Impacts of Central Administration of the Novel Peptide, LEAP-2, in Different Food Intake Models in Conscious Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Implantation of an Intracerebroventricular (ICV) Catheter
2.3. Preparation of Drugs
2.4. Food Intake Analysis
2.5. Experimental Feeding Schedule: Ad Libitum (AL) versus Time-Restricted Feeding (TRF)
2.6. Statistical Analyses
3. Results
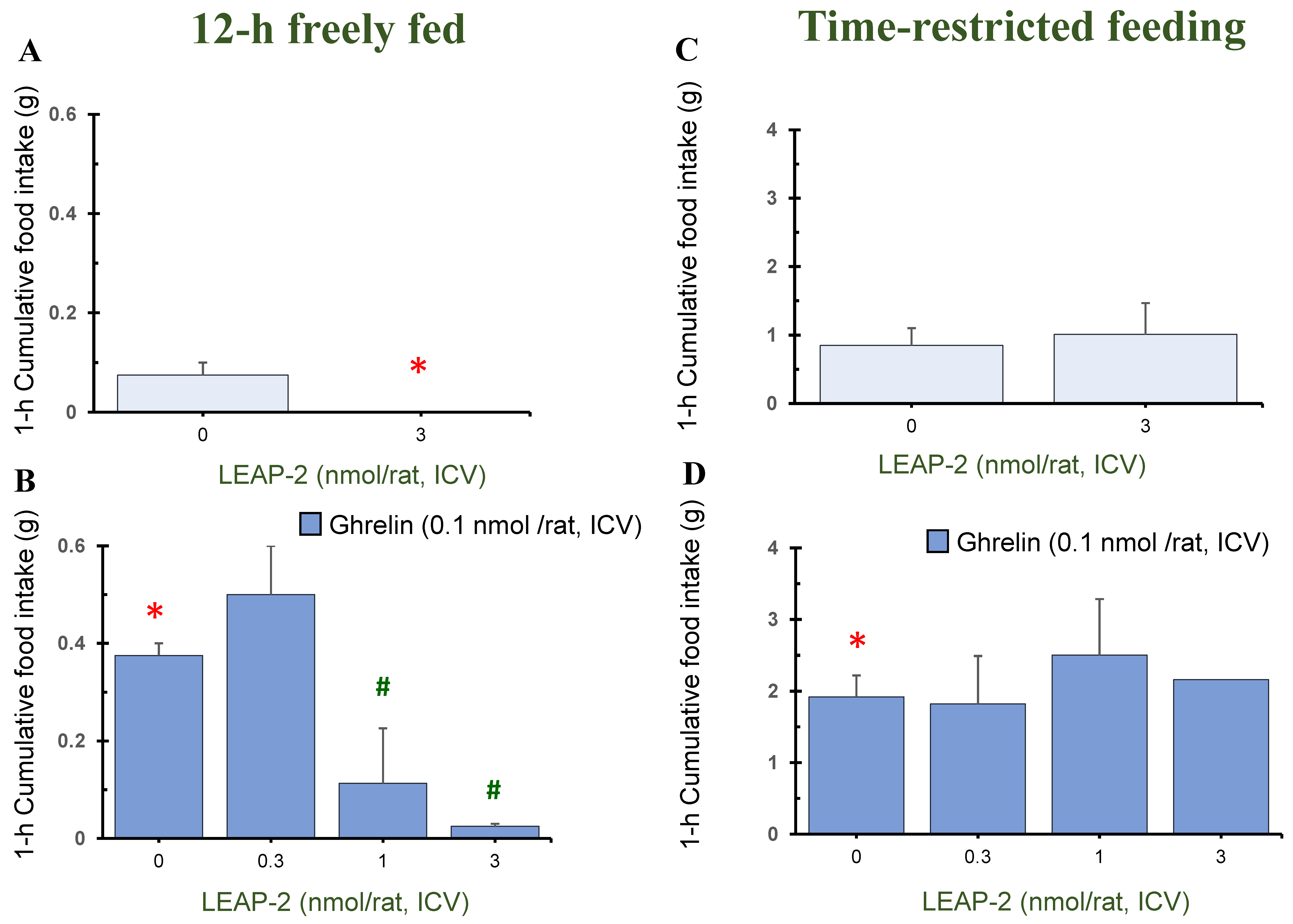
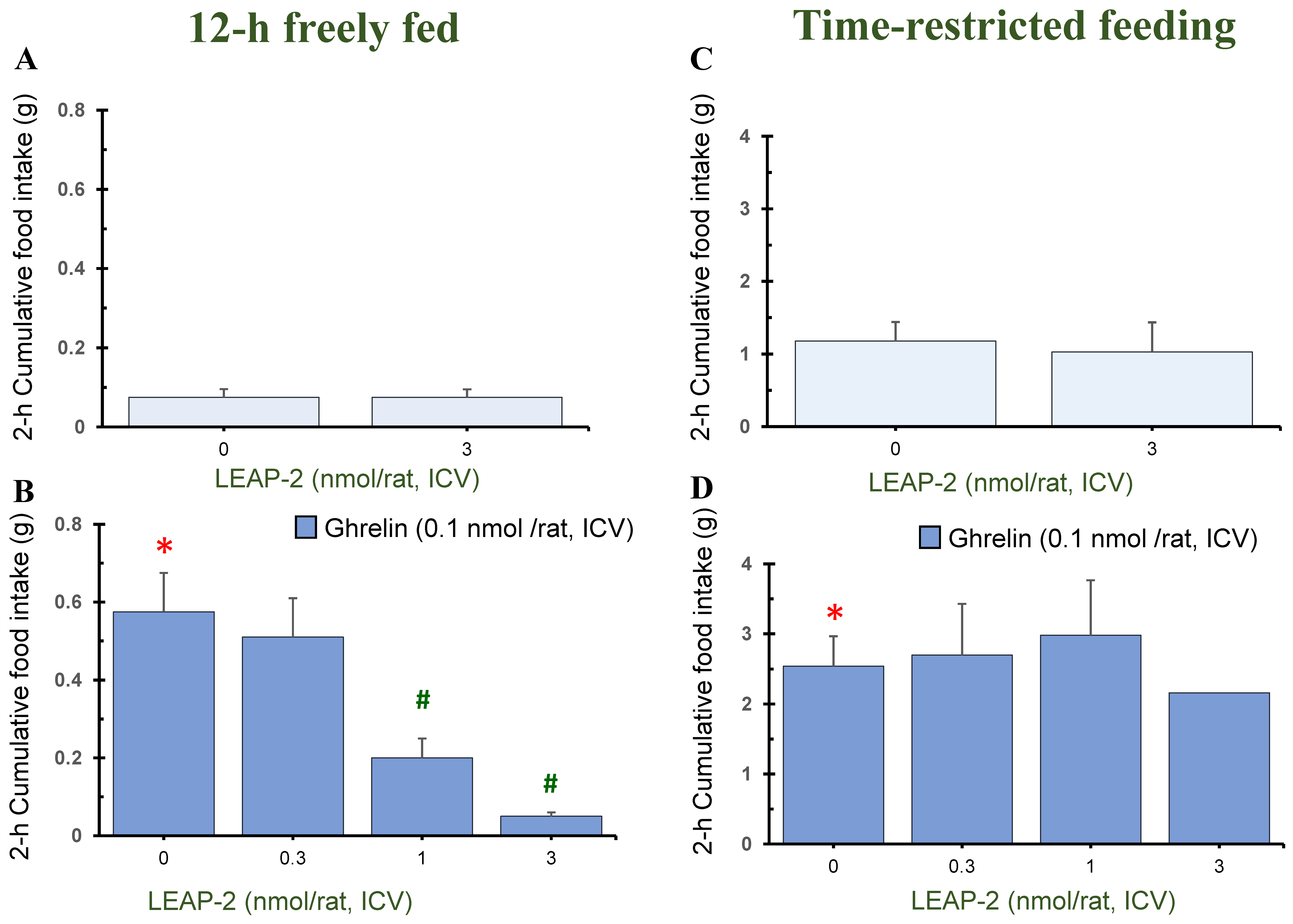
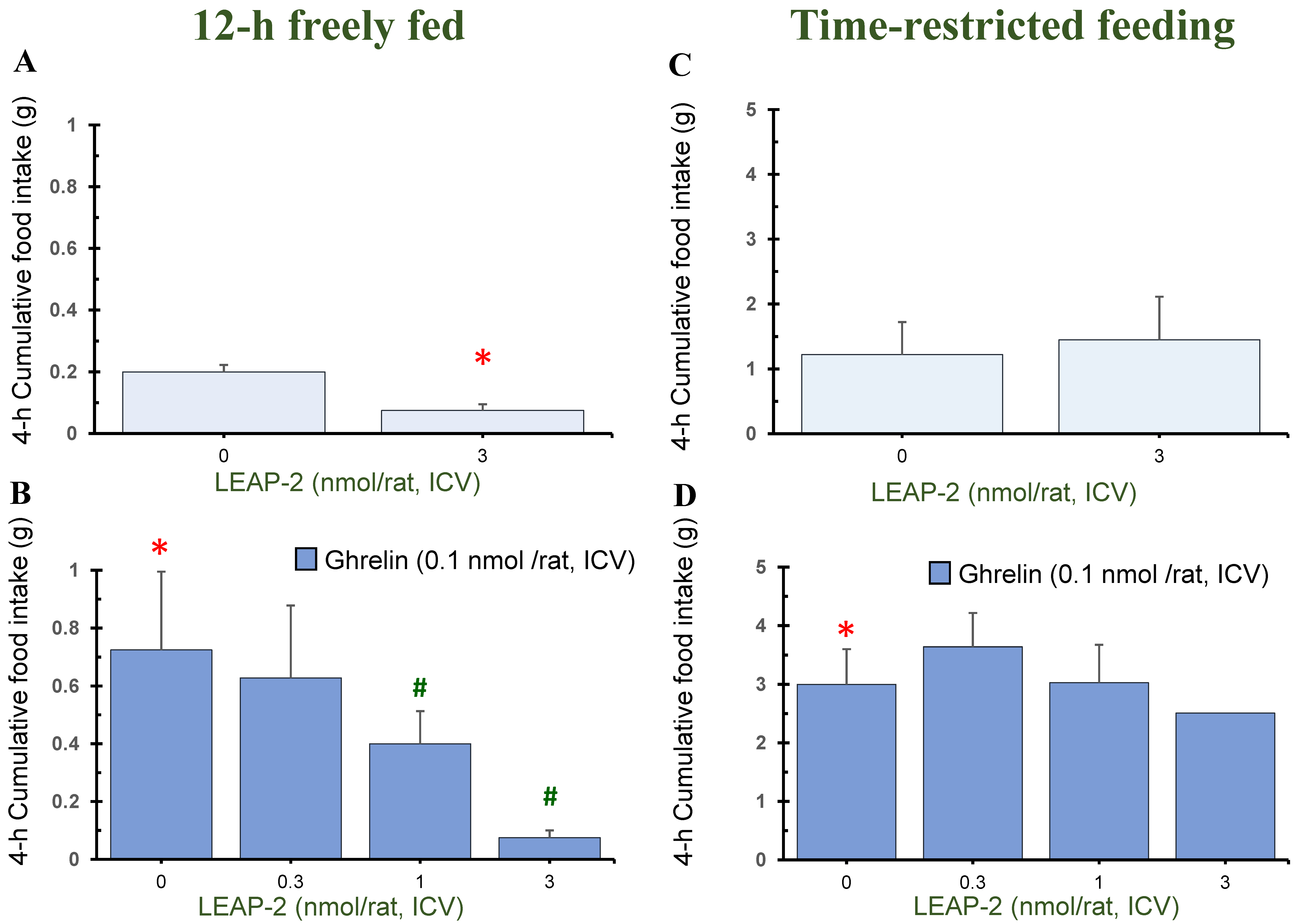
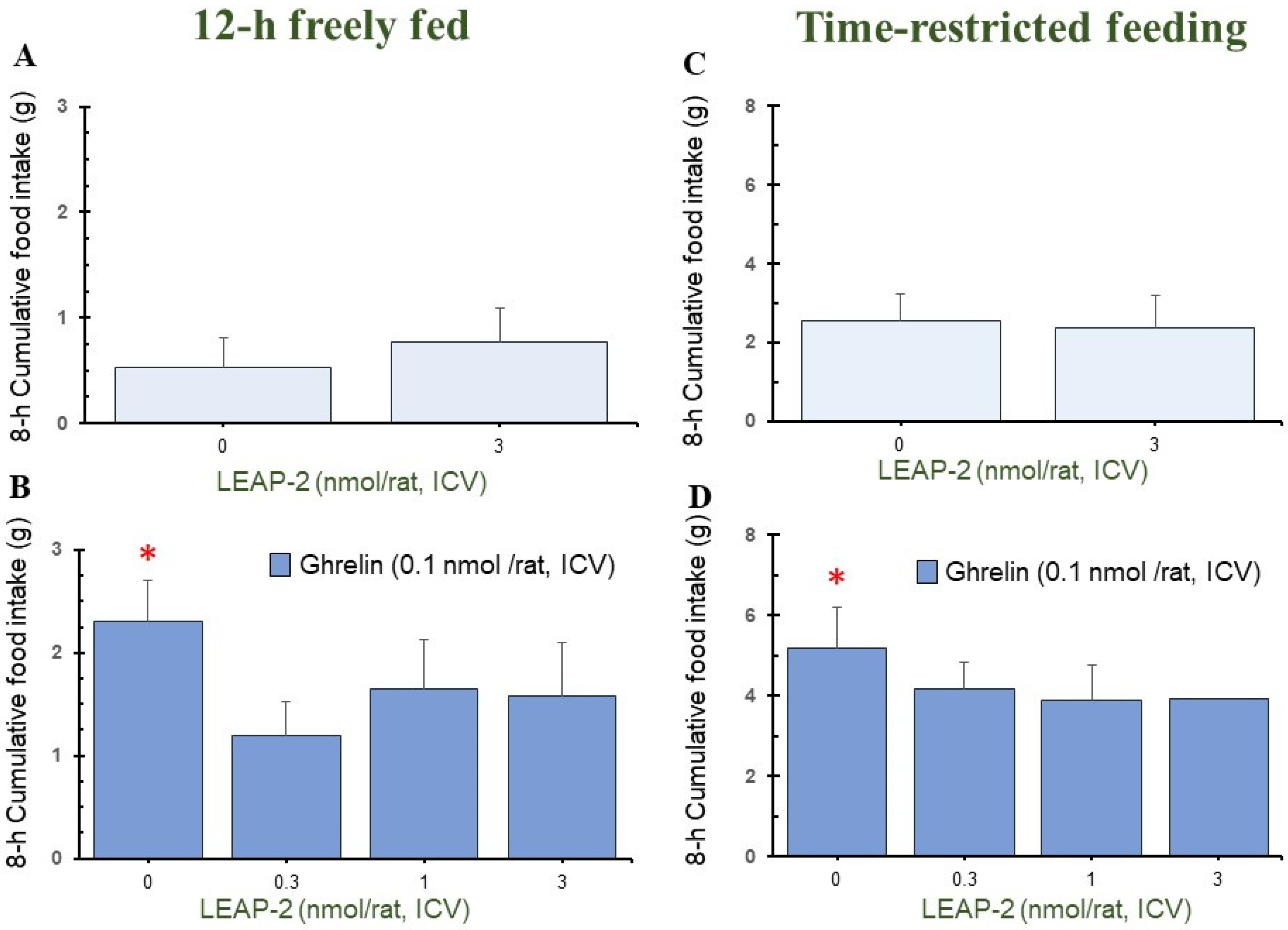
3.1. Action of Pre-Treatment with LEAP-2 via ICV without O-n-Octanoylated Ghrelin
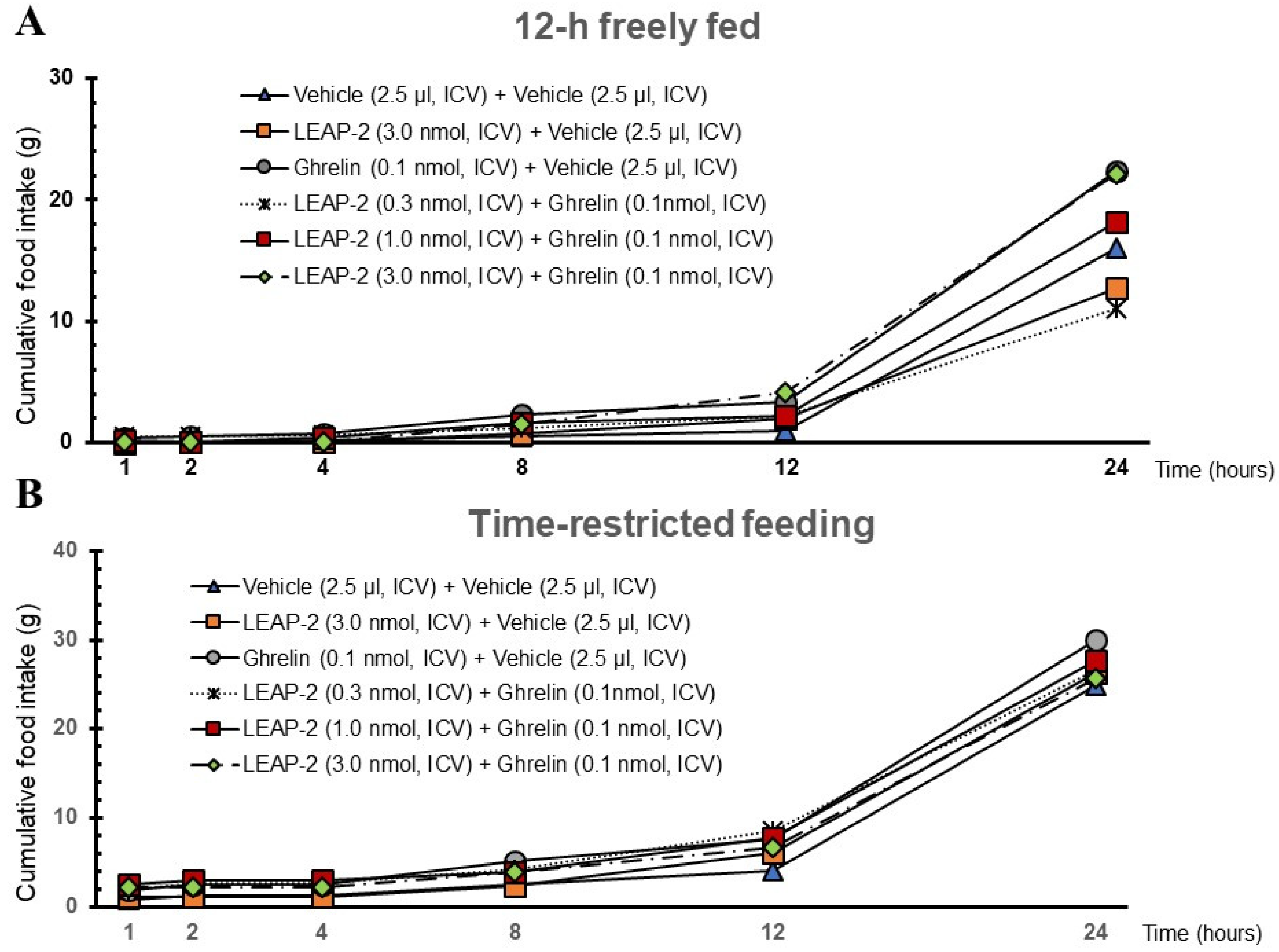
3.2. Effect of Interaction between LEAP-2 and O-n-Octanoylated Ghrelin on Food Intake in a 12 h Freely Fed State
3.3. Effect of Interaction between LEAP-2 and O-n-Octanoylated Ghrelin on Food Intake under TRF State
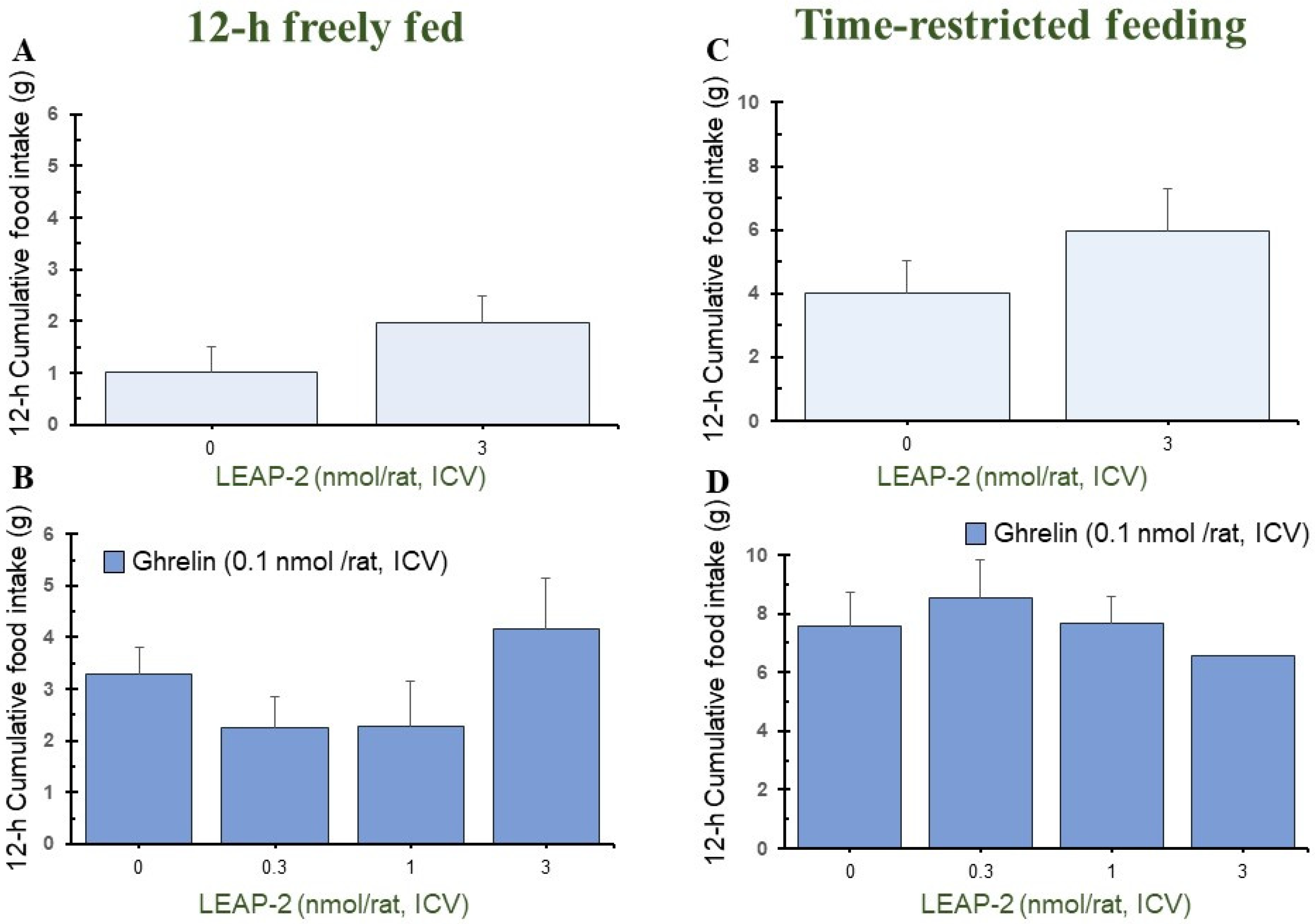
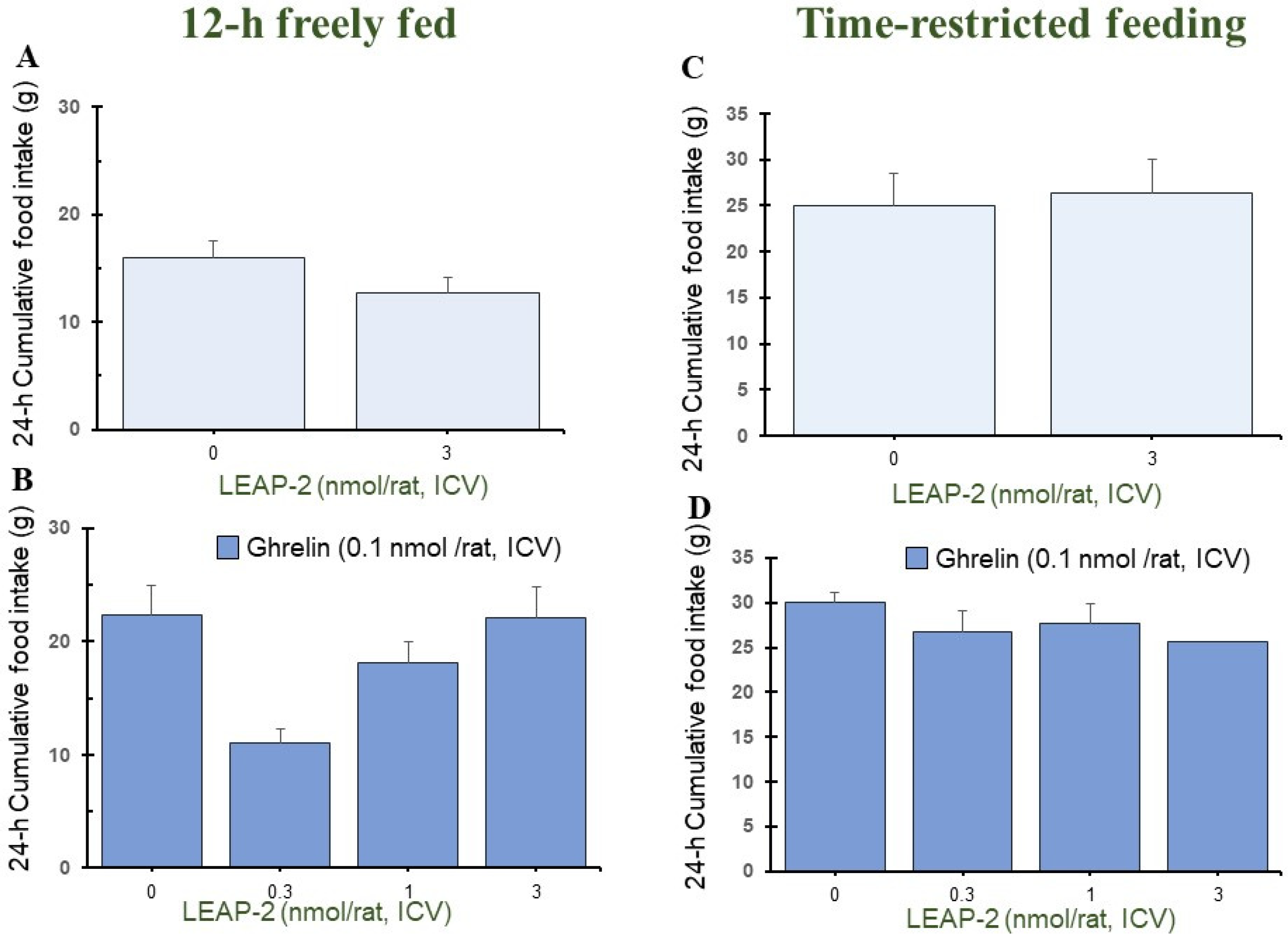
3.4. Temporal Effects of Pre-Treatment ICV Administration of LEAP-2
3.5. Cumulative Food Intake after Administration of LEAP-2 with or without Ghrelin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469.e466. [Google Scholar] [CrossRef]
- Lugilde, J.; Casado, S.; Beiroa, D.; Cuñarro, J.; Garcia-Lavandeira, M.; Álvarez, C.V.; Nogueiras, R.; Diéguez, C.; Tovar, S. LEAP-2 Counteracts Ghrelin-Induced Food Intake in a Nutrient, Growth Hormone and Age Independent Manner. Cells 2022, 11, 324. [Google Scholar] [CrossRef]
