Prognostic Implications of Insulin Resistance in Heart Failure in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Insulin Resistance Assessment
2.3. Clinical Covariates and Outcomes
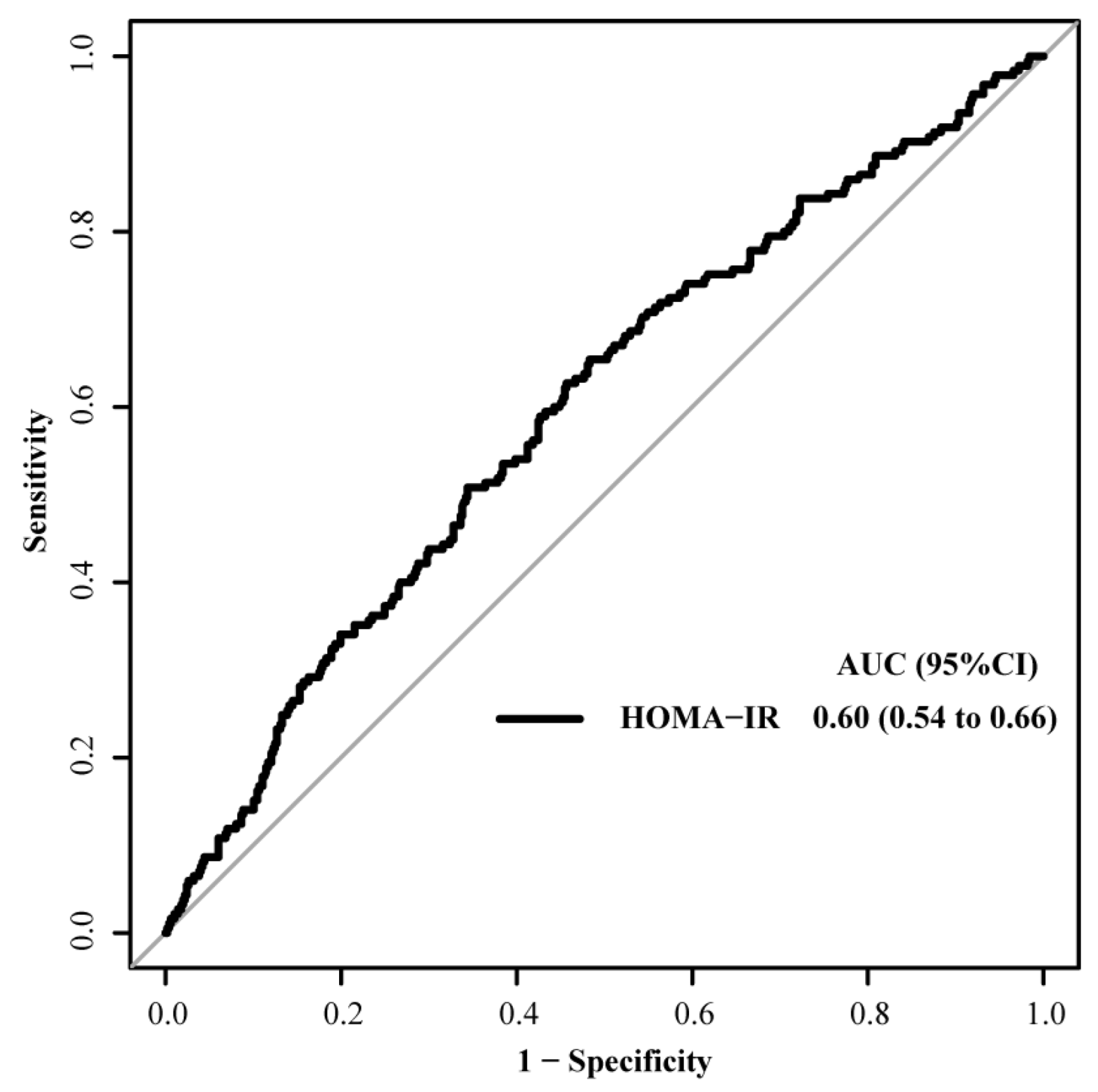
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
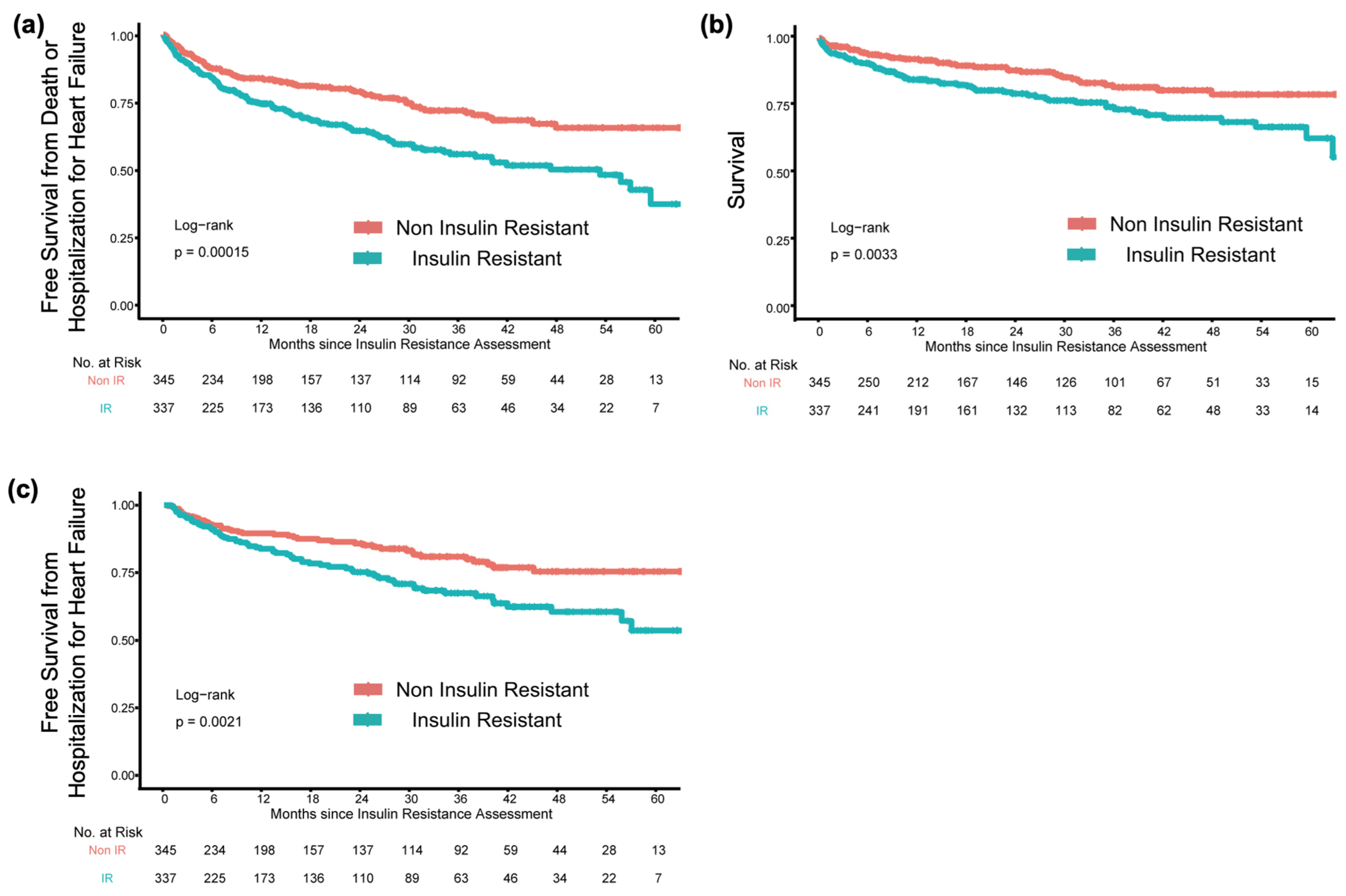
3.2. Clinical Outcomes According to IR Grouping
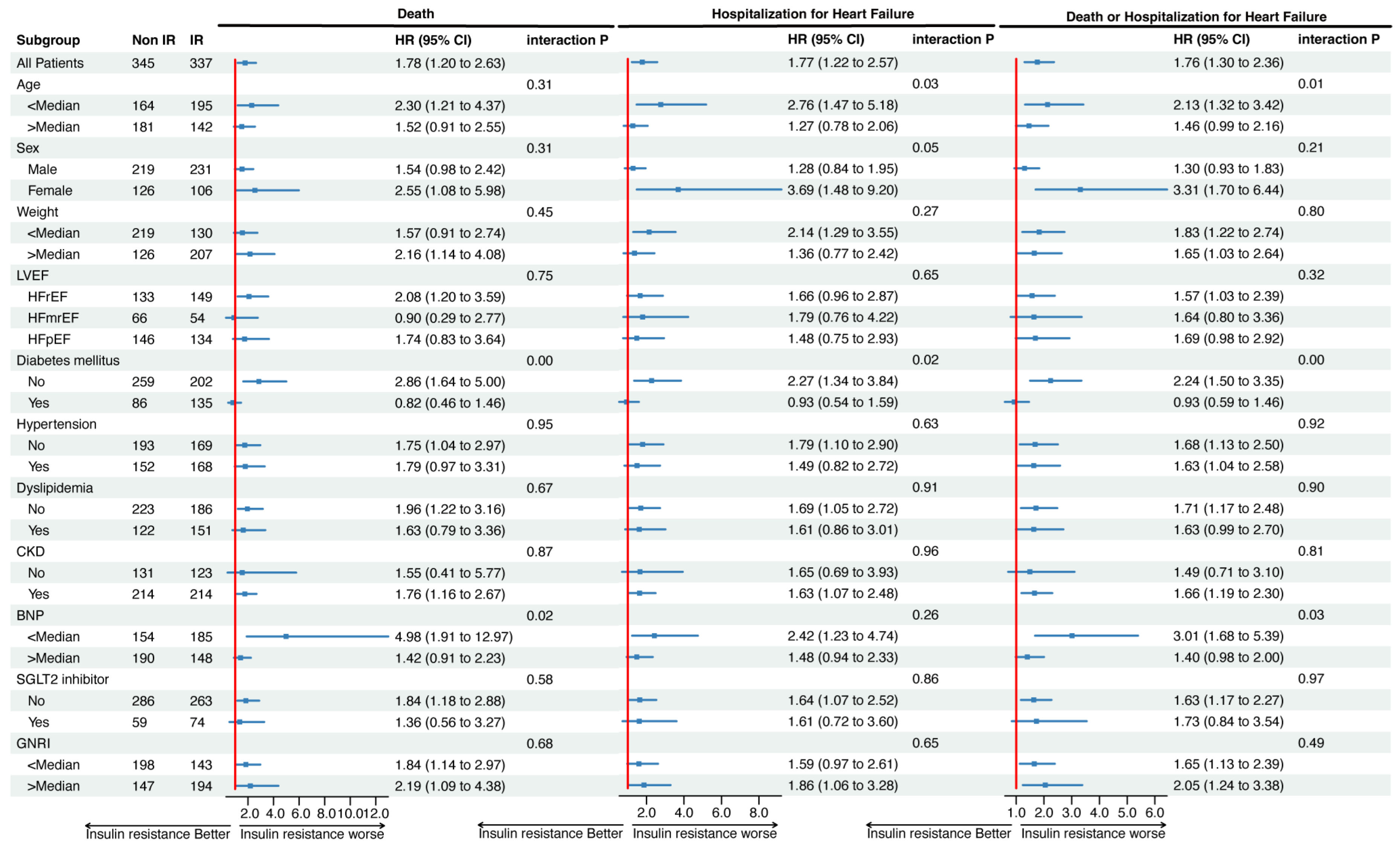
3.3. Association of IR with Clinical Outcomes in Each Subgroup
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L.; et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement from the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019, 140, e294–e324. [Google Scholar] [CrossRef]
- Bahrami, H.; Bluemke, D.A.; Kronmal, R.; Bertoni, A.G.; Lloyd-Jones, D.M.; Shahar, E.; Szklo, M.; Lima, J.A.C. Novel metabolic risk factors for incident heart failure and their relationship with obesity, the MESA (Multi-Ethnic Study of Atherosclerosis) Study. J. Am. Coll. Cardiol. 2008, 51, 1775–1783. [Google Scholar] [CrossRef]
- Allen, L.A.; Magid, D.J.; Gurwitz, J.H.; Smith, D.H.; Goldberg, R.J.; Saczynski, J.; Thorp, M.L.; Hsu, G.; Sung, S.H.; Go, A.S. Risk factors for adverse outcomes by left ventricular ejection fraction in a contemporary heart failure population. Circ. Heart Fail. 2013, 6, 635–646. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef]
- Erqou, S.; Adler, A.I.; Challa, A.A.; Fonarow, G.C.; Echouffo-Tcheugui, J.B. Insulin resistance and incident heart failure: A meta-analysis. Eur. J. Heart Fail. 2022, 24, 1139–1141. [Google Scholar] [CrossRef]
- Son, T.K.; Toan, N.H.; Thang, N.; Tuong, H.L.T.; Tien, H.A.; Thuy, N.H.; Minh, H.V.; Valensi, P. Prediabetes and insulin resistance in a population of patients with heart failure and reduced or preserved ejection fraction but without diabetes, overweight or hypertension. Cardiovasc. Diabetol. 2022, 21, 75. [Google Scholar] [CrossRef]
