Association of Placental Pathology with Physical and Neuronal Development of Infants: A Narrative Review and Reclassification of the Literature by the Consensus Statement of the Amsterdam Placental Workshop Group
Abstract
1. Introduction
2. Materials and Methods
3. Results
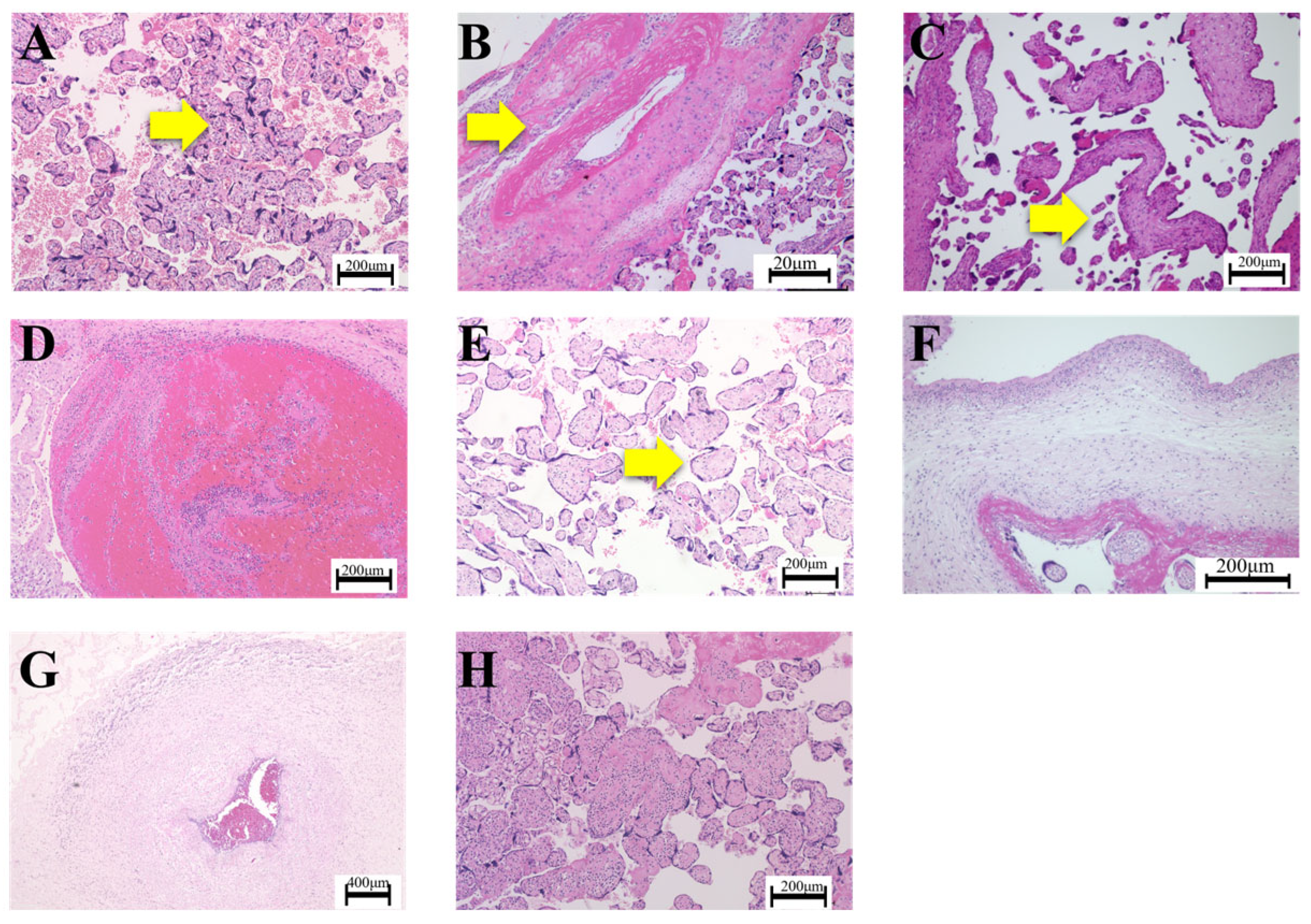
3.1. Articles Reclassified Using APWGCS
| First Author (Publication Year) | Country | Study Period | Sample Size | Selection Criteria | Postnatal Evaluation Period | Reference of Placental Pathology |
|---|---|---|---|---|---|---|
| Yaguchi C et al. (2018) [28] | Japan | 2007–2011 | 258 | Singleton pregnancy. General population between 29 and 42 wks | 18 m.o. | APWGCS |
| Ueda M et al. (2020) [30] | Japan | 2007–2011 | 258 | Singleton pregnancy. General population between 29 and 42 wks | 40 m.o. | APWGCS |
| Parra-Saavedra M et al. (2014) [38] | Spain | 2010–2012 | 83 | SGA infants delivered at >34 wks | 2 y.o. | International Federation of Placenta Associations and Society for Pediatric Pathology (perinatal section) |
| Chalak L, et al. (2021) [43] | USA | N/A | 321 | newborn infants with hypoxic–ischemic encephalopathy (HIE), Delivered at 36 wks | 2 y.o. | APWGCS |
| Vinnars MT et al. (2015) [39] | Sweden | 2004–2007 | 139 | Delivered at 22–26 wks | 2.5 y.o. | N/A |
| Mir IN et al. (2020) [40] | USA | 2009–2012 | 241 | Delivered at <29 wks | 2 y.o. | Modified Redline classification |
| Roescher AM et al. (2011) [41] | The Netherlands | N/A | 40 | Singleton pregnancy. Delivered at 25.4–31.7 wks | 24 h | Royal College of Obstetricians and Gynaecologists, Royal College of Pathologists, and College of American Pathologists |
| van Vliet EOG et al. (2012) [42] | The Netherlands | 2001–2003 | 72 | Singleton pregnancy. Delivered at <32 wks or birthweight of <1500 g | 2 y.o./7 y.o. | N/A |
| Gardella B, et al. (2021) [26] | Italy | 2007–2015 | 20 | Singleton pregnancy. FGR delivered at ≤34 wks and birthweight of ≤1500 g | 2 y.o. | APWGCS |
3.2. Maternal Vascular Malperfusion (MVM) and Infantile Outcomes
3.3. Fetal Vascular Malperfusion (FVM) and Infantile Outcomes
3.4. Maternal Inflammatory Response (MIR) and Infantile Outcomes
3.5. Fetal Inflammatory Response (FIR) and Infantile Outcomes
3.6. Villitis of Unknown Etiology (VUE) and Infantile Outcomes
3.7. Numbers of Non-Specific Placental Pathological Lesions and Infantile Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. What is the placenta? Am. J. Obstet. Gynecol. 2015, 213, S6.e1–S6.e4. [Google Scholar] [CrossRef] [PubMed]
- Tarrade, A.; Panchenko, P.; Junien, C.; Gabory, A. Placental contribution to nutritional programming of health and diseases: Epigenetics and sexual dimorphism. J. Exp. Biol. 2015, 218, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Benirschke, K.; Burton, G.; Baergen, R. Pathology of the Human Placenta, 6th ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Jansson, T.; Powell, T.L. Role of the placenta in fetal programming: Underlying mechanisms and potential interventional