Prenatal Iodine Intake and Maternal Pregnancy and Postpartum Depressive and Anhedonia Symptoms: Findings from a Multiethnic US Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposures
2.3. Outcomes
2.4. Data Analysis
3. Results
3.1. Characteristics of Study Participants and Prenatal Iodine Intake
3.2. Regression Results
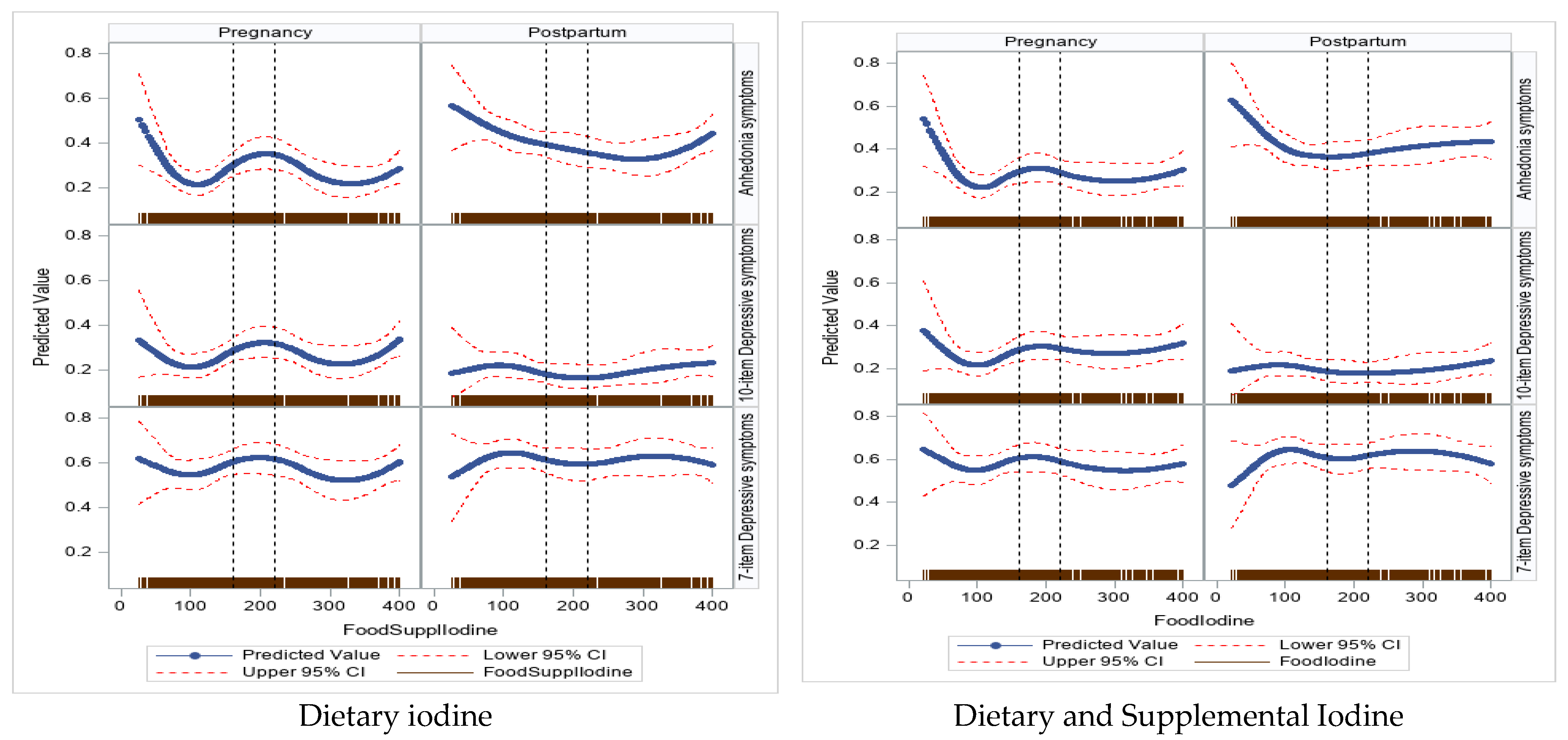
3.2.1. Iodine Intake and Global EPDS Score—Antepartum
3.2.2. Postpartum
3.2.3. Iodine Intake and Anhedonia and Depressive Subscales Symptom Scores—Antepartum
3.2.4. Postpartum
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shorey, S.; Chee, C.Y.I.; Ng, E.D.; Chan, Y.H.; Tam, W.W.S.; Chong, Y.S. Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 104, 235–248. [Google Scholar] [CrossRef]
- Gollan, J.K.; Yang, A.; Ciolino, J.D.; Sit, D.; Wisner, K.L. Postpartum anhedonia: Emergent patterns in bipolar and unipolar depression. Psychiatry Res. 2021, 306, 114274. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, C.; Luo, Y.; Gao, S.; Sun, J.; Chen, Q.; Lv, X.; Cao, J.; Lei, Z.; Fang, J. Altered neural activity in the reward-related circuit associated with anhedonia in mild to moderate Major Depressive Disorder. J. Affect. Disord. 2024, 345, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.; Marano, G. Is peripartum anhedonia a missing target? Bipolar Disord. 2024, 26, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Putnam, K.T.; Wilcox, M.; Robertson-Blackmore, E.; Sharkey, K.; Bergink, V.; Munk-Olsen, T.; Deligiannidis, K.M.; Payne, J.; Altemus, M.; Newport, J.; et al. Clinical phenotypes of perinatal depression and time of symptom onset: Analysis of data from an international consortium. Lancet Psychiatry 2017, 4, 477–485. [Google Scholar] [CrossRef]
- Vliegen, N.; Casalin, S.; Luyten, P. The course of postpartum depression: A review of longitudinal studies. Harv. Rev. Psychiatry 2014, 22, 1–22. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Eberhard-Gran, M.; Eskild, A.; Samuelsen, S.O.; Tambs, K. A short matrix-version of the Edinburgh Depression Scale. Acta Psychiatr. Scand. 2007, 116, 195–200. [Google Scholar] [CrossRef]
- Conroy, S.; Pariante, C.M.; Marks, M.N.; Davies, H.A.; Farrelly, S.; Schacht, R.; Moran, P. Maternal psychopathology and infant development at 18 months: The impact of maternal personality disorder and depression. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 51–61. [Google Scholar] [CrossRef]
- Leung, B.M.; Kaplan, B.J. Perinatal depression: Prevalence, risks, and the nutrition link—A review of the literature. J. Am. Diet. Assoc. 2009, 109, 1566–1575. [Google Scholar] [CrossRef]
