The Association of Plasma Homocysteine Concentrations with a 10-Year Risk of All-Cause and Cardiovascular Mortality in a Community-Based Chinese Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Demographic and Clinical Characteristics
2.3. Specimen Collection and Laboratory Testing
2.4. Ascertainment of Mortality
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
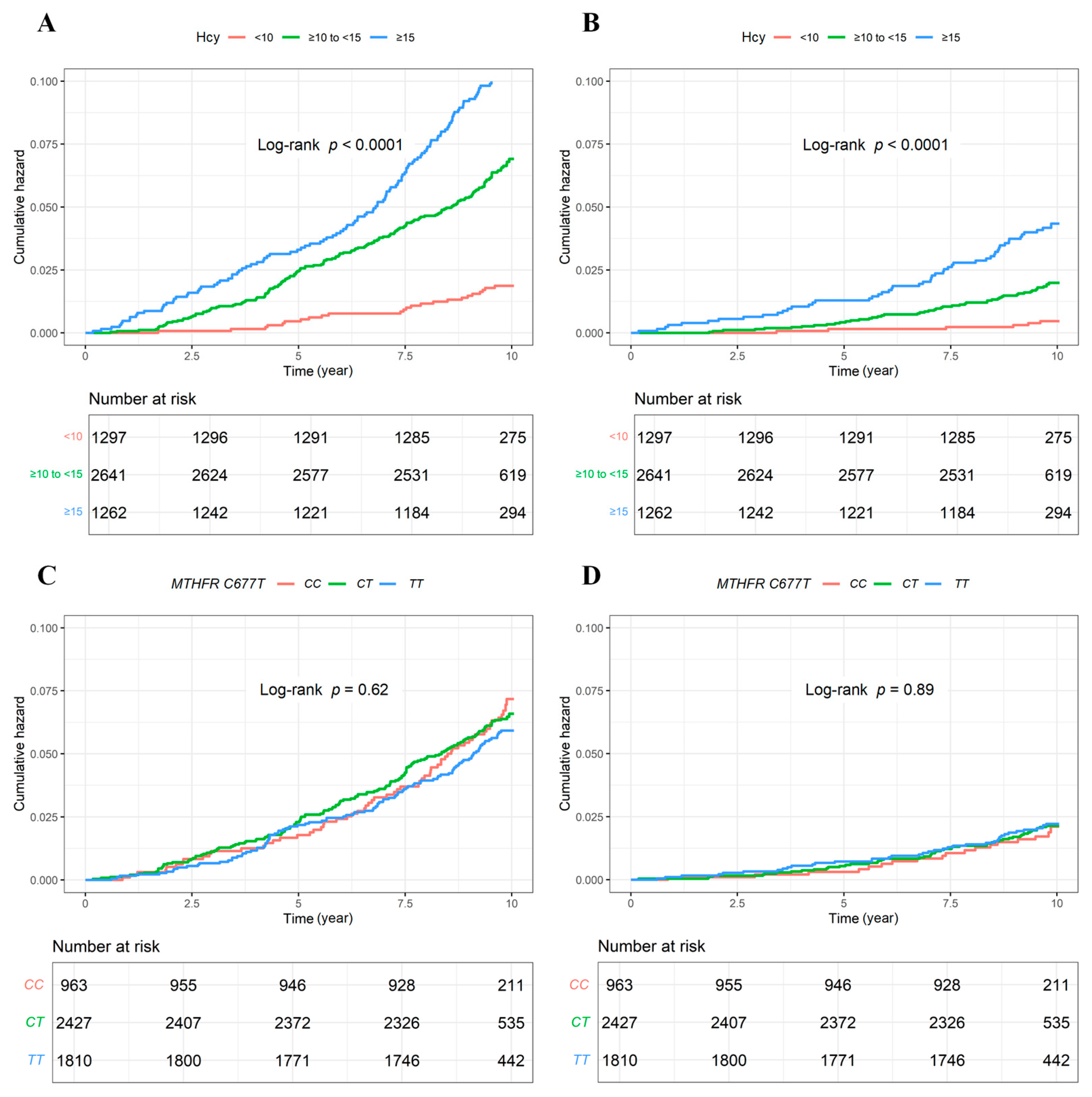
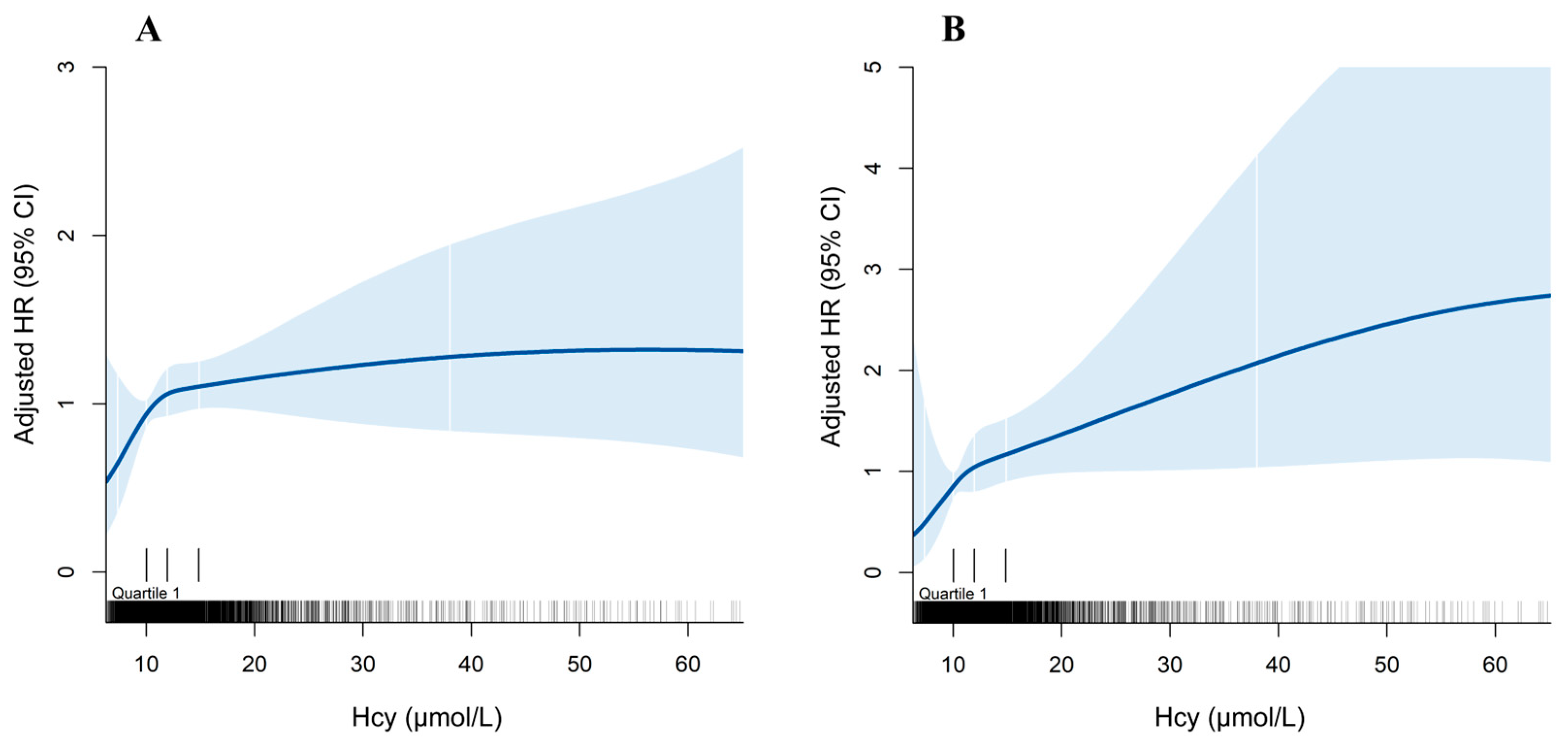
3.2. All-Cause and CV Mortality
3.3. Stratification Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Lamuño, D.; Arrieta-Blanco, F.J.; Fuentes, E.D.; Forga-Visa, M.T.; Morales-Conejo, M.; Peña-Quintana, L.; Vitoria-Miñana, I. Hyperhomocysteinemia in Adult Patients: A Treatable Metabolic Condition. Nutrients 2023, 16, 135. [Google Scholar] [CrossRef]
- Hu, H.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.; Liu, X.; Sun, H. Catalpol Inhibits Homocysteine-induced Oxidation and Inflammation via Inhibiting Nox4/NF-kappaB and GRP78/PERK Pathways in Human Aorta Endothelial Cells. Inflammation 2019, 42, 64–80. [Google Scholar] [CrossRef]
- Du, X.; Ma, X.; Tan, Y.; Shao, F.; Li, C.; Zhao, Y.; Miao, Y.; Han, L.; Dang, G.; Song, Y.; et al. B cell-derived anti-beta 2 glycoprotein I antibody mediates hyperhomocysteinemia-aggravated hypertensive glomerular lesions by triggering ferroptosis. Signal Transduct. Target. Ther. 2023, 8, 103. [Google Scholar] [CrossRef]
- Han, L.; Miao, Y.; Zhao, Y.; Zhang, X.; Ma, X.; Du, X.; Kong, W.; Xu, Q.; Liu, J.; Dai, K.; et al. The binding of autotaxin to integrins mediates hyperhomocysteinemia-potentiated platelet activation and thrombosis in mice and humans. Blood Adv. 2022, 6, 46–61. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, E364–E467. [Google Scholar] [CrossRef]
- Peng, H.Y.; Man, C.F.; Xu, J.; Fan, Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: A meta-analysis of prospective studies. J. Zhejiang Univ. Sci. B 2015, 16, 78–86. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, A.; Zhong, F. Association between Homocysteine Levels and All-cause Mortality: A Dose-Response Meta-Analysis of Prospective Studies. Sci. Rep. 2017, 7, 4769. [Google Scholar] [CrossRef]