approaches. Clin. Sci. 2007, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, P.E.; Lemaire, M.; Fneich, S.; Voisin, S.; Jouin, M.; Junien, C.; Gabory, A. Epigenetics and Nutrition: Maternal nutrition impacts on placental development and health of offspring. Biol. Aujourdhui 2015, 209, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, E.; Hula, N.; Spaans, F.; Cooke, C.M.; Davidge, S.T. Placenta-targeted treatment strategies: An opportunity to impact fetal development and improve offspring health later in life. Pharmacol. Res. 2020, 157, 104836. [Google Scholar] [CrossRef] [PubMed]
- Aljunaidy, M.M.; Morton, J.S.; Cooke, C.M.; Davidge, S.T. Prenatal hypoxia and placental oxidative stress: Linkages to developmental origins of cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R395–R399. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Poston, L.; Gluckman, P.D. DOHaD—The challenge of translating the science to policy. J. Dev. Orig. Health Dis. 2019, 10, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Vacher, C.M.; Bonnin, A.; Mir, I.N.; Penn, A.A. Editorial: Advances and perspectives in neuroplacentology. Front. Endocrinol. 2023, 14, 1206072. [Google Scholar] [CrossRef] [PubMed]
- Kratimenos, P.; Penn, A.A. Placental programming of neuropsychiatric disease. Pediatr. Res. 2019, 86, 157–164. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.J.; Barak, S.; Penn, A.A. A new pipeline for clinico-pathological and molecular placental research utilizing FFPE tissues. Placenta 2021, 112, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Tsuchiya, K.J.; Yaguchi, C.; Horikoshi, Y.; Furuta-Isomura, N.; Oda, T.; Kohmura-Kobayashi, Y.; Tamura, N.; Uchida, T.; Itoh, H. The fetal/placental weight ratio is associated with the incidence of atopic dermatitis in female infants during the first 14months: The Hamamatsu Birth Cohort for Mothers and Children (HBC Study). Int. J. Womens Dermatol. 2020, 6, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Thornburg, K.L.; Osmond, C.; Kajantie, E.; Eriksson, J.G. Beyond birthweight: The maternal and placental origins of chronic disease. J. Dev. Orig. Health Dis. 2010, 1, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Gardella, B.; Dominoni, M.; Scatigno, A.L.; Cesari, S.; Fiandrino, G.; Orcesi, S.; Spinillo, A. What is known about neuroplacentology in fetal growth restriction and in preterm infants: A narrative review of literature. Front. Endocrinol. 2022, 13, 936171. [Google Scholar] [CrossRef] [PubMed]
- La Verde, M.; Torella, M.; Ronsini, C.; Riemma, G.; Cobellis, L.; Marrapodi, M.M.; Capristo, C.; Rapisarda, A.M.C.; Morlando, M.; De Franciscis, P. The association between fetal Doppler and uterine artery blood volume flow in term pregnancies: A pilot study. Ultraschall Med. 2024, 45, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Arizawa, M. Clinical Placentology (Japanese), 1st ed.; Kinpodo: Tokyo, Japan, 2013. [Google Scholar]