- Doehner, W.; Rauchhaus, M.; Ponikowski, P.; Godsland, I.F.; von Haehling, S.; Okonko, D.O.; Leyva, F.; Proudler, A.J.; Coats, A.J.S.; Anker, S.D. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J. Am. Coll. Cardiol. 2005, 46, 1019–1026. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Vardeny, O.; Gupta, D.K.; Claggett, B.; Burke, S.; Shah, A.; Loehr, L.; Rasmussen-Torvik, L.; Selvin, E.; Chang, P.P.; Aguilar, D.; et al. Insulin Resistance and Incident Heart Failure The ARIC Study (Atherosclerosis Risk in Communities). JACC Heart Fail. 2013, 1, 531–536. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Bergman, R.N.; Ider, Y.Z.; Bowden, C.R.; Cobelli, C. Quantitative estimation of insulin sensitivity. Am. J. Physiol. 1979, 236, e667–e677. [Google Scholar] [CrossRef]
- Garofolo, M.; Gualdani, E.; Scarale, M.G.; Bianchi, C.; Aragona, M.; Campi, F.; Lucchesi, D.; Daniele, G.; Miccoli, R.; Francesconi, P.; et al. Insulin resistance and risk of major vascular events and all-cause mortality in type 1 diabetes: A 10-year follow-up study. Diabetes Care 2020, 43, e139–e141. [Google Scholar] [CrossRef]
- Penno, G.; Solini, A.; Orsi, E.; Bonora, E.; Fondelli, C.; Trevisan, R.; Vedovato, M.; Cavalot, F.; Zerbini, G.; Lamacchia, O.; et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: A prospective cohort study. BMC Med. 2021, 19, 66. [Google Scholar] [CrossRef]
- Barr, E.L.; Cameron, A.J.; Balkau, B.; Zimmet, P.Z.; Welborn, T.A.; Tonkin, A.M.; Shaw, J.E. HOMA insulin sensitivity index and the risk of all-cause mortality and cardiovascular disease events in the general population: The Australian diabetes, obesity and lifestyle study (AusDiab). Diabetologia 2010, 53, 79–88. [Google Scholar] [CrossRef]