- Ma, X.; Xue, X.; Zhang, J.; Liang, S.; Xu, C.; Wang, Y.; Zhu, J. Liver Expressed Antimicrobial Peptide 2 is Associated with Steatosis in Mice and Humans. Exp. Clin. Endocrinol. Diabetes 2021, 129, 601–610. [Google Scholar] [CrossRef]
- M’Kadmi, C.; Cabral, A.; Barrile, F.; Giribaldi, J.; Cantel, S.; Damian, M.; Mary, S.; Denoyelle, S.; Dutertre, S.; Péraldi-Roux, S.; et al. N-Terminal Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Region Exhibits Inverse Agonist Activity toward the Ghrelin Receptor. J. Med. Chem. 2019, 62, 965–973. [Google Scholar] [CrossRef]
- Lu, X.; Huang, L.; Huang, Z.; Feng, D.; Clark, R.J.; Chen, C. LEAP-2: An Emerging Endogenous Ghrelin Receptor Antagonist in the Pathophysiology of Obesity. Front. Endocrinol. 2021, 12, 717544. [Google Scholar] [CrossRef]
- Islam, M.N.; Mita, Y.; Maruyama, K.; Tanida, R.; Zhang, W.; Sakoda, H.; Nakazato, M. Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J. Endocrinol. 2020, 244, 13–23. [Google Scholar] [CrossRef]
- Chen, C.Y.; Asakawa, A.; Fujimiya, M.; Lee, S.D.; Inui, A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol. Rev. 2009, 61, 430–481. [Google Scholar] [CrossRef]
- Mizutani, M.; Atsuchi, K.; Asakawa, A.; Matsuda, N.; Fujimura, M.; Inui, A.; Kato, I.; Fujimiya, M. Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G974–G980. [Google Scholar] [CrossRef]
- Chen, C.Y.; Fujimiya, M.; Laviano, A.; Chang, F.Y.; Lin, H.C.; Lee, S.D. Modulation of ingestive behavior and gastrointestinal motility by ghrelin in diabetic animals and humans. J. Chin. Med. Assoc. 2010, 73, 225–229. [Google Scholar] [CrossRef]