- Beebe, L.A.; Cowan, L.D.; Altshuler, G. The epidemiology of placental features: Associations with gestational age and neonatal outcome. Obstet. Gynecol. 1996, 87, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, K.M.; Folkins, A.K. Placental and Clinical Characteristics of Term Small-for-Gestational-Age Neonates: A Case-Control Study. Pediatr. Dev. Pathol. 2016, 19, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Elimian, A.; Verma, U.; Beneck, D.; Cipriano, R.; Visintainer, P.; Tejani, N. Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet. Gynecol. 2000, 96, 333–336. [Google Scholar] [CrossRef]
- Catov, J.M.; Scifres, C.M.; Caritis, S.N.; Bertolet, M.; Larkin, J.; Parks, W.T. Neonatal outcomes following preterm birth classified according to placental features. Am. J. Obstet. Gynecol. 2017, 216, 411.e411–411.e14. [Google Scholar] [CrossRef] [PubMed]
- Redline, R.W.; Roberts, D.J.; Parast, M.M.; Ernst, L.M.; Morgan, T.K.; Greene, M.F.; Gyamfi-Bannerman, C.; Louis, J.M.; Maltepe, E.; Mestan, K.K.; et al. Placental pathology is necessary to understand common pregnancy complications and achieve an improved taxonomy of obstetrical disease. Am. J. Obstet. Gynecol. 2023, 228, 187–202. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Slack, J.C.; Parra-Herran, C. Life After Amsterdam: Placental Pathology Consensus Recommendations and Beyond. Surg. Pathol. Clin. 2022, 15, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Gardella, B.; Dominoni, M.; Caporali, C.; Cesari, S.; Fiandrino, G.; Longo, S.; De Vito, G.B.; Naboni, C.; Tonduti, D.; Perotti, G.; et al. Placental features of fetal vascular malperfusion and infant neurodevelopmental outcomes at 2 years of age in severe fetal growth restriction. Am. J. Obstet. Gynecol. 2021, 225, 413.e11. [Google Scholar] [CrossRef] [PubMed]
- Takagai, S.; Tsuchiya, K.J.; Itoh, H.; Kanayama, N.; Mori, N.; Takei, N. Cohort Profile: Hamamatsu Birth Cohort for Mothers and Children (HBC Study). Int. J. Epidemiol. 2016, 45, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, C.; Itoh, H.; Tsuchiya, K.J.; Furuta-Isomura, N.; Horikoshi, Y.; Matsumoto, M.; Jeenat, F.U.; Keiko, M.K.; Kohmura-Kobatashi, Y.; Tamura, N.; et al. Placental pathology predicts infantile physical development during first 18 months in Japanese population: Hamamatsu birth cohort for mothers and children (HBC Study). PLoS ONE 2018, 13, e0194988. [Google Scholar] [CrossRef]
- Nimkar, S.; Joshi, S.; Kinikar, A.; Valvi, C.; Devaleenal, D.B.; Thakur, K.; Bendre, M.; Khwaja, S.; Ithape, M.; Kattagoni, K.; et al. Mullen Scales of Early Learning Adaptation for Assessment of Indian Children and Application to Tuberculous Meningitis. J. Trop. Pediatr. 2021, 67, fmaa034. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Tsuchiya, K.J.; Yaguchi, C.; Furuta-Isomura, N.; Horikoshi, Y.; Matsumoto, M.; Suzuki, M.; Oda, T.; Kawai, K.; Itoh, T.; et al. Placental pathology predicts infantile neurodevelopment. Sci. Rep. 2022, 12, 2578. [Google Scholar] [CrossRef]
