Insights into the Mechanisms of Action of Akkermansia muciniphila in the Treatment of Non-Communicable Diseases
Abstract
1. Introduction
Search Strategy
2. Modes of Action
3. Gut Diseases
3.1. Inflammatory Bowel Disease (IBD)
3.2. Irritable Bowel Syndrome (IBS)-like Symptoms in IBD Patients
4. Metabolic Diseases
5. Cancer
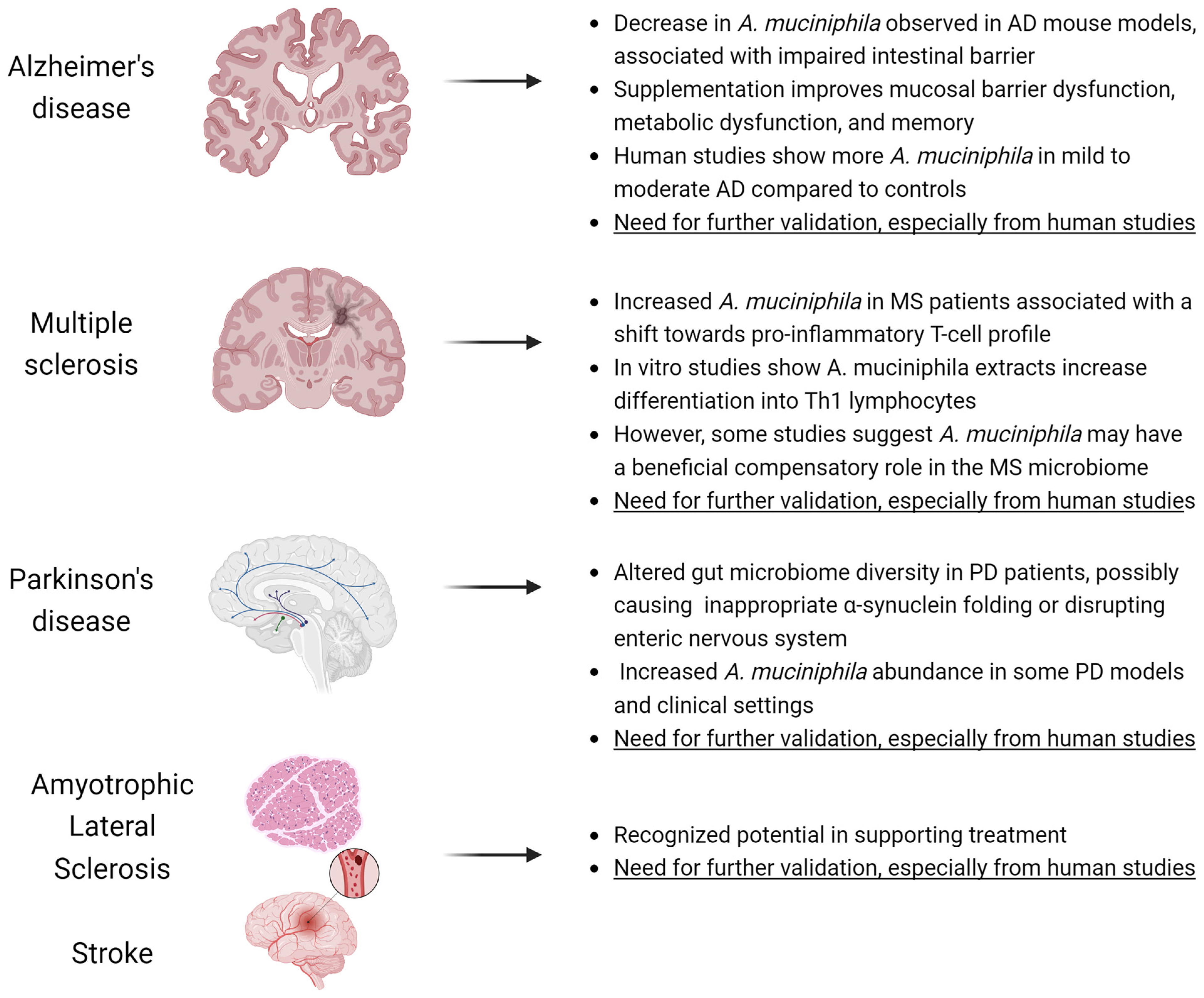
6. Neurodegenerative Diseases
- The studies described in MS and PD prove correlation, not causation.
- Bacterial contents in tests are given in relative abundances, which in practice means that their number (absolute value) is not necessarily higher [136].
- Pasteurized A. muciniphila does not colonize the gut so does not induce changes in the composition of the microbiota [37].
- No authority, including the EFSA, has denied the product’s placing on the market on the basis of these correlative reports alone [22].