- Jenkins DJ, A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.I.; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J. Am. Coll. Cardiol. 2018, 71, 2570–2584. [Google Scholar] [CrossRef]
- Zhao, W.; Gao, F.; Lv, L.; Chen, X. The interaction of hypertension and homocysteine increases the risk of mortality among middle-aged and older population in the United States. J. Hypertens. 2022, 40, 254–263. [Google Scholar] [CrossRef]
- Lu, J.; Chen, K.; Chen, W.; Liu, C.; Jiang, X.; Ma, Z.; Li, D.; Shen, Y.; Tian, H. Association of Serum Homocysteine with Cardiovascular and All-Cause Mortality in Adults with Diabetes: A Prospective Cohort Study. Oxid Med. Cell Longev. 2022, 2022, 2156483. [Google Scholar] [CrossRef]
- Wang, X.; Peng, H.; Xia, C.; Zhou, Y.; Shen, L.; Cheng, X.; Yang, C.; Yang, Y.; Long, L. Association of B vitamin intake and total homocysteine levels with all-cause and cause-specific mortality in central obesity. Nutrition 2023, 116, 112189. [Google Scholar] [CrossRef]
- Song, J.H.; Huh, H.; Bae, E.; Lee, J.; Lee, J.P.; Lee, J.S.; Kim, G.S.; Yoo, K.D. Association between homocysteinemia and mortality in CKD: A propensity-score matched analysis using NHANES-National Death Index. Medicine 2022, 101, e30334. [Google Scholar] [CrossRef]
- Zarembska, E.; Slusarczyk, K.; Wrzosek, M. The Implication of a Polymorphism in the Methylenetetrahydrofolate Reductase Gene in Homocysteine Metabolism and Related Civilisation Diseases. Int. J. Mol. Sci. 2023, 25, 193. [Google Scholar] [CrossRef]
- Husemoen, L.L.N.; Skaaby, T.; Jørgensen, T.; Thuesen, B.H.; Fenger, M.; Grarup, N.; Sandholt, C.H.; Hansen, T.; Pedersen, O.; Linneberg, A. MTHFR C677T genotype and cardiovascular risk in a general population without mandatory folic acid fortification. Eur. J. Nutr. 2014, 53, 1549–1559. [Google Scholar] [CrossRef]
- Yang, Q.; Bailey, L.; Clarke, R.; Flanders, W.D.; Liu, T.; Yesupriya, A.; Khoury, M.J.; Friedman, J.M. Prospective study of methylenetetrahydrofolate reductase (MTHFR) variant C677T and risk of all-cause and cardiovascular disease mortality among 6000 US adults. Am. J. Clin. Nutr. 2012, 95, 1245–1253. [Google Scholar] [CrossRef]
