Longitudinal Analysis of One-Carbon Metabolism-Related Metabolites in Maternal and Cord Blood of Japanese Pregnant Women
Abstract
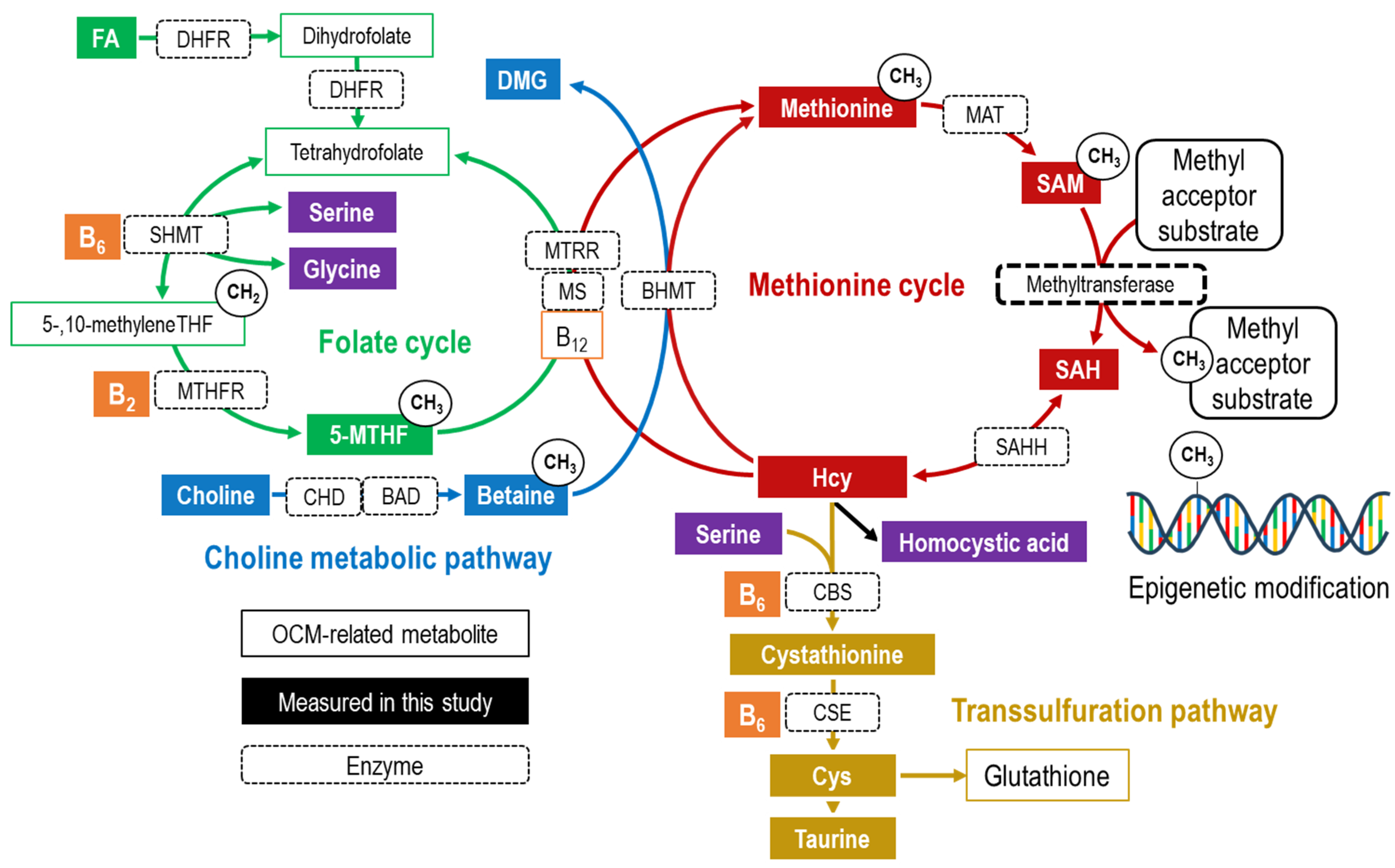
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Information on Mothers and Children
2.4. Measurement of Serum OCM-Related Metabolites
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Serum Concentrations of OCM-Related Metabolites across Blood Sampling Periods
3.3. Relationship between Maternal Blood at Birth and Cord Blood in OCM-Related Metabolites
3.3.1. Comparison of Serum OCM-Related Metabolite Concentrations between Maternal Blood at Birth and Cord Blood
3.3.2. Correlation of Serum OCM-Related Metabolite Concentrations between Maternal and Cord Blood at Birth
3.4. Cross-Sectional Relationships between OCM-Related Metabolites in Maternal Blood at Each Blood Collection Period and Cord Blood
3.4.1. Correlation between Serum 5-MTHF or Betaine Concentration and SAM Concentration
3.4.2. Longitudinal Changes in Correlation Coefficients for Homocysteine Metabolism during Pregnancy
3.4.3. 5-MTHF or Betaine Concentration Associated with the tHcy/tCys Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef] [PubMed]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-carbon metabolism: Linking nutritional biochemistry to epigenetic programming of long-term development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, J.; Gruca, L.L.; Bennett, C.; Parimi, P.S.; Duenas, C.; Marczewski, S.; Fierro, J.L.; Kalhan, S.C. Methionine metabolism in human pregnancy. Am. J. Clin. Nutr. 2010, 91, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Nijhout, H.F.; Reed, M.C. One-carbon metabolism during the menstrual cycle and pregnancy. PLoS Comput. Biol. 2021, 17, e1009708. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, K.M.; Williams, B.A.; Elango, R.; Barr, S.I.; Karakochuk, C.D. Pregnancy-induced alterations of 1-carbon metabolism and significance for maternal nutrition requirements. Nutr. Rev. 2022, 80, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Shoji, K.; Tajima, A.; Horiguchi, S.; Fukuoka, H.; Nishikawa, M.; Kagawa, Y.; Kawabata, T. Serum 5-methyltetrahydrofolate status is associated with one-carbon metabolism-related metabolite concentrations and enzyme activity indicators in young women. Int. J. Mol. Sci. 2023, 24, 10993. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of nutrition for development-folate review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Guerra-Shinohara, E.M.; Morita, O.E.; Peres, S.; Pagliusi, R.A.; Sampaio Neto, L.F.; D’Almeida, V.; Irazusta, S.P.; Allen, R.H.; Stabler, S.P. Low ratio of S-adenosylmethionine to S-adenosylhomocysteine is associated with vitamin deficiency in Brazilian pregnant women and newborns. Am. J. Clin. Nutr. 2004, 80, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.R.; Stabler, S.P.; Machado, A.L.; Braga, R.C.; Hirata, R.D.; Hirata, M.H.; Sampaio-Neto, L.F.; Allen, R.H.; Guerra-Shinohara, E.M. Association