7. Mental Illnesses
8. Modulating the Quantity of A. muciniphila
9. Limitations
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin Degrader Akkermansia muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Van Herreweghen, F.; De Paepe, K.; Marzorati, M.; Van de Wiele, T. Mucin as a Functional Niche Is a More Important Driver of in Vitro Gut Microbiota Composition and Functionality than Supplementation of Akkermansia m Uciniphila. Appl. Environ. Microbiol. 2021, 87, e02647-20. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Mesmin, L.; Chassaing, B.; Desvaux, M.; De Paepe, K.; Gresse, R.; Sauvaitre, T.; Forano, E.; de Wiele, T.V.; Schüller, S.; Juge, N.; et al. Experimental Models to Study Intestinal Microbes-Mucus Interactions in Health and Disease. FEMS Microbiol. Rev. 2019, 43, 457–489. [Google Scholar] [CrossRef] [PubMed]
- Karcher, N.; Nigro, E.; Punčochář, M.; Blanco-Míguez, A.; Ciciani, M.; Manghi, P.; Zolfo, M.; Cumbo, F.; Manara, S.; Golzato, D.; et al. Genomic Diversity and Ecology of Human-Associated Akkermansia Species in the Gut Microbiome Revealed by Extensive Metagenomic Assembly. Genome Biol. 2021, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M. Microbe Profile: Akkermansia muciniphila: A Conserved Intestinal Symbiont That Acts as the Gatekeeper of Our Mucosa. Microbiology 2017, 163, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, M.; Xu, T.; Zhou, X.; Qian, M.; Yang, Z.; Cao, K.; Han, X. Akkermansia muciniphila Identified as Key Strain to Function in Pathogen Invasion and Intestinal Stem Cell Proliferation through Wnt Signaling Pathway. eLife 2023, 12, RP92906. [Google Scholar] [CrossRef]
- Miller, R.S.; Hoskins, L.C. Mucin Degradation in Human Colon Ecosystems. Fecal Population Densities of Mucin-Degrading Bacteria Estimated by a “Most Probable Number” Method. Gastroenterology 1981, 81, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. Valid Publication of the Names of Forty-Two Phyla of Prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Derrien, M.; Isolauri, E.; de Vos, W.M.; Salminen, S. Intestinal Integrity and Akkermansia muciniphila, a Mucin-Degrading Member of the Intestinal Microbiota Present in Infants, Adults, and the Elderly. Appl. Environ. Microbiol. 2007, 73, 7767–7770. [Google Scholar] [CrossRef]
- Geerlings, S.Y.; Kostopoulos, I.; de Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Loviselli, A.; Velluzzi, F.; Manzin, A. Gut Microbiota Markers and Dietary Habits Associated with Extreme Longevity in Healthy Sardinian Centenarians. Nutrients 2022, 14, 2436. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The Gut Microbiome as a Modulator of Healthy Ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Yue, Y.; Ma, C.; Dong, L.; Chen, F. Pasteurized Akkermansia muciniphila Ameliorate the LPS-Induced Intestinal Barrier Dysfunction via Modulating AMPK and NF-κB through TLR2 in Caco-2 Cells. Nutrients 2022, 14, 764. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The Outer Membrane Protein Amuc_1100 of Akkermansia muciniphila Promotes Intestinal 5-HT Biosynthesis and Extracellular Availability through TLR2 Signalling. Food Funct. 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live Biotherapeutic Products: The Importance of a Defined Regulatory Framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Safety of Pasteurised Akkermansia muciniphila as a Novel Food Pursuant to Regulation (EU) 2015/2283|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6780 (accessed on 9 April 2023).
- Novel Food—European Commission. Available online: https://food.ec.europa.eu/safety/novel-food_en (accessed on 25 April 2024).