- Chen, C.Y.; Inui, A.; Asakawa, A.; Fujino, K.; Kato, I.; Chen, C.C.; Ueno, N.; Fujimiya, M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology 2005, 129, 8–25. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef]
- Das, M.; Kumar, D.; Sauceda, C.; Oberg, A.; Ellies, L.G.; Zeng, L.; Jih, L.J.; Newton, I.G.; Webster, N.J.G. Time-Restricted Feeding Attenuates Metabolic Dysfunction-Associated Steatohepatitis and Hepatocellular Carcinoma in Obese Male Mice. Cancers 2024, 16, 1513. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Fatima, S.; Zaman, M.H.; Khan, S.A.; Safdar, M.; Alam, I.; Feng, Q. Time-restricted feeding regulates molecular mechanisms with involvement of circadian rhythm to prevent metabolic diseases. Nutrition 2021, 89, 111244. [Google Scholar] [CrossRef]
- Wang, X.P.; Xing, C.Y.; Zhang, J.X.; Zhou, J.H.; Li, Y.C.; Yang, H.Y.; Zhang, P.F.; Zhang, W.; Huang, Y.; Long, J.G.; et al. Time-restricted feeding alleviates cardiac dysfunction induced by simulated microgravity via restoring cardiac FGF21 signaling. FASEB J. 2020, 34, 15180–15196. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Grinshpan, L.S.; Ivancovsky-Wajcman, D.; Goldenshluger, A.; Gepner, Y. One size does not fit all; practical, personal tailoring of the diet to NAFLD patients. Liver Int. 2022, 42, 1731–1750. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wai-Sun Wong, V.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). Obes. Facts 2024, 7, 1–70. [Google Scholar] [CrossRef]
- Zelber-Sagi, S. Guidelines and Future Perspectives of MAFLD. Obes. Facts 2024, 17 (Suppl. 1), 18–19. [Google Scholar] [CrossRef]