between decreased vitamin levels and MTHFR, MTR and MTRR gene polymorphisms as determinants for elevated total homocysteine concentrations in pregnant women. Eur. J. Clin. Nutr. 2008, 62, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Tserga, A.; Binder, A.M.; Michels, K.B. Impact of folic acid intake during pregnancy on genomic imprinting of IGF2/H19 and 1-carbon metabolism. FASEB J. 2017, 31, 5149–5158. [Google Scholar] [CrossRef] [PubMed]
- Adaikalakoteswari, A.; Webster, C.; Goljan, I.; Saravanan, P. Simultaneous detection of five one-carbon metabolites in plasma using stable isotope dilution liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1012, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sepulveda, A.; Espana-Perrot, P.P.; Fernandez, X.B.; Ahumada, V.; Bustos, V.; Arraztoa, J.A.; Dobierzewska, A.; Figueroa-Diesel, H.; Rice, G.E.; Illanes, S.E. Levels of key enzymes of methionine-homocysteine metabolism in preeclampsia. BioMed Res. Int. 2013, 2013, 731962. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, T.; Zhao, X.; Guan, Z.; Wang, Z.; Zhu, Z.; Xie, Q.; Wang, J.; Niu, B. Quantification of folate metabolites in serum using ultraperformance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 962, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Luo, G.A.; Liang, Q.L.; Wang, Y.; Yang, H.H.; Wang, Y.M.; Zheng, X.Y.; Song, X.M.; Chen, G.; Zhang, T.; et al. Neural tube defects and disturbed maternal folate- and homocysteine-mediated one-carbon metabolism. Exp. Neurol. 2008, 212, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Lopreato, F.R.; Stabler, S.P.; Carvalho, F.R.; Hirata, R.D.; Hirata, M.H.; Robi, D.L.; Sampaio-Neto, L.F.; Allen, R.H.; Guerra-Shinohara, E.M. Relationships between gene polymorphisms of folate-related proteins and vitamins and metabolites in pregnant women and neonates. Clin. Chim. Acta 2008, 398, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.K.; Park, H.J.; Hausman, D.B.; Fleming, J.M.; Bland, V.L.; Rosa, G.; Kennedy, E.M.; Caudill, M.A.; Malysheva, O.; Kauwell, G.P.A.; et al. Association between one-carbon metabolism indices and DNA methylation status in maternal and cord blood. Sci. Rep. 2018, 8, 16873. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Fukuoka, H.; Kawabata, T.; Shoji, K.; Mori, C.; Sakurai, K.; Nishikawa, M.; Ohkubo, T.; Oshida, K.; Yanagisawa, N.; et al. Distribution of 5-methyltetrahydrofolate and folic acid levels in maternal and cord blood serum: Longitudinal evaluation of Japanese pregnant women. Nutrients 2020, 12, 1633. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Miyaso, H.; Eguchi, A.; Matsuno, Y.; Yamamoto, M.; Todaka, E.; Fukuoka, H.; Hata, A.; Mori, C. Chiba study of Mother and Children’s Health (C-MACH): Cohort study with omics analyses. BMJ Open. 2016, 6, e010531. [Google Scholar] [CrossRef]