- Ausk, K.J.; Boyko, E.J.; Ioannou, G.N. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care 2010, 33, 1179–1185. [Google Scholar] [CrossRef]
- Welsh, P.; Preiss, D.; Lloyd, S.M.; Craen AJ de Jukema, J.W.; Westendorp, R.G.; Buckley, B.M.; Kearney, P.M.; Briggs, A.; Stott, D.J.; Ford, I.; et al. Contrasting associations of insulin resistance with diabetes, cardiovascular disease and all-cause mortality in the elderly: PROSPER long-term follow-up. Diabetologia 2014, 57, 2513–2520. [Google Scholar] [CrossRef]
- Pan, K.; Chlebowski, R.T.; Mortimer, J.E.; Gunther, M.J.; Rohan, T.; Vitolins, M.Z.; Adams-Campbell, L.L.; Ho, G.Y.F.; Cheng, T.D.; Nelson, R.A. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the women’s health initiative. Cancer 2020, 126, 3638–3647. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Arnold, A.M.; Aurigemma, G.P.; Polak, J.F.; Tracy, R.P.; Kitzman, D.W.; Gardin, J.M.; Rutledge, J.E.; Boineau, R.C. Predictors of congestive heart failure in the elderly: The cardiovascular health study. J. Am. Coll. Cardiol. 2000, 35, 1628–1637. [Google Scholar] [CrossRef]
- Chokshi, A.; Drosatos, K.; Cheema, F.H.; Ji, R.; Khawaja, T.; Yu, S.; Kato, T.; Khan, R.; Takayama, H.; Knöll, R.; et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 2012, 125, 2844–2853. [Google Scholar] [CrossRef]
- Schulze, P.C.; Biolo, A.; Gopal, D.; Shahzad, K.; Balog, J.; Fish, M.; Siwik, D.; Colucci, W.S. Dynamics in insulin resistance and plasma levels of adipokines in patients with acute decompensated and chronic stable heart failure. J. Card. Fail. 2011, 17, 1004–1011. [Google Scholar] [CrossRef]