- Choi, C.K.; Kweon, S.S.; Lee, Y.H.; Nam, H.S.; Choi, S.W.; Kim, H.Y.; Shin, M.H. Association Between Plasma Homocysteine Level and Mortality: A Mendelian Randomization Study. Korean Circ. J. 2023, 53, 710–719. [Google Scholar] [CrossRef]
- Fan, F.; Qi, L.; Jia, J.; Xu, X.; Liu, Y.; Yang, Y.; Qin, X.; Li, J.; Li, H.; Zhang, Y.; et al. Noninvasive Central Systolic Blood Pressure Is More Strongly Related to Kidney Function Decline Than Peripheral Systolic Blood Pressure in a Chinese Community-Based Population. Hypertension 2016, 67, 1166–1172. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef]
- Liu, T.; Momin, M.; Zhou, H.; Zheng, Q.; Fan, F.; Jia, J.; Liu, M.; Bao, M.; Li, J.; Huo, Y.; et al. Exome-Wide Association Study Identifies East Asian-Specific Missense Variant MTHFR C136T Influencing Homocysteine Levels in Chinese Populations RH: ExWAS of tHCY in a Chinese Population. Front. Genet 2021, 12, 717621. [Google Scholar] [CrossRef]
- Tang, C.S.; Zhang, H.; Cheung, C.Y.; Xu, M.; Ho, J.C.; Zhou, W.; Cherny, S.S.; Zhang, Y.; Holmen, O.; Au, K.W.; et al. Exome-wide association analysis reveals novel coding sequence variants associated with lipid traits in Chinese. Nat. Commun. 2015, 6, 10206. [Google Scholar] [CrossRef]
- Paganelli, F.; Mottola, G.; Fromonot, J.; Marlinge, M.; Deharo, P.; Guieu, R.; Ruf, J. Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link? Int. J. Mol. Sci. 2021, 22, 1690. [Google Scholar] [CrossRef]
- Sacco, R.L.; Adams, R.; Albers, G.; Alberts, M.J.; Benavente, O.; Furie, K.; Goldstein, L.B.; Gorelick, P.; Halperin, J.; Harbaugh, R.; et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: A statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: Co-sponsored by the Council on Cardiovascular Radiology and Intervention: The American Academy of Neurology affirms the value of this guideline. Circulation 2006, 113, e409–e449. [Google Scholar]