- Sun, Y.; Ferguson, M.; Reeves, M.M.; Kelly, J.T. Maternal Dietary Patterns and Risk of Postpartum Depression: A Systematic Review. Matern. Child. Health J. 2023, 27, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Dama, M.; Steiner, M.; Van Lieshout, R. Thyroid peroxidase autoantibodies and perinatal depression risk: A systematic review. J. Affect. Disord. 2016, 198, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Minaldi, E.; D’andrea, S.; Castellini, C.; Martorella, A.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Thyroid autoimmunity and risk of post-partum depression: A systematic review and meta-analysis of longitudinal studies. J. Endocrinol. Investig. 2020, 43, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Sylvén, S.M.; Elenis, E.; Michelakos, T.; Larsson, A.; Olovsson, M.; Poromaa, I.S.; Skalkidou, A. Thyroid function tests at delivery and risk for postpartum depressive symptoms. Psychoneuroendocrinology 2013, 38, 1007–1013. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The Importance of Adequate Iodine during Pregnancy and Infancy. World Rev. Nutr. Diet. 2016, 115, 118–124. [Google Scholar] [PubMed]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of iodine deficiency and excess in pregnant women: An overview of current knowns and unknowns. Am. J. Clin. Nutr. 2016, 104 (Suppl. S3), 918s–923s. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001. [Google Scholar]
- Brantsæter, A.L.; Garthus-Niegel, S.; Brandlistuen, R.E.; Caspersen, I.H.; Meltzer, H.M.; Abel, M.H. Mild-to-moderate iodine deficiency and symptoms of emotional distress and depression in pregnancy and six months postpartum—Results from a large pregnancy cohort. J. Affect. Disord. 2022, 318, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Moleti, M.; Di Bella, B.; Giorgianni, G.; Mancuso, A.; De Vivo, A.; Alibrandi, A.; Trimarchi, F.; Vermiglio, F. Maternal thyroid function in different conditions of iodine nutrition in pregnant women exposed to mild-moderate iodine deficiency: An observational study. Clin. Endocrinol. 2011, 74, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, C.; Teng, Y.; Guan, Y.; Zhang, L.; Jia, X.; Cui, D.; Li, J.; Guan, H. The Effect of Iodine-Containing Vitamin Supplementation during Pregnancy on Thyroid Function in Late Pregnancy and Postpartum Depression in an Iodine-Sufficient Area. Biol. Trace Elem. Res. 2020, 198, 1–7. [Google Scholar] [CrossRef]
- Block, G.; Hartman, A.M.; Dresser, C.M.; Carroll, M.D.; Gannon, J.; Gardner, L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986, 124, 453–469. [Google Scholar] [CrossRef]
- Parrott, M.S.; Bodnar, L.M.; Simhan, H.N.; Harger, G.; Markovic, N.; Roberts, J.M. Maternal cereal consumption and adequacy of micronutrient intake in the periconceptional period. Public Health Nutr. 2009, 12, 1276–1283. [Google Scholar] [CrossRef]
- Boucher, B.; Cotterchio, M.; Kreiger, N.; Nadalin, V.; Block, T.; Block, G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006, 9, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wirfält, A.K.; Jeffery, R.W.; Elmer, P.J. Comparison of food frequency questionnaires: The reduced Block and Willett questionnaires differ in ranking on nutrient intakes. Am. J. Epidemiol. 1998, 148, 1148–1156. [Google Scholar] [CrossRef]