- McGregor, D.O.; Dellow, W.J.; Lever, M.; George, P.M.; Robson, R.A.; Chambers, S.T. Dimethylglycine accumulates in uremia and predicts elevated plasma homocysteine concentrations. Kidney Int. 2001, 59, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- King, W.D.; Ho, V.; Dodds, L.; Perkins, S.L.; Casson, R.I.; Massey, T.E. Relationships among biomarkers of one-carbon metabolism. Mol. Biol. Rep. 2012, 39, 7805–7812. [Google Scholar] [CrossRef] [PubMed]
- Ulvik, A.; Hustad, S.; McCann, A.; Midttun, O.; Nygard, O.K.; Ueland, P.M. Ratios of one-carbon metabolites are functional markers of b-vitamin status in a Norwegian coronary angiography screening cohort. J. Nutr. 2017, 147, 1167–1173. [Google Scholar] [CrossRef]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Visentin, C.E.; Masih, S.; Plumptre, L.; Malysheva, O.; Nielsen, D.E.; Sohn, K.J.; Ly, A.; Lausman, A.Y.; Berger, H.; Croxford, R.; et al. Maternal Choline status, but not fetal genotype, influences cord plasma choline metabolite concentrations. J. Nutr. 2015, 145, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Ozarda Ilcol, Y.; Uncu, G.; Ulus, I.H. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch. Physiol. Biochem. 2002, 110, 393–399. [Google Scholar] [CrossRef]
- Velzing-Aarts, F.V.; Holm, P.I.; Fokkema, M.R.; van der Dijs, F.P.; Ueland, P.M.; Muskiet, F.A. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am. J. Clin. Nutr. 2005, 81, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Roig, S.; Cavalle-Busquets, P.; Fernandez-Ballart, J.D.; Ballesteros, M.; Berrocal-Zaragoza, M.I.; Salat-Batlle, J.; Ueland, P.M.; Murphy, M.M. Low folate status enhances pregnancy changes in plasma betaine and dimethylglycine concentrations and the association between betaine and homocysteine. Am. J. Clin. Nutr. 2013, 97, 1252–1259. [Google Scholar] [CrossRef]
- Wu, B.T.; Innis, S.M.; Mulder, K.A.; Dyer, R.A.; King, D.J. Low plasma vitamin B-12 is associated with a lower pregnancy-associated rise in plasma free choline in Canadian pregnant women and lower postnatal growth rates in their male infants. Am. J. Clin. Nutr. 2013, 98, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Coopman, M.F.; Tan, A.; Schroder, T.H.; Sinclair, G.; Vallance, H.D.; Lamers, Y. Serum betaine and dimethylglycine are higher in south Asian compared with European pregnant women in Canada, with betaine and total homocysteine inversely associated in early and midpregnancy, independent of ethnicity. J. Nutr. 2019, 149, 2145–2155. [Google Scholar] [CrossRef]
- Resseguie, M.; Song, J.; Niculescu, M.D.; da Costa, K.A.; Randall, T.A.; Zeisel, S.H. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. Faseb. J. 2007, 21, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Jiang, X.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Brenna, J.T.; Stabler, S.P.; Allen, R.H.; Gregory, J.F., 3rd; Caudill, M.A. Pregnancy alters choline dynamics: Results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am. J. Clin. Nutr. 2013, 98, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Park, E.I.; Garrow, T.A. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organization of the human gene. J. Biol. Chem. 1999, 274, 7816–7824. [Google Scholar] [CrossRef] [PubMed]