- Wong, A.K.F.; Symon, R.; AlZadjali, M.A.; Ang, D.S.C.; Ogston, S.; Choy, A.; Petrie, J.R.; Struthers, A.D.; Lang, C.C. The effect of metformin on insulin resistance and exercise parameters in patients with heart failure. Eur. J. Heart Fail. 2012, 14, 1303–1310. [Google Scholar] [CrossRef]
- Larsen, A.H.; Jessen, N.; Nørrelund, H.; Tolbod, L.P.; Harms, H.J.; Feddersen, S.; Nielsen, F.; Brøsen, K.; Hansson, N.H.; Frøkiær, J.; et al. A randomised, double-blind, placebo-controlled trial of metformin on myocardial efficiency in insulin-resistant chronic heart failure patients without diabetes. Eur. J. Heart Fail. 2020, 22, 1628–1637. [Google Scholar] [CrossRef]
| All | HOMA-IR < 2.5 | HOMA-IR ≥ 2.5 | p Value | |
|---|---|---|---|---|
| n | 682 | 345 | 337 | |
| Age (median [IQR]) | 70 [59–77] | 71 [61–78] | 68 [57–76] | 0.004 |
| Male sex (%) | 450 (66.0) | 219 (63.5) | 231 (68.5) | 0.19 |
| Height (median [IQR]) | 162 [155, 168] | 161 [153, 167] | 163 [156, 169] | 0.005 |
| Weight (median [IQR]) | 60 [51, 70] | 57 [48, 65] | 64 [54, 74] | <0.001 |
| BSA (median [IQR]) | 1.63 [1.49–1.77] | 1.58 [1.45–1.73] | 1.69 [1.56–1.82] | <0.001 |
| BMI (median [IQR]) | 22.8 [20.3–25.7] | 21.8 [19.8–24.1] | 24.3 [21.3–27.1] | <0.001 |
| Ischaemic aetiology (%) | 134 (19.6) | 47 (13.6) | 87 (25.8) | <0.001 |
| Hypertension (%) | 320 (46.9) | 152 (44.1) | 168 (49.9) | 0.15 |
| Dyslipidaemia (%) | 273 (40.0) | 122 (35.4) | 151 (44.8) | 0.02 |
| DM (%) | 221 (32.4) | 86 (24.9) | 135 (40.1) | <0.001 |
| AF or AFL (%) | 134 (19.6) | 67 (19.4) | 67 (19.9) | 0.96 |
| CKD (%) | 428 (62.8) | 214 (62.0) | 214 (62.5) | 0.75 |
| CIED (%) | 200 (29.3) | 104 (30.1) | 96 (28.5) | 0.70 |
| CRT (%) | 95 (13.9) | 46 (13.3) | 49 (14.5) | 0.73 |
| Unplanned hospitalisation (%) | 257 (37.7) | 137 (39.7) | 120 (35.6) | 0.31 |
| LVEF spectrum | 0.28 | |||