- Völgyi, E.; Carroll, K.N.; Hare, M.E.; Ringwald-Smith, K.; Piyathilake, C.; Yoo, W.; Tylavsky, F.A. Dietary patterns in pregnancy and effects on nutrient intake in the Mid-South: The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study. Nutrients 2013, 5, 1511–1530. [Google Scholar] [CrossRef] [PubMed]
- Roseland, J.M.; Spungen, J.H.; Patterson, K.Y.; Ershow, A.G.; Gahche, J.J.; Pehrsson, P. USDA, FDA, and ODS-NIH Database for the Iodine Content of Common Foods. In Release; U.S. Department of Agriculture Agricultural Research Service: West Beltsville, MD, USA, 2022. [Google Scholar]
- Ershow, A.G.; Haggans, C.J.; Roseland, J.M.; Patterson, K.Y.; Spungen, J.H.; Gahche, J.J.; Merkel, J.M.; Pehrsson, P.R. Databases of Iodine Content of Foods and Dietary Supplements-Availability of New and Updated Resources. J. Acad. Nutr. Diet. 2022, 122, 1229–1231. [Google Scholar] [CrossRef]
- Pehrsson, P.R.; Roseland, J.M.; Patterson, K.Y.; Phillips, K.M.; Spungen, J.H.; Andrews, K.W.; Gusev, P.A.; Gahche, J.J.; Haggans, C.J.; Merkel, J.M.; et al. Iodine in Foods and Dietary Supplements: A Collaborative Database Developed by NIH, FDA and USDA. J. Food Compost. Anal. 2022, 109, 104369. [Google Scholar] [CrossRef]
- Abt, E.; Kumar, S.; Weyant, R.J. Periodontal disease and medical maladies: What do we really know? J. Am. Dent. Assoc. 2022, 153, 9–13. [Google Scholar] [CrossRef]
- Ershow, A.G.; Skeaff, S.A.; Merkel, J.M.; Pehrsson, P.R. Development of Databases on Iodine in Foods and Dietary Supplements. Nutrients 2018, 10, 100. [Google Scholar] [CrossRef]
- Milagres, R.; Souza, E.C.G.; Peluzio, M.; Franceschini, S.; Duarte, M.S.L. Food Iodine Content Table compiled from international databases. Rev. Nutr. 2020, 33, e190222. [Google Scholar] [CrossRef]
- Lautarescu, A.; Victor, S.; Lau-Zhu, A.; Counsell, S.J.; Edwards, A.D.; Craig, M.C. The factor structure of the Edinburgh Postnatal Depression Scale among perinatal high-risk and community samples in London. Arch. Womens Ment. Health 2022, 25, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Flom, J.D.; Chiu, Y.-H.M.; Tamayo-Ortiz, M.; Schnaas, L.; Curtin, P.C.; Wright, R.J.; O Wright, R.; Téllez-Rojo, M.M.; Rosa, M.J. Subconstructs of the Edinburgh Postpartum Depression Scale in a postpartum sample in Mexico City. J. Affect. Disord. 2018, 238, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.M.; Sheffield, P.E.; Hsu, H.-H.L.; Goldstein, J.; Curtin, P.C.; Wright, R.J. Subconstructs of the Edinburgh Postnatal Depression Scale in a multi-ethnic inner-city population in the U.S. Arch. Womens Ment. Health 2017, 20, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Levis, B.; Negeri, Z.; Sun, Y.; Benedetti, A.; Thombs, B.D. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: Systematic review and meta-analysis of individual participant data. BMJ (Clin. Res. Ed.) 2020, 371, m4022. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Yonkers, K.A.; Vigod, S.; Ross, L.E. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet. Gynecol. 2011, 117, 961–977. [Google Scholar] [CrossRef]