- Buzzetti, E.; Linden, A.; Best, L.M.; Madden, A.M.; Roberts, D.; Chase, T.J.G.; Freeman, S.C.; Cooper, N.J.; Sutton, A.J.; Fritche, D.; et al. Lifestyle modifications for nonalcohol-related fatty liver disease: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 6, Cd013156. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.-J.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults With Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Mu, X.; Shen, J. The effects of time restricted feeding on weight loss and other changes of anthropometric parameters among physically active individuals. Sci. Sports 2024, 39, 87–95. [Google Scholar] [CrossRef]
- Phillips, N.E.; Mareschal, J.; Schwab, N.; Manoogian, E.N.C.; Borloz, S.; Ostinelli, G.; Gauthier-Jaques, A.; Umwali, S.; Gonzalez Rodriguez, E.; Aeberli, D.; et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 2021, 13, 1042. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Ting, C.H.; Chi, C.W.; Li, C.P.; Chen, C.Y. Differential modulation of endogenous cannabinoid CB1 and CB2 receptors in spontaneous and splice variants of ghrelin-induced food intake in conscious rats. Nutrition 2015, 31, 230–235. [Google Scholar] [CrossRef]
- Yeh, C.; Ting, C.H.; Doong, M.L.; Chi, C.W.; Lee, S.D.; Chen, C.Y. Intracerebroventricular urocortin 3 counteracts central acyl ghrelin-induced hyperphagic and gastroprokinetic effects via CRF receptor 2 in rats. Drug Des. Dev. Ther. 2016, 10, 3281–3290. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tsai, C.Y.; Lee, W.J.; Liaw, W.J.; Chiang, C.H.; Ho, S.T.; Lee, S.D. Intracerebroventricular O-n-octanoylated ghrelin and its splice variant-induced feeding is blocked by insulin, independent of obestatin or CRF receptor, in satiated rats. Nutrition 2012, 28, 812–820. [Google Scholar] [CrossRef]
