tunnel syndrome [37]. |
| Cycle duration [56,57] | Patients with acquired brain injury demonstrate a slower cycle duration, consistent with negative features of upper motor neuron syndrome [57]. In post-stroke patients, the cycle time was longer in both affected and unaffected limbs compared with healthy subjects. The increased time to complete cycles suggests slower motor recruitment patterns when performing handgrip contraction and relaxation in the post-stroke group [56]. |
| Time between cycles [56] | In post-stroke patients, the time between cycles was longer in both affected and unaffected limbs compared with healthy individuals. A longer time between repeated cycles suggests difficulty in resuming the contraction phase of the task after relaxation, potentially due to slower muscle recruitment, biomechanical changes after the previous contraction phase, and/or motor planning difficulties [56]. |
| Maximum force velocity [56,57] Minimum force velocity [56,57] | In post-stroke patients, the time required to reach maximum and minimum velocities (from the onset of muscle contraction) was slower than in healthy individuals. Therefore, the maximum velocity of force (maximum rate of contraction) was severely impaired, coinciding with negative upper motor neuron features such as weakness, reduced motor control, and fatigue. The post-stroke group also showed a longer time to reach maximum relaxation, that is, reduced minimum force velocity [56]. Patients with acquired brain injury had lower maximum and minimum force velocity values when compared to healthy individuals [57]. |
| Rate of grip force Rate of grip force development (RGFD) [41,52] Maximal rate of grip force development (Max RGFD) [52] Maximal rate of grip force development normalized (Max RGFD) [52] | The rate of force development is frequently used to assess muscle force generation. It has important functional significance, as rapid and strong muscle contractions are required in sports and a high rate of contractile force development exerted during the initial phase of muscle contraction can be very important for successful performance. Age-related decreases in grip force generation capacity have been found for maximal RFFD and normalized maximal RFFD, possibly due to altered muscle contraction capacity [52] RGFD was moderately and positively correlated with maximal grip strength in endurance athletes over 35 years of age [41]. |
| Final force value [53] | The final force values were closely related to the average integrated area [53]. |
| Inflection point [53,54] | Physiological factors such as muscle fiber recruitment and muscle oxygenation related to strength effort differ at pre- and post-inflection points [53]. The inflection point is a useful parameter for dividing force and contraction velocity because it relates to the force–time parameters that evaluate the maximum development phase, but not to the peak value. |
| Int0.25 s Int0.5 s Int1 s [54] | The areas integrated up to 0.25 and 0.5 s were related to muscle contraction velocity and potentially assess explosive muscle function [54]. |
| Submaximal control 25% [40,41] | Submaximal strength was moderately and negatively correlated with maximal HGS [41]. |
| Response time [51] | Response time was associated with some items related to activities of daily living (assessed by the Barthel Index) [51]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Santos, T.; Guerra, R.S.; Valdiviesso, R.; Amaral, T.F. Hand Grip Force–Time Curve Indicators Evaluated by Dynamometer: A Systematic Review. Nutrients 2024, 16, 1951. https://doi.org/10.3390/nu16121951
Silva-Santos T, Guerra RS, Valdiviesso R, Amaral TF. Hand Grip Force–Time Curve Indicators Evaluated by Dynamometer: A Systematic Review. Nutrients. 2024; 16(12):1951. https://doi.org/10.3390/nu16121951
Chicago/Turabian StyleSilva-Santos, Tânia, Rita S. Guerra, Rui Valdiviesso, and Teresa F. Amaral. 2024. "Hand Grip Force–Time Curve Indicators Evaluated by Dynamometer: A Systematic Review" Nutrients 16, no. 12: 1951. https://doi.org/10.3390/nu16121951
APA StyleSilva-Santos, T., Guerra, R. S., Valdiviesso, R., & Amaral, T. F. (2024). Hand Grip Force–Time Curve Indicators Evaluated by Dynamometer: A Systematic Review. Nutrients, 16(12), 1951. https://doi.org/10.3390/nu16121951