- Basraon, S.; Costantine, M.M. Mood disorders in pregnant women with thyroid dysfunction. Clin. Obstet. Gynecol. 2011, 54, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Siegmann, E.M.; Müller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Grömer, T.W. Association of Depression and Anxiety Disorders with Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, V.; Altshuler, L.; Whybrow, P. Psychoneuroendocrinology of mood disorders. The hypothalamic-pituitary-thyroid axis. Psychiatr. Clin. N. Am. 1998, 21, 277–292. [Google Scholar] [CrossRef]
- Bath, S.C. The effect of iodine deficiency during pregnancy on child development. Proc. Nutr. Soc. 2019, 78, 150–160. [Google Scholar] [CrossRef]
- Abel, M.H.; Korevaar, T.I.M.; Erlund, I.; Villanger, G.D.; Caspersen, I.H.; Arohonka, P.; Alexander, J.; Meltzer, H.M.; Brantsaeter, A.L. Iodine Intake is Associated with Thyroid Function in Mild to Moderately Iodine Deficient Pregnant Women. Thyroid 2018, 28, 1359–1371. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Ovesen, L.; Bülow, I.; Jørgensen, T.; Knudsen, N.; Laurberg, P.; Perrild, H. Dietary iodine intake and urinary iodine excretion in a Danish population: Effect of geography, supplements and food choice. Br. J. Nutr. 2002, 87, 61–69. [Google Scholar] [CrossRef]
- Andersen, S.; Karmisholt, J.; Pedersen, K.M.; Laurberg, P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br. J. Nutr. 2008, 99, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.; Azizi, F.; Hedayati, M.; Mirmiran, P.; O’Herlihy, C.; Smyth, P.P. Is placental iodine content related to dietary iodine intake? Clin. Endocrinol. 2011, 75, 261–264. [Google Scholar] [CrossRef]
- Murphy, S.P.; Guenther, P.M.; Kretsch, M.J. Using the dietary reference intakes to assess intakes of groups: Pitfalls to avoid. J. Am. Diet. Assoc. 2006, 106, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Winer, E.S.; Veilleux, J.C.; Ginger, E.J. Development and validation of the Specific Loss of Interest and Pleasure Scale (SLIPS). J. Affect. Disord. 2014, 152–154, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Gard, D.E.; Gard, M.G.; Kring, A.M.; John, O.P. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J. Res. Personal. 2006, 40, 1086–1102. [Google Scholar] [CrossRef]
- Treadway, M.T.; Zald, D.H. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011, 35, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Guintivano, J.; Manuck, T.; Meltzer-Brody, S. Predictors of Postpartum Depression: A Comprehensive Review of the Last Decade of Evidence. Clin. Obstet. Gynecol. 2018, 61, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Q.; Wang, N.; Li, S.; Zhang, H.; Zhu, Y.; Guo, H.; Wang, F.; He, L.; Xia, S.; et al. Serum and urinary essential trace elements in association with major depressive disorders: A case-control study. Front. Psychiatry 2023, 14, 1297411. [Google Scholar] [CrossRef]