- Ting, C.H.; Chen, Y.C.; Liaw, W.J.; Lin, H.C.; Chen, C.Y. Peripheral injection of pancreatic polypeptide enhances colonic transit without eliciting anxiety or altering colonic secretion in rats. Neuropeptides 2016, 55, 67–71. [Google Scholar] [CrossRef]
- Huang, H.H.; Chen, L.Y.; Doong, M.L.; Chang, S.C.; Chen, C.Y. α-melanocyte stimulating hormone modulates the central acyl ghrelin-induced stimulation of feeding, gastrointestinal motility, and colonic secretion. Drug Des. Dev. Ther. 2017, 11, 2377–2386. [Google Scholar] [CrossRef]
- Chen, C.Y.; Doong, M.L.; Li, C.P.; Liaw, W.J.; Lee, H.F.; Chang, F.Y.; Lin, H.C.; Lee, S.D. A novel simultaneous measurement method to assess the influence of intracerebroventricular obestatin on colonic motility and secretion in conscious rats. Peptides 2010, 31, 1113–1117. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chien, E.J.; Chang, F.Y.; Lu, C.L.; Luo, J.C.; Lee, S.D. Impacts of peripheral obestatin on colonic motility and secretion in conscious fed rats. Peptides 2008, 29, 1603–1608. [Google Scholar] [CrossRef]
- Lawrence, C.B.; Snape, A.C.; Baudoin, F.M.; Luckman, S.M. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 2002, 143, 155–162. [Google Scholar] [CrossRef]
- Nakazato, M.; Murakami, N.; Date, Y.; Kojima, M.; Matsuo, H.; Kangawa, K.; Matsukura, S. A role for ghrelin in the central regulation of feeding. Nature 2001, 409, 194–198. [Google Scholar] [CrossRef]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001, 86, 5992. [Google Scholar] [CrossRef]