- Song, S.; Song, B.M.; Park, H.Y. Associations of Serum Folate and Homocysteine Concentrations with All-Cause, Cardiovascular Disease, and Cancer Mortality in Men and Women in Korea: The Cardiovascular Disease Association Study. J. Nutr. 2023, 153, 760–770. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, X.; Guan, G.; Zhang, Y.; Hui, R.; Xing, Y.; Wang, J.; Zhu, L. Non-linear associations of serum and red blood cell folate with risk of cardiovascular and all-cause mortality in hypertensive adults. Hypertens. Res. 2023, 46, 1504–1515. [Google Scholar] [CrossRef]
- Mendonca, N.; Jagger, C.; Granic, A.; Martin-Ruiz, C.; Mathers, J.C.; Seal, C.J.; Hill, T.R. Elevated Total Homocysteine in All Participants and Plasma Vitamin B12 Concentrations in Women Are Associated with All-Cause and Cardiovascular Mortality in the Very Old: The Newcastle 85+ Study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1258–1264. [Google Scholar] [CrossRef]
- Pusceddu, I.; Herrmann, W.; Kleber, M.E.; Scharnagl, H.; Hoffmann, M.M.; Winklhofer-Roob, B.M.; Marz, W.; Herrmann, M. Subclinical inflammation, telomere shortening, homocysteine, vitamin B6, and mortality: The Ludwigshafen Risk and Cardiovascular Health Study. Eur. J. Nutr. 2020, 59, 1399–1411. [Google Scholar] [CrossRef]
- Mo, T.; Long, P.; Wang, Y.; Peng, R.; Niu, R.; Wang, Q.; Jiang, J.; Shi, L.; Yang, H.; Xu, C.; et al. Genetic susceptibility, homocysteine levels, and risk of all-cause and cause-specific mortality: A prospective cohort study. Clin. Chim. Acta 2023, 538, 1–8. [Google Scholar] [CrossRef]
- Wu, D.F.; Yin, R.X.; Deng, J.L. Homocysteine, hyperhomocysteinemia and H-type hypertension. Eur. J. Prev. Cardiol. 2024, zwae022. [Google Scholar] [CrossRef]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202. [Google Scholar] [CrossRef]
- Li, R.; Weng, H.; Pan, Y.; Meng, X.; Liao, X.; Wang, M.; Zhang, Y.; Sui, Y.; Zuo, L.; Wang, Y.; et al. Relationship between homocysteine levels and post-stroke cognitive impairment in female and male population: From a prospective multicenter study. J. Transl. Intern. Med. 2021, 9, 264–272. [Google Scholar] [CrossRef]
- Mccaddon, A.; Miller, J.W. Homocysteine—A retrospective and prospective appraisal. Front. Nutr. 2023, 10, 1179807. [Google Scholar] [CrossRef]
- Kozakova, M.; Morizzo, C.; Penno, G.; Shore, A.C.; Nilsson, J.; Palombo, C. Plasma Homocysteine and Cardiovascular Organ Damage in a Population with a High Prevalence of Risk Factors. J. Clin. Endocrinol. Metab. 2020, 105, E2815–E2824. [Google Scholar] [CrossRef]