- Varsi, K.; Ueland, P.M.; Torsvik, I.K.; Bjørke-Monsen, A.L. Maternal serum cobalamin at 18 weeks of pregnancy predicts infant cobalamin status at 6 months-a prospective, observational study. J. Nutr. 2018, 148, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.E.; Wu, Q.; Wang, X.; Deng, L.; Caudill, M.A.; Rozen, R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J. Nutr. 2010, 140, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Midttun, Ø.; McCann, A.; Aarseth, O.; Krokeide, M.; Kvalheim, G.; Meyer, K.; Ueland, P.M. Combined measurement of 6 fat-soluble vitamins and 26 water-soluble functional vitamin markers and amino acids in 50 μL of serum or plasma by high-throughput mass spectrometry. Anal. Chem. 2016, 88, 10427–10436. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Kopec, J.; Rembeza, E.; Bezerra, G.A.; Oberholzer, A.E.; Suormala, T.; Lutz, S.; Chalk, R.; Borkowska, O.; Baumgartner, M.R.; et al. Structural basis for the regulation of human 5,10-methylenetetrahydrofolate reductase by phosphorylation and S-adenosylmethionine inhibition. Nat. Commun. 2018, 9, 2261. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Reed, M.C.; Anderson, D.F.; Mattingly, J.C.; James, S.J.; Ulrich, C.M. Long-range allosteric interactions between the folate and methionine cycles stabilize DNA methylation reaction rate. Epigenetics 2006, 1, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B. Homocysteine: Overview of biochemistry, molecular biology, and role in disease processes. Semin. Vasc. Med. 2005, 5, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Janosik, M.; Kery, V.; Gaustadnes, M.; Maclean, K.N.; Kraus, J.P. Regulation of human cystathionine beta-synthase by S-adenosyl-L-methionine: Evidence for two catalytically active conformations involving an autoinhibitory domain in the C-terminal region. Biochemistry 2001, 40, 10625–10633. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.M.; Hoffman, J.L. Fractionation and kinetic properties of rat liver and kidney methionine adenosyltransferase isozymes. Biochemistry 1983, 22, 1636–1641. [Google Scholar] [CrossRef]
- Markham, G.D.; Pajares, M.A. Structure-function relationships in methionine adenosyltransferases. Cell. Mol. Life Sci. 2009, 66, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Martin, J.J. Inactivation of betaine-homocysteine methyltransferase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun. 1984, 118, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. The metabolism of homocysteine: Pathways and regulation. Eur. J. Pediatr. 1998, 157 (Suppl. S2), S40–S44. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Dudman, N.P.; Perry, M.A.; Young, K.; Wang, X.L. Interrelations between plasma homocysteine and intracellular S-adenosylhomocysteine. Biochem. Biophys. Res. Commun. 