- Fogarty, N.M.; Ferguson-Smith, A.C.; Burton, G.J. Syncytial knots (Tenney-Parker changes) in the human placenta: Evidence of loss of transcriptional activity and oxidative damage. Am. J. Pathol. 2013, 183, 144–152. [Google Scholar] [CrossRef]
- Desa, D.J. Intimal cushions in foetal placental veins. J. Pathol. 1973, 110, 347–352. [Google Scholar] [CrossRef]
- Conti, N.; Torricelli, M.; Voltolini, C.; Vannuccini, S.; Clifton, V.L.; Bloise, E.; Petraglia, F. Term histologic chorioamnionitis: A heterogeneous condition. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 188, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Chaemsaithong, P.; Romero, R.; Shaman, M.; Kim, C.J.; Kim, J.S.; Qureshi, F.; Jacques, S.M.; Ahmed, A.I.; Chaiworapongsa, T.; et al. Placental lesions associated with acute atherosis. J. Matern. Fetal Neonatal Med. 2015, 28, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Redline, R.W. Villitis of unknown etiology: Noninfectious chronic villitis in the placenta. Hum. Pathol. 2007, 38, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Masaki, N.; Kohmura-Kobayashi, Y.; Yaguchi, C.; Hayasaka, T.; Itoh, H.; Setou, M.; Kanayama, N. Decrease in Sphingomyelin (d18:1/16:0) in Stem Villi and Phosphatidylcholine (16:0/20:4) in Terminal Villi of Human Term Placentas with Pathohistological Maternal Malperfusion. PLoS ONE 2015, 10, e0142609. [Google Scholar] [CrossRef] [PubMed]
- Furuta, N.; Yaguchi, C.; Itoh, H.; Morishima, Y.; Tamura, N.; Kato, M.; Uchida, T.; Suzuki, K.; Sugihara, K.; Kawabata, Y.; et al. Immunohistochemical detection of meconium in the fetal membrane, placenta and umbilical cord. Placenta 2012, 33, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Parra-Saavedra, M.; Crovetto, F.; Triunfo, S.; Savchev, S.; Peguero, A.; Nadal, A.; Parra, G.; Gratacos, E.; Figueras, F. Neurodevelopmental outcomes of near-term small- for-gestationl age infants with and without signs of placental underperfusion. Placenta 2014, 35, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Vinnars, M.T.; Vollmer, B.; Nasiell, J.; Papadogiannakis, N.; Westgren, M. Association between cerebral palsy and microscopically verified placental infarction in extremely preterm infants. Acta Obstet. Gynecol. Scand. 2015, 94, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.N.; Chalak, L.F.; Brown, L.S.; Johnson-Welch, S.; Heyne, R.; Rosenfeld, C.R.; Kapadia, V.S. Impact of multiple placental pathologies on neonatal death, bronchopulmonary dysplasia, and neurodevelopmental impairment in preterm infants. Pediatr. Res. 2020, 87, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Roescher, A.M.; Hitzert, M.M.; Timmer, A.; Verhagen, E.A.; Erwich, J.J.; Bos, A.F. Placental pathology is associated with illness severity in preterm infants in the first twenty-four hours after birth. Early Hum. Dev. 2011, 87, 315–319. [Google Scholar] [CrossRef]
- van Vliet, E.O.; de Kieviet, J.F.; van der Voorn, J.P.; Been, J.V.; Oosterlaan, J.; van Elburg, R.M. Placental pathology and long-term neurodevelopment of very preterm infants. Am. J. Obstet. Gynecol. 2012, 206, 489.e1–489.e7. [Google Scholar] [CrossRef] [PubMed]