| HFrEF (%) | 282 (41.3) | 133 (38.6) | 149 (44.2) | |
| HFmrEF (%) | 120 (17.6) | 66 (19.1) | 54 (16.0) | |
| HFpEF (%) | 280 (41.1) | 146 (42.3) | 134 (39.8) | |
| LVEF (median [IQR]) | 44 [31–58] | 45 [33–59] | 43 [30–57] | 0.22 |
| LVDd (median [IQR]) | 53 [46–60] | 52 [46–59] | 54 [46–61] | 0.08 |
| LAD (median [IQR]) | 43 [39–50] | 43 [39–49] | 44 [40–50] | 0.12 |
| BNP (median [IQR]) | 371 [148–778] | 447 [201–900] | 297 [117–646] | <0.001 |
| eGFR (median [IQR]) | 51 [35–68] | 52 [35–68] | 50 [34–67] | 0.50 |
| Albumin (median [IQR]) | 3.9 [3.4–4.2] | 3.8 [3.4–4.2] | 3.9 [3.4–4.2] | 0.36 |
| Sodium (median [IQR]) | 139 [137–141] | 139 [137–141] | 139 [137–141] | 1.00 |
| CRP (median [IQR]) | 0.19 [0.08–0.66] | 0.16 [0.06–0.59] | 0.22 [0.10–0.74] | 0.007 |
| GNRI | 100.8 [92.4, 109.4] | 98.2 [90.6, 105.5] | 103.5 [94.7, 112.3] | <0.001 |
| HOMA-IR (median [IQR]) | 2.47 [1.41–5.64] | 1.42 [0.93–1.84] | 5.72 [3.52–10.50] | <0.001 |
| RAAS inhibitor (%) | 421 (61.7) | 202 (58.6) | 219 (65.0) | 0.10 |
| ACEI (%) | 281 (41.2) | 133 (38.6) | 148 (43.9) | 0.18 |
| ARB (%) | 115 (16.9) | 55 (15.9) | 60 (17.8) | 0.59 |
| ARNI (%) | 26 (3.8) | 14 (4.1) | 12 (3.6) | 0.89 |
| BB (%) | 554 (81.2) | 267 (77.4) | 287 (85.2) | 0.01 |
| MRA (%) | 405 (59.4) | 207 (60.0) | 198 (58.8) | 0.80 |
| SGLT2 inhibitor (%) | 133 (19.5) | 59 (17.1) | 74 (22.0) | 0.13 |
| Statin (%) | 229 (33.6) | 90 (26.1) | 139 (41.2) | <0.001 |
| Insulin (%) | 35 (5.1) | 4 (1.2) | 31 (14.5) | <0.001 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | |
| Death | 1.78 | 1.20 to 2.63 | 0.00461 | 1.86 | 1.22 to 2.83 | 0.0041 |
| HHF | 1.77 | 1.22 to 2.57 | 0.00293 | 1.91 | 1.28 to 2.83 | 0.00162 |
| Death or HHF | 1.76 | 1.30 to 2.36 | 0.000247 | 1.91 | 1.39 to 2.62 | 0.0000778 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value | |