2000, 271, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fux, R.; Kloor, D.; Hermes, M.; Röck, T.; Proksch, B.; Grenz, A.; Delabar, U.; Bücheler, R.; Igel, S.; Mörike, K.; et al. Effect of acute hyperhomocysteinemia on methylation potential of erythrocytes and on DNA methylation of lymphocytes in healthy male volunteers. Am. J. Physiol. Renal. Physiol. 2005, 289, F786–F792. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Melnyk, S.; Pogribna, M.; Pogribny, I.P.; Hine, R.J.; James, S.J. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J. Biol. Chem. 2000, 275, 29318–29323. [Google Scholar] [CrossRef]
- Ehrlich, M. Expression of various genes is controlled by DNA methylation during mammalian development. J. Cell. Biochem. 2003, 88, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Finnell, R.H.; Spiegelstein, O.; Wlodarczyk, B.; Triplett, A.; Pogribny, I.P.; Melnyk, S.; James, J.S. DNA methylation in Folbp1 knockout mice supplemented with folic acid during gestation. J. Nutr. 2002, 132, 2457S–2461S. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Gruca, L.L.; Parimi, P.S.; O’Brien, A.; Dierker, L.; Burkett, E. Serine metabolism in human pregnancy. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E733–E740. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.L.; Hellmuth, C.; Uhl, O.; Buss, C.; Wadhwa, P.D.; Koletzko, B.; Entringer, S. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS ONE 2015, 10, e0145794. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Plumptre, L.; Brosnan, M.E.; Pongnopparat, T.; Masih, S.P.; Visentin, C.E.; Berger, H.; Lamers, Y.; Caudill, M.A.; Malysheva, O.V.; et al. Formate concentrations in maternal plasma during pregnancy and in cord blood in a cohort of pregnant Canadian women: Relations to genetic polymorphisms and plasma metabolites. Am. J. Clin. Nutr. 2019, 110, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Marczewski, S.E. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord 2012, 13, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y. Lean Body mass harbors sensing mechanisms that allow safeguarding of methionine homeostasis. Nutrients 2017, 9, 1035. [Google Scholar] [CrossRef] [PubMed]
- Tochitani, S. Taurine: A Maternally derived nutrient linking mother and offspring. Metabolites 2022, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Elliott, A.; Bowling, F.G. Sulphate in pregnancy. Nutrients 2015, 7, 1594–1606. [Google Scholar] [CrossRef] [PubMed]
- Cikot, R.J.; Steegers-Theunissen, R.P.; Thomas, C.M.; de Boo, T.M.; Merkus, H.M.; Steegers, E.A. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br. J. Nutr. 2001, 85, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Coopman, M.F.; Farias, D.R.; Franco-Sena, A.B.; Vaz, J.S.; Kac, G.; Lamers, Y. Maternal plasma pyridoxal 5’-phosphate concentration is inversely associated with plasma cystathionine concentration across all trimesters in healthy pregnant women. J. Nutr. 2019, 149, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Scott, J.M.; McPartlin, J.M.; Fernandez-Ballart, J.D. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am. J. Clin. Nutr. 2002, 76, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Byg, K.E.; Hvas, A.M.; Bergholt, T.; Eriksen, L. Erythrocyte folate, plasma folate and plasma homocysteine during normal pregnancy and postpartum: A longitudinal study comprising 404 Danish women. Eur. J. Haematol. 2006, 76, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, N.; Reyes, L.; González-Medina, A.; Alonso-Aperte, E.; Varela-Moreiras, G. Physiologic changes in homocysteine metabolism in pregnancy: A longitudinal study in Spain. Nutrition 2011, 27, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Visentin, C.E.; Masih, S.P.; Plumptre, L.; Schroder, T.H.; Sohn, K.J.; Ly, A.; Lausman, A.Y.; Berger, H.; Croxford, R.; Lamers, Y.; et al. Low serum vitamin b-12 concentrations are prevalent in a cohort of pregnant Canadian women. J. Nutr. 2016, 146, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, H.; Tang, A.; Xiang, Z. Changes of serum homocysteine levels during pregnancy and the establishment of reference intervals in pregnant Chinese women. Clin. Chim. Acta 2019, 489, 1–4. [Google Scholar] [CrossRef]
- Viskova, H.; Vesela, K.; Janosikova, B.; Krijt, J.; Visek, J.A.; Calda, P. Plasma cysteine concentrations in uncomplicated pregnancies. Fetal Diagn. Ther. 2007, 22, 254–258. [Google Scholar] [CrossRef]
- Sadre-Marandi, F.; Dahdoul, T.; Reed, M.C.; Nijhout, H.F. Sex differences in hepatic one-carbon metabolism. BMC Syst. Biol. 2018, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, A.; Chmurzynska, A. Folate and choline absorption and uptake: Their role in fetal development. Biochimie 2019, 158, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Lofthouse, E.M.; Sengers, B.G.; Lewis, R.M. A systems perspective on placental amino acid transport. J. Physiol. 2018, 596, 5511–5522. [Google Scholar] [CrossRef] [PubMed]
- Tsitsiou, E.; Sibley, C.P.; D’Souza, S.W.; Catanescu, O.; Jacobsen, D.W.; Glazier, J.D. Homocysteine is transported by the microvillous plasma membrane of human placenta. J. Inherit. Metab. Dis. 2011, 34, 57–65. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, S.W.; Glazier, J.D. Homocysteine metabolism in pregnancy and developmental impacts. Front. Cell. Dev. Biol. 2022, 10, 802285. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Inui, K. Novel riboflavin transporter family RFVT/SLC52: Identification, nomenclature, functional characterization and genetic diseases of RFVT/SLC52. Mol. Asp. Med. 2013, 34, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.M.; Reed, M.C.; Nijhout, H.F. A population model of folate-mediated one-carbon metabolism. Nutrients 2013, 5, 2457–2474. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.M.; Reed, M.C.; Nijhout, H.F. The relationship between intracellular and plasma levels of folate and metabolites in the methionine cycle: A model. Mol. Nutr. Food. Res. 2013, 57, 628–636. [Google Scholar] [CrossRef] [PubMed]