- Chalak, L.; Redline, R.W.; Goodman, A.M.; Juul, S.E.; Chang, T.; Yanowitz, T.D.; Maitre, N.; Mayock, D.E.; Lampland, A.L.; Bendel-Stenzel, E.; et al. Acute and Chronic Placental Abnormalities in a Multicenter Cohort of Newborn Infants with Hypoxic-Ischemic Encephalopathy. J. Pediatr. 2021, 237, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Dominoni, M.; Mas, F.D.; Cesari, S.; Fiandrino, G.; Gardella, B. Placental fetal vascular malperfusion, neonatal neurologic morbidity, and infant neurodevelopmental outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2023, 229, 632–640.e2. [Google Scholar] [CrossRef] [PubMed]
| First Author (Publication Year) | Pathological Descriptions for Reclassifying as ‘MVM’ Using APWGCS Criteria | Selection Criteria | Postnatal Evaluation Period | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|
| MVM | Accelerated Villous Maturation | Decidual Arteriopathy | Distal Villous Hypoplasia | Infarction | Other | ||||
| Yaguchi C et al. (2018) [28] | AD | AD | AD | Singleton pregnancy. General population between 29 and 42 wks | 18 m.o. | Negative impact on body weight | |||
| Ueda M et al. (2020) [30] | AD | AD | AD | Singleton pregnancy. General population between 29 and 42 wks | 40 m.o. | Negative impact on Mullen Scale of Early Learning composite scores in neurodevelopment | |||
| Parra-Saavedra M et al. (2014) [38] | Placental under-perfusion | Excessive intervillous fibrin syncytial knots involving terminal villi and villous agglutination (>50%) | Acute atherosis and mural hypertrophy | Undergrowth/distal villous hypoplasia | Intervillous fibrin deposition and villous infarcts | Specific vascular alterations indicative of maternal vascular loss of integrity include arterial rupture (abruption placenta) and venous rupture (acute and chronic marginal abruption). Maldevelopmental vascular alterations include chorioangioma and chorioangiosis | SGA infants delivered at >34 wks | 2 y.o. | # Negative impact on Bayley neurodevelopmental scores (Cognitive, Language, Motor) compared to MIR (chorioamnionitis) |
| Chalak L, et al. (2021) [43] | AD | Newborn infants with HIE, delivered at 36 wks | 2 y.o. | $ Negative impact on base deficit within the first hour of birth | |||||
| Vinnars MT et al. (2015) [39] | Abnormal villous maturation | Decidual arteriopathy | Placental infarction, placental abruption | Intervillous thrombosis | Delivered at 22–26 wks | 2.5 y.o. | Negative impact on the occurrence of Cerebral palsy | ||
| Mir IN, et al. (2020) [40] | Severe maternal decidual vasculopathy | Distal villous hypoplasia | Infarcts | Delivered at <29 wks | 2 y.o. | No specific association, although non-specific multiple pathological lesions had a negative association with neurodevelopmental impairment and/or bronchopulmonary dysplasia | |||
| Roescher AM et al. (2011) [41] | Maternal vascular under-perfusion | Singleton pregnancy. Delivered at 25.4–31.7 wks | 24 h | No association | |||||