- Druce, M.R.; Wren, A.M.; Park, A.J.; Milton, J.E.; Patterson, M.; Frost, G.; Ghatei, M.A.; Small, C.; Bloom, S.R. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. 2005, 29, 1130–1136. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Hosoda, H.; Kojima, M.; Matsuo, H.; Kangawa, K. Ghrelin and des-acyl ghrelin: Two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000, 279, 909–913. [Google Scholar] [CrossRef]
- Sato, T.; Nakamura, Y.; Shiimura, Y.; Ohgusu, H.; Kangawa, K.; Kojima, M. Structure, regulation and function of ghrelin. J. Biochem. 2012, 151, 119–128. [Google Scholar] [CrossRef]
- Yang, J.; Brown, M.S.; Liang, G.; Grishin, N.V.; Goldstein, J.L. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008, 132, 387–396. [Google Scholar] [CrossRef]
- Cornejo, M.P.; Castrogiovanni, D.; Schiöth, H.B.; Reynaldo, M.; Marie, J.; Fehrentz, J.A.; Perello, M. Growth hormone secretagogue receptor signalling affects high-fat intake independently of plasma levels of ghrelin and LEAP2, in a 4-day binge eating model. J. Neuroendocrinol. 2019, 31, e12785. [Google Scholar] [CrossRef]
- Barrile, F.; M’Kadmi, C.; De Francesco, P.N.; Cabral, A.; García Romero, G.; Mustafá, E.R.; Cantel, S.; Damian, M.; Mary, S.; Denoyelle, S.; et al. Development of a novel fluorescent ligand of growth hormone secretagogue receptor based on the N-Terminal Leap2 region. Mol. Cell Endocrinol. 2019, 498, 110573. [Google Scholar] [CrossRef]