| Maternal Blood | Cord Blood (n = 121) | Cord Blood/Maternal Blood at Birth Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Pregnancy (n = 146) | Late Pregnancy (n = 131) | At Birth (n = 116) | |||||||||
| Analytes | Unit | Median | (25th, 75th) | Median | (25th, 75th) | Median | (25th, 75th) | Median | (25th, 75th) | Median | (25th, 75th) |
| 5-MTHF † | nmol/L | 32.2 a | (20.3, 52.8) | 17.0 b | (11.6, 31.7) | 14.1 c | (9.8, 23.2) | 44.7 *** | (36.5, 64.2) | 3.23 | (2.18, 4.42) |
| FA † | nmol/L | 0.620 a | (0.095, 1.221) | 0.620 | (0.127, 1.205) | 0.433 b | (0 †, 1.052) | 0.530 | (0 †, 1.043) | 0.90 | (0.39, 1.47) |
| Choline | µmol/L | 7.39 a | (6.35, 9.00) | 7.98 b | (6.83, 9.80) | 11.30 c | (9.45, 12.84) | 28.25 *** | (25.05, 32.35) | 2.56 | (2.18, 3.06) |
| Betaine | µmol/L | 21.1 a | (17.3, 25.3) | 13.8 b | (11.8, 17.1) | 13.5 b | (11.6, 16.0) | 26.9 *** | (24.2, 31.2) | 1.95 | (1.67, 2.30) |
| DMG | µmol/L | 1.77 a | (1.24, 2.43) | 1.70 a | (1.13, 2.44) | 2.22 b | (1.64, 3.32) | 3.17 *** | (2.57, 4.12) | 1.36 | (1.16, 1.70) |
| Betaine/DMG | µmol/L | 11.71 a | (9.11, 16.20) | 8.37 b | (6.25, 11.93) | 6.04 c | (4.40, 8.40) | 8.64 *** | (6.63, 11.57) | - | |
| Methionine | µmol/L | 18.7 | (16.4, 23.8) | 19.2 | (17, 22.8) | 20.5 | (17.9, 23.5) | 29.8 *** | (27.6, 33.3) | 1.49 | (1.32, 1.67) |
| SAM | nmol/L | 59.2 | (49.5, 67.6) | 58.6 | (50.7, 67.2) | 60.2 | (51.0, 69.3) | 113.5 *** | (99.9, 129.8) | 1.91 | (1.65, 2.23) |
| SAH | nmol/L | 11.2 a | (9.4, 13.5) | 12.4 b | (10.0, 14.4) | 23.8 c | (18.8, 30.5) | 45.3 *** | (38.2, 55.4) | 1.89 | (1.58, 2.31) |
| SAM/SAH | µmol/L | 5.34 a | (4.11 6.18) | 4.74 b | (3.88, 5.84) | 2.70 c | (1.88, 3.37) | 2.56 | (1.95, 3.14) | - | |
| tHcy † | µmol/L | 5.38 a | (4.58, 6.36) | 5.61 b | (4.74, 6.96) | 7.16 c | (5.88, 9.16) | 6.02 *** | (5.01, 7.75) | 0.85 | (0.76, 0.95) |
| Homocysteic acid | µmol/L | 0 ‡ | (0 ‡,0 ‡) | 0 ‡ | (0 ‡,0 ‡) | 0 ‡ | (0 ‡,0 ‡) | 0 ‡ | (0 ‡,0 ‡) | - | |
| Cystathionine | nmol/L | 103 a | (76, 133) | 213 b | (165, 287) | 214 b | (171, 291) | 327 *** | (245, 402) | 1.37 | (1.18, 1.68) |
| tCys | µmol/L | 240 a | (219, 258) | 213 b | (199, 229) | 241 a | (218, 264) | 213 *** | (197, 231) | 0.90 | (0.82, 0.97) |
| tHcy/tCys | µmol/L | 0.0230 a | (0.0194, 0.0259) | 0.0268 b | (0.0231, 0.0322) | 0.0302 c | (0.0246, 0.0355) | 0.0295 *** | (0.0236, 0.0347) | - | |
| Taurine | µmol/L | 66.7 a | (54.6, 95.6) | 60.0 b | (48.1, 77.5) | 75.1 a | (51.9, 105.3) | 187.0 *** | (146.5, 230.8) | 2.55 | (1.80, 3.76) |
| Serine | µmol/L | 99 a | (88, 110) | 104 b | (91, 115) | 114 c | (98, 129) | 156 *** | (143, 169) | 1.35 | (1.23, 1.54) |
| Glycine | µmol/L | 153 a | (139, 174) | 151 a | (131, 172) | 172 b | (146, 207) | 260 *** | (235, 283) | 1.51 | (1.30, 1.72) |
| Riboflavin | nmol/L | 9.92 a | (2.32, 18.18) | 7.20 b | (1.99, 17.65) | 7.98 b | (2.69, 14.59) | 55.65 *** | (34.13, 79.28) | 5.63 | (3.41, 9.56) |