- Cui, R.; Moriyama, Y.; Koike, K.A.; Date, C.; Kikuchi, S.; Tamakoshi, A.; Iso, H.; Group, J.S. Serum total homocysteine concentrations and risk of mortality from stroke and coronary heart disease in Japanese: The JACC study. Atherosclerosis 2008, 198, 412–418. [Google Scholar] [CrossRef]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef]
- Xu, B.; Kong, X.; Xu, R.; Song, Y.; Liu, L.; Zhou, Z.; Gu, R.; Shi, X.; Zhao, M.; Huang, X.; et al. Homocysteine and all-cause mortality in hypertensive adults without pre-existing cardiovascular conditions: Effect modification by MTHFR C677T polymorphism. Medicine 2017, 96, e5862. [Google Scholar] [CrossRef]
- GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Xu, X.; Shi, Z.; Zhou, L.; Lin, J.; Atlantis, E.; Chen, X.; Hussain, A.; Wang, Y.; Wang, Y. Impact of COVID-19 on risks and deaths of non-communicable diseases in the Western Pacific region. Lancet Reg. Health West. Pac. 2024, 43, 100795. [Google Scholar] [CrossRef]
| Characteristics | Overall | Plasma Hcy Concentrations | p-Value | ||
|---|---|---|---|---|---|
| <10 μmol/L | ≥10 to <15 μmol/L | ≥15 μmol/L | |||
| N | 5200 | 1297 | 2641 | 1262 | |
| Age (year), mean (SD) | 57.14 (8.93) | 53.79 (7.47) | 57.81 (8.67) | 59.18 (9.88) | <0.001 |
| Sex, N (%) | <0.001 | ||||
| Male | 1970 (37.9) | 137 (10.6) | 962 (36.4) | 871 (69.0) | |
| Female | 3230 (62.1) | 1160 (89.4) | 1679 (63.6) | 391 (31.0) | |
| Plasma Hcy (μmol/L), median (IQR) | 11.95 (10.00, 14.89) | 8.82 (8.08, 9.49) | 11.97 (11.02, 13.23) | 18.63 (16.51, 25.19) | <0.001 |
| Serum folate (ng/mL), median (IQR) | 6.18 (4.98, 8.19) | 7.70 (6.05, 10.23) | 6.32 (5.20, 8.07) | 4.88 (4.22, 5.89) | <0.001 |
| MTHFR C677T, N (%) | <0.001 | ||||
| CC | 963 (18.5) | 322 (24.8) | 503 (19.0) | 138 (10.9) | |
| CT | 2427 (46.7) | 686 (52.9) | 1325 (50.2) | 416 (33.0) | |
| TT | 1810 (34.8) | 289 (22.3) | 813 (30.8) | 708 (56.1) | |
| BMI (kg/m2), mean (SD) | 26.09 (3.38) | 25.95 (3.52) | 26.08 (3.34) | 26.28 (3.28) | 0.046 |
| eGFR (mL/min/1.73 m2), mean (SD) | 94.28 (13.13) | 101.18 (9.15) | 94.03 (11.63) | 87.73 (15.79) | <0.001 |