- Shankar, K.; Metzger, N.P.; Singh, O.; Mani, B.K.; Osborne-Lawrence, S.; Varshney, S.; Gupta, D.; Ogden, S.B.; Takemi, S.; Richard, C.P.; et al. LEAP2 deletion in mice enhances ghrelin’s actions as an orexigen and growth hormone secretagogue. Mol. Metab. 2021, 53, 101327. [Google Scholar] [CrossRef]
- Tezenas du Montcel, C.; Duriez, P.; Cao, J.; Lebrun, N.; Ramoz, N.; Viltart, O.; Gorwood, P.; Tolle, V. The role of dysregulated ghrelin/LEAP-2 balance in anorexia nervosa. iScience 2023, 26, 107996. [Google Scholar] [CrossRef]
- Mani, B.K.; Puzziferri, N.; He, Z.; Rodriguez, J.A.; Osborne-Lawrence, S.; Metzger, N.P.; Chhina, N.; Gaylinn, B.; Thorner, M.O.; Thomas, E.L.; et al. LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Investig. 2019, 129, 3909–3923. [Google Scholar] [CrossRef]
- Sundaram, S.; Yan, L. Time-restricted feeding reduces adiposity in mice fed a high-fat diet. Nutr. Res. 2016, 36, 603–611. [Google Scholar] [CrossRef]
- Sorrell, J.; Yates, E.; Rivir, M.; Woods, S.C.; Hogenesch, J.B.; Perez-Tilve, D. The central melanocortin system mediates the benefits of time-restricted feeding on energy balance. Physiol. Behav. 2020, 227, 113132. [Google Scholar] [CrossRef]
- Akamizu, T.; Takaya, K.; Irako, T.; Hosoda, H.; Teramukai, S.; Matsuyama, A.; Tada, H.; Miura, K.; Shimizu, A.; Fukushima, M.; et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur. J. Endocrinol. 2004, 150, 447–455. [Google Scholar] [CrossRef]
- Hagemann, C.A.; Jensen, M.S.; Holm, S.; Gasbjerg, L.S.; Byberg, S.; Skov-Jeppesen, K.; Hartmann, B.; Holst, J.J.; Dela, F.; Vilsbøll, T.; et al. LEAP2 reduces postprandial glucose excursions and ad libitum food intake in healthy men. Cell Rep. Med. 2022, 3, 100582. [Google Scholar] [CrossRef]