| van Vliet EOG et al. (2012) [42] | Placental under-perfusion | Increased syncytial knots, villous agglutination | Distal villous hypoplasia | Intervillous fibrin | Singleton pregnancy. Delivered at <32 wks or birthweight of <1500 g | 2 y.o./7 y.o. | Negative association with Mental Developmental Index score at 2 y.o. but not at 7 y.o., compared to MIR | ||
| Gardella B et al. (2021) [26] | AD | AD | AD | AD | AD | Arterial or venous abruption | Singleton pregnancy. FGR delivered at ≤34 wks and birthweight of ≤1500 g | 2 y.o. | No association |
| First Author (Publication Year) | Pathological Descriptions for Reclassifying as FVM Using APWGCS Criteria | Selection Criteria | Postnatal Evaluation Period | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| FVM | Avascular Villi | Fetal Thrombosis | Villous Stromal Vascular Karyorrhexis | Consideration | ||||
| Yaguchi C et al. (2018) [28] | AD | AD | AD | Singleton pregnancy. General population between 29 and 42 wks | 18 m.o. | No association | ||
| Ueda M et al. (2020) [30] | AD | AD | AD | Singleton pregnancy. General population between 29 and 42 wks | 40 m.o. | Positive impact on Mullen Scale of Early Learning composite scores in neurodevelopment | ||
| Parra-Saavedra M et al. (2014) [38] | Fetal vascular supply | Villous avascularity | Thrombosis of chorionic plate and stem villous channels | SGA infants delivered at >34 wks | 2 y.o. | # Negative association with Bayley neurodevelopmental scores (Cognitive, Language, Motor) compared to MIR (chorioamnionitis) | ||
| Chalak L, et al. (2021) [43] | AD | Newborn infants with HIE, delivered at 36 wks | 2 y.o. | $ Negative impact on base deficit within the first hour of birth | ||||
| Vinnars MT et al. (2015) [39] | Fetal thrombosis | Delivered at 22–26 wks | 2.5 y.o. | No association | ||||
| Mir IN et al. (2020) [40] | Fetal thrombotic vasculopathy | AD | Segmental or complete obstruction | Villous stromal vascular karyorrhexis | Delivered at <29 wks | 2 y.o. | No specific association, although non-specific multiple pathological lesions had negative association with neurodevelopmental impairment and/or bronchopulmonary dysplasia | |
| Roescher AM et al. (2011) [41] | Fetal thrombotic vasculopathy | Singleton pregnancy. Delivered at 25.4–31.7 wks | 24 h | Negative impact on the Score of Neonatal Acute Physiology Perinatal Extension (SNAPPE) | ||||
| van Vliet EOG et al. (2012) [42] | N/A | Singleton pregnancy. Delivered at <32 wks or birthweight of <1500 g | 2 y.o./7 y.o. | N/A | ||||
| Gardella B et al. (2021) [26] | AD | AD | Intramural fibrin of large placental vessels | Intermittent cord obstruction, ectasia | Singleton pregnancy. FGR delivered at ≤34 wks or birthweight of ≤1500 g | 2 y.o. | Negative impact on neurodevelopment | |
| First Author (Publication Year) | Pathological Descriptions for Reclassifying as ‘MIR’ of APWGCS | Selection Criteria | Postnatal Evaluation Period | Outcomes |
|---|---|---|---|---|
| Yaguchi C et al. (2018) [28] | AD | Singleton pregnancy. General population between 29 and 42 wks | 18 m.o. | No association |