| N | Percent | Food and Supplement Iodine (µg/Day), Median (IQR) # | p-Value | |
|---|---|---|---|---|
| Overall | 837 | 100 | 187 (116, 315) | |
| Maternal factors | ||||
| Age at birth, years, mean (SD) * | 29.8 (5.92) | |||
| Age at birth, years | 0.4 | |||
| 18–<25 | 194 | 23.2 | 175 (111, 324) | |
| 25–<35 | 473 | 56.5 | 188 (114, 310) | |
| ≥35 | 170 | 20.3 | 198 (127, 320) | |
| Race/ethnicity | 0.05 | |||
| Non-Hispanic White | 142 | 17.3 | 151 (110, 269) | |
| Black/Hispanic Black | 352 | 43 | 198 (115, 337) | |
| Non-Black Hispanic | 288 | 35.1 | 195 (126, 323) | |
| Other | 37 | 4.52 | 153 (107, 281) | |
| Missing | 18 | |||
| Education | 0.6 | |||
| High school or less | 321 | 39.2 | 185 (111, 331) | |
| More than high school | 496 | 60.7 | 191 (118, 311) | |
| Missing | 20 | |||
| Depression pre-pregnancy | 0.02 | |||
| Yes | 208 | 25.3 | 204 (131, 378) | |
| No | 614 | 74.7 | 181 (112, 304) | |
| Missing | 15 | |||
| Pregnancy depressive symptoms (Global EPDS) | 0.05 | |||
| <10 | 538 | 71.5 | 177 (112, 312) | |
| ≥10 | 214 | 28.5 | 203 (127, 345) | |
| Missing | 85 | |||
| Postpartum depressive symptoms (Global EPDS) | 0.6 | |||
| <10 | 454 | 79.2 | 191 (117, 319) | |
| ≥10 | 119 | 20.8 | 168 (116, 324) | |
| Missing | 264 | |||
| Pregnancy anhedonia symptoms | 0.6 | |||
| 0 | 552 | 73.5 | 185 (115, 324) | |
| 1–6 | 199 | 26.5 | 193 (120, 317) | |
| Missing | 86 | |||
| Postpartum anhedonia symptoms | 0.04 | |||
| 0 | 391 | 68.2 | 198 (126, 319) | |
| 1–6 | 182 | 31.8 | 158 (108, 321) | |
| Missing | 264 | |||
| Pregnancy depressive subscale symptoms | 0.5 | |||
| <4 | 339 | 45.1 | 180 (111, 318) | |
| ≥4 | 413 | 54.9 | 193 (118, 322) | |
| Missing | 85 | |||
| Postpartum depressive subscale symptoms | 0.8 | |||
| <4 | 292 | 51.0 | 188 (117, 323) | |
| ≥4 | 281 | 49.0 | 187 (118, 315) | |
| Missing | 264 | |||
| Thyroid disease § | 0.4 | |||
| Yes | 52 | 6.33 | 170 (113, 278) | |
| No | 769 | 93.7 | 190 (116, 320) | |
| Missing | 16 | |||
| Child sex | 0.8 | |||
| Female | 422 | 50.5 | 181 (116, 323) | |
| Male | 413 | 49.5 | 192 (115, 305) | |
| Missing | 2 |
| Prenatal Anhedonia Symptoms | Prenatal Global EPDS Score | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||
| Dietary Iodine Intake | Crude | Adjusted | Crude | Adjusted |
| <100 µg/day | 1.03 (0.61, 1.73) | 0.99 (0.58, 1.67) | 0.87 (0.52, 1.47) | 0.82 (0.48, 1.40) |
| 100–<160 µg/day | 1.14 (0.71, 1.84) | 1.17 (0.72, 1.90) | 0.97 (0.60, 1.57) | 0.96 (0.59, 1.57) |
| 160–220 µg/day | Ref. | Ref. | Ref. | Ref. |
| >220–<400 µg/day | 1.05 (0.63, 1.75) | 1.04 (0.62, 1.74) | 1.13 (0.68, 1.86) | 1.09 (0.65, 1.83) |