| eGFR classification (mL/min/1.73 m2), N (%) | <0.001 | ||||
| ≥90 | 3606 (69.4) | 1147 (88.6) | 1816 (68.8) | 643 (51.0) | |
| <90 | 1590 (30.6) | 148 (11.4) | 824 (31.2) | 618 (49.0) | |
| Current smoking, N (%) | 1025 (19.7) | 82 (6.3) | 470 (17.8) | 473 (37.5) | <0.001 |
| Current drinking, N (%) | 1230 (23.7) | 143 (11.0) | 581 (22.0) | 506 (40.1) | <0.001 |
| Prevalence of disease, N (%) | |||||
| Hypertension | 2657 (51.1) | 547 (42.2) | 1362 (51.6) | 748 (59.3) | <0.001 |
| Diabetes | 1289 (24.8) | 304 (23.4) | 687 (26.0) | 298 (23.6) | 0.115 |
| Dyslipidemia | 3733 (71.8) | 930 (71.7) | 1911 (72.4) | 892 (70.7) | 0.551 |
| CVD | 292 (5.6) | 49 (3.8) | 143 (5.4) | 100 (7.9) | <0.001 |
| Medication, N (%) | |||||
| Antihypertensive drugs | 1689 (32.7) | 346 (26.8) | 865 (33.0) | 478 (38.1) | <0.001 |
| Hypoglycemic drugs | 572 (11.0) | 141 (10.9) | 321 (12.2) | 110 (8.8) | 0.006 |
| Lipid-lowering drugs | 555 (10.8) | 141 (11.0) | 288 (11.0) | 126 (10.1) | 0.643 |
| Death endpoint, N (%) | |||||
| All-cause mortality | 320 (6.2) | 24 (1.9) | 172 (6.5) | 124 (9.8) | <0.001 |
| CV mortality | 107 (2.1) | 6 (0.5) | 50 (1.9) | 51 (4.0) | <0.001 |
| Endpoints | N | No. of Deaths, N (%) | Crude | Adjusted * | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |||
| All-cause mortality | ||||||
| Linear trend | ||||||
| Hcy | 5200 | 320 (6.2) | 1.02 (1.01, 1.03) | <0.001 | 1.01 (0.99, 1.02) | 0.363 |
| Classified clinical threshold | ||||||
| <10 μmol/L | 1297 | 24 (1.9) | ref | ref | ||
| ≥10 to <15 μmol/L | 2641 | 172 (6.5) | 3.61 (2.35, 5.53) | <0.001 | 1.74 (1.12, 2.71) | 0.014 |
| ≥15 μmol/L | 1262 | 124 (9.8) | 5.55 (3.58, 8.59) | <0.001 | 1.64 (1.01, 2.68) | 0.047 |
| Pooled clinical threshold | ||||||
| <10 μmol/L | 1297 | 24 (1.9) | ref | ref | ||
| ≥10 μmol/L | 3903 | 296 (7.6) | 4.23 (2.79, 6.41) | <0.001 | 1.72 (1.11, 2.68) | 0.015 |
| MTHFR C677T | ||||||
| CC | 963 | 64 (6.6) | ref | ref | ||
| CT | 2427 | 152 (6.3) | 0.94 (0.71, 1.27) | 0.702 | 1.05 (0.78, 1.42) | 0.729 |
| TT | 1810 | 104 (5.7) | 0.86 (0.63, 1.18) | 0.350 | 1.03 (0.75, 1.42) | 0.850 |
| CV mortality | ||||||
| Linear trend | ||||||