| Ueda M et al. (2020) [30] | AD | Singleton pregnancy. General population between 29 and 42 wks | 40 m.o. | No association |
| Parra-Saavedra M et al. (2014) [38] | N/A | SGA infants delivered at >34 wks | 2 y.o. | N/A |
| Chalak L, et al. (2021) [43] | AD | Newborn infants with HIE, delivered at 36 wks | 2 y.o. | N/A |
| Vinnars MT et al. (2015) [39] | Acute chorioamnionitis | Delivered at 22–26 wks | 2.5 y.o. | No association |
| Mir IN et al. (2020) [40] | Chorioamnionitis | Delivered at <29 wks | 2 y.o. | No specific association, although non-specific multiple pathological lesions had negative association with neurodevelopmental impairment and/or bronchopulmonary dysplasia |
| Roescher AM et al. (2011) [41] | Ascending intrauterine infection | Singleton pregnancy. Delivered at 25.4–31.7 wks | 24 h | No association |
| van Vliet EOG et al. (2012) [42] | Chorioamnionitis | Singleton pregnancy. Delivered at <32 wks or birthweight of <1500 g | 2 y.o./7 y.o. | Positive association with Bayley neurodevelopmental scores (Cognitive, Language, Motor) compared to MVM |
| Gardella B et al. (2021) [26] | Acute chorioamnionitis (stage and grade) | Singleton pregnancy. FGR delivered at ≤34 wks and birthweight of ≤1500 g | 2 y.o. | No association |
| First Author (Publication Year) | Pathological Descriptions for Reclassifying as ‘FIR’ Using APWGCS Criteria | Selection Criteria | Postnatal Evaluation Period | Outcomes |
|---|---|---|---|---|
| Yaguchi C et al. (2018) [28] | AD | Singleton pregnancy. General population between 29 and 42 wks | 18 m.o. | No association |
| Ueda M et al. (2020) [30] | AD | Singleton pregnancy. General population between 29 and 42 wks | 40 m.o. | Positive association with Mullen Scale of Early Learning composite scores in neurodevelopment |
| Parra-Saavedra M et al. (2014) [38] | N/A | SGA infants delivered at >34 wks | 2 y.o. | N/A |
| Chalak L, et al. (2021) [43] | AD | Newborn infants with HIE, delivered at 36 wks | 2 y.o. | N/A |
| Vinnars MT et al. (2015) [39] | N/A | Delivered at 22–26 wks | 2.5 y.o. | N/A |
| Mir IN et al. (2020) [40] | Vasculitis in the umbilical vessels and/or chorionic plate vessels | Delivered at <29 wks | 2 y.o. | No specific association, although non-specific multiple pathological lesions had a negative association with neurodevelopmental impairment and/or bronchopulmonary dysplasia |
| Roescher AM et al. (2011) [41] | N/A | Singleton pregnancy. Delivered at 25.4–31.7 wks | 24 h | N/A |
| van Vliet EOG et al. (2012) [42] | Chorioamnionitis with an additional fetal response | Singleton pregnancy. Delivered at <32 wks or birthweight of <1500 g | 2 y.o./7 y.o. | N/A |
| Gardella B et al. (2021) [26] | Fetal inflammatory acute response (stage and grade) | Singleton pregnancy. FGR delivered at ≤34 wks and birthweight of ≤1500 g | 2 y.o. | No association |
| First Author (Publication Year) | Pathological Descriptions for Reclassifying as VUE Using APWGCS Criteria | Selection Criteria | Postnatal Evaluation Period | Outcomes |