| ≥400 µg/day | 1.40 (0.80, 2.47) | 1.29 (0.72, 2.30) | 1.35 (0.77, 2.36) | 1.16 (0.65, 2.08) |
| Dietary and supplemental iodine | ||||
| <100 µg/day | 0.98 (0.58, 1.67) | 0.96 (0.56, 1.65) | 0.89 (0.52, 1.53) | 0.86 (0.49, 1.50) |
| 100–<160 µg/day | 1.00 (0.62, 1.65) | 1.04 (0.63, 1.71) | 0.93 (0.56, 1.53) | 0.93 (0.56, 1.56) |
| 160–220 µg/day | Ref. | Ref. | Ref. | Ref. |
| >220–<400 µg/day | 0.97 (0.60, 1.58) | 0.97 (0.59, 1.60) | 1.08 (0.66, 1.76) | 1.07 (0.65, 1.77) |
| ≥400 µg/day | 1.19 (0.69, 2.05) | 1.12 (0.64, 1.94) | 1.33 (0.78, 2.29) | 1.18 (0.68, 2.06) |
| Postpartum Anhedonia Symptoms | Postpartum Global EPDS Score | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||
| Dietary Iodine Intake | Crude | Adjusted | Crude | Adjusted |
| <100 µg/day | 1.74 (1.09, 2.78) | 1.74 (1.08, 2.79) | 1.20 (0.68, 2.13) | 1.20 (0.66, 2.19) |
| 100–<160 µg/day | 1.23 (0.79, 1.90) | 1.25 (0.80, 1.99) | 1.15 (0.67, 1.98) | 1.17 (0.67, 2.05) |
| 160–220 µg/day | Ref. | Ref. | Ref. | Ref. |
| >220–<400 µg/day | 1.32 (0.83, 2.10) | 1.31 (0.82, 2.10) | 1.16 (0.66, 2.06) | 1.12 (0.62, 2.01) |
| ≥400 µg/day | 1.53 (0.90, 2.60) | 1.47 (0.86, 2.51) | 1.21 (0.63, 2.30) | 1.03 (0.53, 2.02) |
| Dietary and supplemental iodine intake | ||||
| <100 µg/day | 1.72 (1.06, 2.79) | 1.74 (1.07, 2.83) | 1.35 (0.73 (2.49) | 1.39 (0.74, 2.63) |
| 100–<160 µg/day | 1.23 (0.78, 1.94) | 1.26 (0.80, 1.99) | 1.38 (0.78, 2.45) | 1.44 (0.80, 2.59) |
| 160–220 µg/day | Ref. | Ref. | Ref. | Ref. |
| >220–<400 µg/day | 0.94 (0.60, 1.48) | 0.93 (0.59, 1.47) | 1.35 (0.77, 2.39) | 1.31 (0.73, 2.35) |
| ≥400 µg/day | 1.29 (0.78, 2.14) | 1.22 (0.73, 2.04) | 1.32 (0.70, 2.50) | 1.12 (0.58, 2.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinkugbe, A.A.; Chiu, Y.-H.M.; Kannan, S.; Bergink, V.; Wright, R.J. Prenatal Iodine Intake and Maternal Pregnancy and Postpartum Depressive and Anhedonia Symptoms: Findings from a Multiethnic US Cohort. Nutrients 2024, 16, 1771. https://doi.org/10.3390/nu16111771
Akinkugbe AA, Chiu Y-HM, Kannan S, Bergink V, Wright RJ. Prenatal Iodine Intake and Maternal Pregnancy and Postpartum Depressive and Anhedonia Symptoms: Findings from a Multiethnic US Cohort. Nutrients. 2024; 16(11):1771. https://doi.org/10.3390/nu16111771
Chicago/Turabian StyleAkinkugbe, Aderonke A., Yueh-Hsiu Mathilda Chiu, Srimathi Kannan, Veerle Bergink, and Rosalind J. Wright. 2024. "Prenatal Iodine Intake and Maternal Pregnancy and Postpartum Depressive and Anhedonia Symptoms: Findings from a Multiethnic US Cohort" Nutrients 16, no. 11: 1771. https://doi.org/10.3390/nu16111771
APA StyleAkinkugbe, A. A., Chiu, Y.-H. M., Kannan, S., Bergink, V., & Wright, R. J. (2024). Prenatal Iodine Intake and Maternal Pregnancy and Postpartum Depressive and Anhedonia Symptoms: Findings from a Multiethnic US Cohort. Nutrients, 16(11), 1771. https://doi.org/10.3390/nu16111771