| Hcy | 5200 | 107 (2.1) | 1.03 (1.01, 1.04) | <0.001 | 1.02 (1.00, 1.03) | 0.036 |
| Classified clinical threshold | ||||||
| <10 μmol/L | 1297 | 6 (0.5) | ref | ref | ||
| ≥10 to <15 μmol/L | 2641 | 50 (1.9) | 4.20 (1.80, 9.81) | <0.001 | 1.80 (0.76, 4.30) | 0.183 |
| ≥15 μmol/L | 1262 | 51 (4.0) | 9.16 (3.93, 21.34) | <0.001 | 2.06 (0.81, 5.20) | 0.128 |
| Pooled clinical threshold | ||||||
| <10 μmol/L | 1297 | 6 (0.5) | ref | ref | ||
| ≥10 μmol/L | 3903 | 101 (2.6) | 5.78 (2.54, 13.18) | <0.001 | 1.85 (0.78, 4.39) | 0.160 |
| MTHFR C677T | ||||||
| CC | 963 | 18 (1.9) | ref | ref | ||
| CT | 2427 | 50 (2.1) | 1.10 (0.64, 1.89) | 0.717 | 1.34 (0.77, 2.31) | 0.297 |
| TT | 1810 | 39 (2.2) | 1.15 (0.66, 2.01) | 0.626 | 1.47 (0.83, 2.61) | 0.186 |
| Subgroup | N | No. of Deaths, N (%) | HR (95% CI) * | p for Interaction | |
|---|---|---|---|---|---|
| Hcy < 10 μmol/L | Hcy ≥ 10 μmol/L | ||||
| Age (year) | 0.530 | ||||
| <65 | 4158 | 12 (1.0) | 103 (3.5) | 1.93 (1.04, 3.56) | |
| ≥65 | 1042 | 12 (10.2) | 193 (20.9) | 1.47 (0.81, 2.69) | |
| Sex | 0.805 | ||||
| Male | 1970 | 5 (3.6) | 189 (10.3) | 1.86 (0.76, 4.58) | |
| Female | 3230 | 19 (1.6) | 107 (5.2) | 1.64 (0.99, 2.71) | |
| BMI (kg/m2) | 0.496 | ||||
| <24 | 1402 | 6 (1.6) | 100 (9.8) | 2.48 (1.07, 5.73) | |
| ≥24 to <28 | 2407 | 12 (2.0) | 133 (7.3) | 1.39 (0.75, 2.57) | |
| ≥28 | 1391 | 6 (1.9) | 63 (5.9) | 1.48 (0.63, 3.46) | |
| eGFR (mL/min/1.73 m2) | 0.608 | ||||
| ≥90 | 3606 | 15 (1.3) | 102 (4.1) | 1.83 (1.05, 3.20) | |
| <90 | 1590 | 9 (6.1) | 194 (13.5) | 1.46 (0.73, 2.90) | |
| Current smoking | 0.262 | ||||
| No | 4175 | 23 (1.9) | 204 (6.9) | 1.57 (1.00, 2.47) | |
| Yes | 1025 | 1 (1.2) | 92 (9.8) | 4.24 (0.59, 30.53) | |
| Current drinking | 0.768 | ||||
| No | 3970 | 21 (1.8) | 208 (7.4) | 1.73 (1.08, 2.77) | |
| Yes | 1230 | 3 (2.1) | 88 (8.1) | 1.43 (0.45, 4.57) | |
| Hypertension | 0.772 | ||||
| No | 2543 | 8 (1.1) | 79 (4.4) | 1.84 (0.88, 3.88) | |
| Yes | 2657 | 16 (2.9) | 217 (10.3) | 1.62 (0.95, 2.74) | |
| Diabetes | 0.496 | ||||
| No | 3911 | 12 (1.2) | 174 (6.0) | 1.94 (1.06, 3.55) | |
| Yes | 1289 | 12 (3.9) | 122 (12.4) | 1.45 (0.78, 2.67) | |
| Dyslipidemia | 0.543 | ||||