|---|---|---|---|---|
| Yaguchi C et al. (2018) [28] | AD | Singleton pregnancy. General population between 29 and 42 wks | 18 m.o. | No association |
| Ueda M et al. (2020) [30] | AD | Singleton pregnancy. General population between 29 and 42 wks | 40 m.o. | No association |
| Parra-Saavedra M et al. (2014) [38] | N/A | SGA infants delivered at >34 wks | 2 y.o. | N/A |
| Chalak L, et al. (2021) [43] | AD | Newborn infants with HIE, delivered at 36 wks | 2 y.o. | $ Negative impact on base deficit within the first hour of birth |
| Vinnars MT et al. (2015) [39] | Chronic villitis | Delivered at 22–26 wks | 2.5 y.o. | No case |
| Mir IN et al. (2020) [40] | Chronic villitis | Delivered at <29 wks | 2 y.o. | No specific association, although non-specific multiple pathological lesions had negative association with neurodevelopmental impairment and/or bronchopulmonary dysplasia |
| Roescher AM et al. (2011) [41] | Chronic villitis of unknown origin, chronic villitis | Singleton pregnancy. Delivered at 25.4–31.7 wks | 24 h | No association |
| van Vliet EOG et al. (2012) [42] | N/A | Singleton pregnancy. Delivered at <32 wks or birthweight of <1500 g | 2 y.o./7 y.o. | No association |
| Gardella B et al. (2021) [26] | Chronic villitis, either of unknownsignificance or infectious villitis | Singleton pregnancy. FGR delivered at ≤34 wks and birthweight of ≤1500 g | 2 y.o. | No association |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaguchi, C.; Ueda, M.; Mizuno, Y.; Fukuchi, C.; Matsumoto, M.; Furuta-Isomura, N.; Itoh, H. Association of Placental Pathology with Physical and Neuronal Development of Infants: A Narrative Review and Reclassification of the Literature by the Consensus Statement of the Amsterdam Placental Workshop Group. Nutrients 2024, 16, 1786. https://doi.org/10.3390/nu16111786
Yaguchi C, Ueda M, Mizuno Y, Fukuchi C, Matsumoto M, Furuta-Isomura N, Itoh H. Association of Placental Pathology with Physical and Neuronal Development of Infants: A Narrative Review and Reclassification of the Literature by the Consensus Statement of the Amsterdam Placental Workshop Group. Nutrients. 2024; 16(11):1786. https://doi.org/10.3390/nu16111786
Chicago/Turabian StyleYaguchi, Chizuko, Megumi Ueda, Yuri Mizuno, Chie Fukuchi, Masako Matsumoto, Naomi Furuta-Isomura, and Hiroaki Itoh. 2024. "Association of Placental Pathology with Physical and Neuronal Development of Infants: A Narrative Review and Reclassification of the Literature by the Consensus Statement of the Amsterdam Placental Workshop Group" Nutrients 16, no. 11: 1786. https://doi.org/10.3390/nu16111786
APA StyleYaguchi, C., Ueda, M., Mizuno, Y., Fukuchi, C., Matsumoto, M., Furuta-Isomura, N., & Itoh, H. (2024). Association of Placental Pathology with Physical and Neuronal Development of Infants: A Narrative Review and Reclassification of the Literature by the Consensus Statement of the Amsterdam Placental Workshop Group. Nutrients, 16(11), 1786. https://doi.org/10.3390/nu16111786