| Age | 1.02 | 1.01 to 1.04 | <0.001 | 1.01 | 1.00 to 1.02 | 0.10 |
| Male sex | 1.58 | 1.13 to 2.21 | <0.01 | 1.36 | 0.90 to 2.07 | 0.14 |
| Weight | 0.99 | 0.95 to 1.02 | 0.41 | 1.00 | 0.98 to 1.02 | 0.95 |
| DM | 1.68 | 1.25 to 2.25 | <0.001 | 1.10 | 0.78 to 1.54 | 0.60 |
| Ischaemic aetiology | 1.94 | 1.41 to 2.66 | <0.001 | 1.17 | 0.82 to 1.67 | 0.39 |
| SGLT2 inhibitor | 1.49 | 1.03 to 2.13 | 0.03 | 1.30 | 0.88 to 1.92 | 0.19 |
| GNRI | 0.96 | 0.94 to 0.97 | <0.001 | 0.98 | 0.96 to 0.99 | <0.01 |
| Sodium | 0.92 | 0.89 to 0.96 | <0.001 | 0.96 | 0.93 to 1.00 | 0.07 |
| log(BNP) | 1.51 | 1.32 to 1.72 | <0.001 | 1.21 | 1.04 to 1.41 | 0.01 |
| eGFR, per 1 mL/min/1.73 m2 | 0.98 | 0.97 to 0.98 | <0.001 | 0.99 | 0.98 to 0.99 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, K.; Nakamura, K.; Akagi, S.; Takaya, Y.; Toda, H.; Miyoshi, T.; Yuasa, S. Prognostic Implications of Insulin Resistance in Heart Failure in Japan. Nutrients 2024, 16, 1888. https://doi.org/10.3390/nu16121888
Iwasaki K, Nakamura K, Akagi S, Takaya Y, Toda H, Miyoshi T, Yuasa S. Prognostic Implications of Insulin Resistance in Heart Failure in Japan. Nutrients. 2024; 16(12):1888. https://doi.org/10.3390/nu16121888
Chicago/Turabian StyleIwasaki, Keiichiro, Kazufumi Nakamura, Satoshi Akagi, Yoichi Takaya, Hironobu Toda, Toru Miyoshi, and Shinsuke Yuasa. 2024. "Prognostic Implications of Insulin Resistance in Heart Failure in Japan" Nutrients 16, no. 12: 1888. https://doi.org/10.3390/nu16121888
APA StyleIwasaki, K., Nakamura, K., Akagi, S., Takaya, Y., Toda, H., Miyoshi, T., & Yuasa, S. (2024). Prognostic Implications of Insulin Resistance in Heart Failure in Japan. Nutrients, 16(12), 1888. https://doi.org/10.3390/nu16121888