| No | 1467 | 5 (1.4) | 88 (8.0) | 2.14 (0.86, 5.35) | |
| Yes | 3733 | 19 (2.0) | 208 (7.4) | 1.57 (0.96, 2.57) | |
| Antihypertensive drugs | 0.370 | ||||
| No | 3481 | 11 (1.2) | 149 (5.9) | 2.05 (1.09, 3.86) | |
| Yes | 1689 | 13 (3.8) | 145 (10.8) | 1.40 (0.78, 2.51) | |
| Hypoglycemic drugs | 0.753 | ||||
| No | 4614 | 17 (1.5) | 233 (6.7) | 1.76 (1.05, 2.96) | |
| Yes | 572 | 7 (5.0) | 63 (14.6) | 1.52 (0.69, 3.36) | |
| Lipid-lowering drugs | 0.131 | ||||
| No | 4597 | 18 (1.6) | 259 (7.5) | 1.96 (1.19, 3.24) | |
| Yes | 555 | 6 (4.3) | 34 (8.2) | 0.88 (0.36, 2.11) | |
| MTHFR C677T | 0.036 | ||||
| CC | 963 | 2 (0.6) | 62 (9.7) | 5.24 (1.27, 21.63) | |
| CT/TT | 4237 | 22 (2.3) | 234 (7.2) | 1.38 (0.87, 2.19) | |
| Serum folate (ng/mL) | 0.104 | ||||
| <6.18 | 2599 | 10 (2.8) | 174 (7.7) | 1.08 (0.56, 2.08) | |
| ≥6.18 | 2601 | 14 (1.5) | 122 (7.4) | 2.22 (1.26, 3.91) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Li, K.; Chen, H.; Jia, J.; Li, J.; Huo, Y.; Fan, F.; Zhang, Y. The Association of Plasma Homocysteine Concentrations with a 10-Year Risk of All-Cause and Cardiovascular Mortality in a Community-Based Chinese Population. Nutrients 2024, 16, 1945. https://doi.org/10.3390/nu16121945
Liang Z, Li K, Chen H, Jia J, Li J, Huo Y, Fan F, Zhang Y. The Association of Plasma Homocysteine Concentrations with a 10-Year Risk of All-Cause and Cardiovascular Mortality in a Community-Based Chinese Population. Nutrients. 2024; 16(12):1945. https://doi.org/10.3390/nu16121945
Chicago/Turabian StyleLiang, Zhe, Kaiyin Li, Hongyu Chen, Jia Jia, Jianping Li, Yong Huo, Fangfang Fan, and Yan Zhang. 2024. "The Association of Plasma Homocysteine Concentrations with a 10-Year Risk of All-Cause and Cardiovascular Mortality in a Community-Based Chinese Population" Nutrients 16, no. 12: 1945. https://doi.org/10.3390/nu16121945
APA StyleLiang, Z., Li, K., Chen, H., Jia, J., Li, J., Huo, Y., Fan, F., & Zhang, Y. (2024). The Association of Plasma Homocysteine Concentrations with a 10-Year Risk of All-Cause and Cardiovascular Mortality in a Community-Based Chinese Population. Nutrients, 16(12), 1945. https://doi.org/10.3390/nu16121945