- Hagemann, C.A.; Zhang, C.; Hansen, H.H.; Jorsal, T.; Rigbolt, K.T.G.; Madsen, M.R.; Bergmann, N.C.; Heimbürger, S.M.N.; Falkenhahn, M.; Theis, S.; et al. Identification and Metabolic Profiling of a Novel Human Gut-derived LEAP2 Fragment. J. Clin. Endocrinol. Metab. 2021, 106, e966–e981. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef]
- Ezpeleta, M.; Gabel, K.; Cienfuegos, S.; Kalam, F.; Lin, S.; Pavlou, V.; Song, Z.; Haus, J.M.; Koppe, S.; Alexandria, S.J.; et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: A randomized controlled trial. Cell Metab. 2023, 35, 56–70.e53. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e105. [Google Scholar] [CrossRef]
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-E.; Chen, C.-Y. Impacts of Central Administration of the Novel Peptide, LEAP-2, in Different Food Intake Models in Conscious Rats. Nutrients 2024, 16, 1946. https://doi.org/10.3390/nu16121946
Lin C-E, Chen C-Y. Impacts of Central Administration of the Novel Peptide, LEAP-2, in Different Food Intake Models in Conscious Rats. Nutrients. 2024; 16(12):1946. https://doi.org/10.3390/nu16121946
Chicago/Turabian StyleLin, Chia-En, and Chih-Yen Chen. 2024. "Impacts of Central Administration of the Novel Peptide, LEAP-2, in Different Food Intake Models in Conscious Rats" Nutrients 16, no. 12: 1946. https://doi.org/10.3390/nu16121946
APA StyleLin, C.-E., & Chen, C.-Y. (2024). Impacts of Central Administration of the Novel Peptide, LEAP-2, in Different Food Intake Models in Conscious Rats. Nutrients, 16(12), 1946. https://doi.org/10.3390/nu16121946