| Pyridoxamine | nmol/L | 0.218 | (0.170, 0.265) | 0.220 | (0.175, 0.269) | 0.233 | (0.195, 0.305) | 0.325 *** | (0.267, 0.433) | 1.37 | (1.02, 1.85) |
| Pyridoxine | nmol/L | 0.135 | (0.092, 0.205) | 0.127 | (0.072, 0.188) | 0.124 | (0.072, 0.169) | 0.211 *** | (0.139, 0.355) | 1.74 | (1.09, 3.59) |
| Analytes | ρ | p-Value |
|---|---|---|
| 5-MTHF † | 0.688 | <0.0001 |
| FA † | 0.372 | <0.0001 |
| Choline | 0.397 | <0.0001 |
| Betaine | 0.366 | <0.0001 |
| DMG | 0.811 | <0.0001 |
| Methionine | 0.466 | <0.0001 |
| SAM | 0.390 | <0.0001 |
| SAH | 0.386 | <0.0001 |
| tHcy † | 0.828 | <0.0001 |
| Cystathionine | 0.593 | <0.0001 |
| tCys | 0.570 | <0.0001 |
| Taurine | 0.169 | 0.0730 |
| Serine | 0.345 | 0.0002 |
| Glycine | 0.579 | <0.0001 |
| Riboflavin | 0.677 | <0.0001 |
| Pyridoxamine | 0.261 | 0.0051 |
| Pyridoxine | 0.411 | <0.0001 |
| Maternal Blood | Cord Blood | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early Pregnancy | Late Pregnancy | At Birth | ||||||||||||||
| SAM | SAH | tHcy † | tHcy/tCys | SAM | SAH | tHcy † | tHcy/tCys | SAM | SAH | tHcy † | tHcy/tCys | SAM | SAH | tHcy † | tHcy/tCys | |
| 5-MTHF † | 0.207 * | 0.075 | −0.356 * | −0.505 * | 0.284 * | 0.099 | −0.518 * | −0.626 * | 0.217 * | 0.104 | −0.544 * | −0.670 * | 0.257 * | 0.000 | −0.394 * | −0.472 |
| Betaine | 0.258 * | 0.116 | −0.241 * | −0.254 * | 0.429 * | 0.140 | −0.355 * | −0.413 * | 0.362 * | 0.196 * | −0.224 * | −0.339 * | 0.333 * | 0.099 | −0.100 | −0.193 |
| Betaine/DMG | −0.094 | −0.232 * | −0.168 * | −0.218 * | 0.128 | −0.163 | −0.372 * | −0.340 * | 0.074 | −0.011 | −0.545 * | −0.509 * | 0.180 * | −0.027 | −0.486 * | −0.458 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, Y.; Fukuoka, H.; Shoji, K.; Mori, C.; Sakurai, K.; Nishikawa, M.; Oshida, K.; Yamashiro, Y.; Kawabata, T. Longitudinal Analysis of One-Carbon Metabolism-Related Metabolites in Maternal and Cord Blood of Japanese Pregnant Women. Nutrients 2024, 16, 1765. https://doi.org/10.3390/nu16111765
Kubo Y, Fukuoka H, Shoji K, Mori C, Sakurai K, Nishikawa M, Oshida K, Yamashiro Y, Kawabata T. Longitudinal Analysis of One-Carbon Metabolism-Related Metabolites in Maternal and Cord Blood of Japanese Pregnant Women. Nutrients. 2024; 16(11):1765. https://doi.org/10.3390/nu16111765
Chicago/Turabian StyleKubo, Yoshinori, Hideoki Fukuoka, Kumiko Shoji, Chisato Mori, Kenichi Sakurai, Masazumi Nishikawa, Kyoichi Oshida, Yuichiro Yamashiro, and Terue Kawabata. 2024. "Longitudinal Analysis of One-Carbon Metabolism-Related Metabolites in Maternal and Cord Blood of Japanese Pregnant Women" Nutrients 16, no. 11: 1765. https://doi.org/10.3390/nu16111765
APA StyleKubo, Y., Fukuoka, H., Shoji, K., Mori, C., Sakurai, K., Nishikawa, M., Oshida, K., Yamashiro, Y., & Kawabata, T. (2024). Longitudinal Analysis of One-Carbon Metabolism-Related Metabolites in Maternal and Cord Blood of Japanese Pregnant Women. Nutrients, 16(11), 1765. https://doi.org/10.3390/nu16111765