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Pham, M.T.; Rajić, A.; Greig, J.D.; Sargeant, J.M.; Papadopoulos, A.; McEwen, S.A. A Scoping Review of Scoping Reviews: Advancing the Approach and Enhancing the Consistency. Res. Synth. Methods 2014, 5, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A Gut-Vascular Barrier Controls the Systemic Dissemination of Bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia muciniphila and Its Role in Regulating Host Functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Segers, A.; de Vos, W.M. Mode of Action of Akkermansia muciniphila in the Intestinal Dialogue: Role of Extracellular Proteins, Metabolites and Cell Envelope Components. Microbiome Res. Rep. 2023, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Choi, Y.; Kim, D.-K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.-S.; Jee, Y.-K.; Gho, Y.S.; et al. Akkermansia muciniphila -Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Ashrafian, F.; Shahriary, A.; Behrouzi, A.; Moradi, H.R.; Keshavarz Azizi Raftar, S.; Lari, A.; Hadifar, S.; Yaghoubfar, R.; Ahmadi Badi, S.; Khatami, S.; et al. Akkermansia muciniphila -Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The Potential of Akkermansia muciniphila in Inflammatory Bowel Disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila Secretes a Glucagon-like Peptide-1-Inducing Protein That Improves Glucose Homeostasis and Ameliorates Metabolic Disease in Mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lv, L.; Shi, D.; Ye, J.; Fang, D.; Guo, F.; Li, Y.; He, X.; Li, L. Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model. Front. Microbiol. 2017, 8, 1804. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The Role of the Probiotic Akkermansia muciniphila in Brain Functions: Insights Underpinning Therapeutic Potential. Crit. Rev. Microbiol. 2023, 49, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, W.; Li, Q.; Chen, Y.; Wu, L.; Dong, Y.; Huang, X.; He, X.; Ou, Z.; Peng, Y. Membrane Protein Amuc_1100 Derived from Akkermansia muciniphila Facilitates Lipolysis and Browning via Activating the AC3/PKA/HSL Pathway. Microbiol. Spectr. 2023, 11, e0432322. [Google Scholar] [CrossRef] [PubMed]
- Grajeda-Iglesias, C.; Durand, S.; Daillère, R.; Iribarren, K.; Lemaitre, F.; Derosa, L.; Aprahamian, F.; Bossut, N.; Nirmalathasan, N.; Madeo, F.; et al. Oral Administration of Akkermansia muciniphila Elevates Systemic Antiaging and Anticancer Metabolites. Aging 2021, 13, 6375–6405. [Google Scholar] [CrossRef]
- Hänninen, A.; Toivonen, R.; Pöysti, S.; Belzer, C.; Plovier, H.; Ouwerkerk, J.P.; Emani, R.; Cani, P.D.; De Vos, W.M. Akkermansia muciniphila Induces Gut Microbiota Remodelling and Controls Islet Autoimmunity in NOD Mice. Gut 2018, 67, 1445–1453. [Google Scholar] [CrossRef]
- Xie, S.; Li, J.; Lyu, F.; Xiong, Q.; Gu, P.; Chen, Y.; Chen, M.; Bao, J.; Zhang, X.; Wei, R.; et al. Novel Tripeptide RKH Derived from Akkermansia muciniphila Protects against Lethal Sepsis. Gut 2023, 73, 78–91. [Google Scholar] [CrossRef]
- Dunleavy, K.A.; Raffals, L.E.; Camilleri, M. Intestinal Barrier Dysfunction in Inflammatory Bowel Disease: Underpinning Pathogenesis and Therapeutics. Dig. Dis. Sci. 2023, 68, 4306–4320. [Google Scholar] [CrossRef]
- De Palma, G.; Reed, D.E.; Bercik, P. Diet–Microbial Cross–Talk Underlying Increased Visceral Perception. Gut Microbes 2023, 15, 2166780. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular Permeability and Tight Junction Regulation in Gut Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Lo Sasso, G.; Khachatryan, L.; Kondylis, A.; Battey, J.N.D.; Sierro, N.; Danilova, N.A.; Grigoryeva, T.V.; Markelova, M.I.; Khusnutdinova, D.R.; Laikov, A.V.; et al. Inflammatory Bowel Disease–Associated Changes in the Gut: Focus on Kazan Patients. Inflamm. Bowel Dis. 2021, 27, 418–433. [Google Scholar] [CrossRef]
- Earley, H.; Lennon, G.; Balfe, Á.; Coffey, J.C.; Winter, D.C.; O’Connell, P.R. The Abundance of Akkermansia muciniphila and Its Relationship with Sulphated Colonic Mucins in Health and Ulcerative Colitis. Sci. Rep. 2019, 9, 15683. [Google Scholar] [CrossRef] [PubMed]
- Presti, A.L.; Chierico, F.D.; Altomare, A.; Zorzi, F.; Cella, E.; Putignani, L.; Luca Guarino, M.P.; Monteleone, G.; Cicala, M.; Angeletti, S.; et al. Exploring the Genetic Diversity of the 16S rRNA Gene of Akkermansia muciniphila in IBD and IBS. Future Microbiol. 2019, 14, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Siles, M.; Enrich-Capó, N.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Alterations in the Abundance and Co-Occurrence of Akkermansia muciniphila and Faecalibacterium Prausnitzii in the Colonic Mucosa of Inflammatory Bowel Disease Subjects. Front. Cell. Infect. Microbiol. 2018, 8, 281. [Google Scholar] [CrossRef]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.W.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. Early Changes in Microbial Community Structure Are Associated with Sustained Remission After Nutritional Treatment of Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.M.H.; Karlsson, H.; Crespo, J.G.; Johansson, M.E.V.; Eklund, L.; Sjövall, H.; Hansson, G.C. Altered O-Glycosylation Profile of MUC2 Mucin Occurs in Active Ulcerative Colitis and Is Associated with Increased Inflammation. Inflamm. Bowel Dis. 2011, 17, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, P.; Wu, X.; Lu, G.; Marcella, C.; Ji, X.; Ji, G.; Zhang, F. Alterations of Akkermansia muciniphila in the Inflammatory Bowel Disease Patients with Washed Microbiota Transplantation. Appl. Microbiol. Biotechnol. 2020, 104, 10203–10215. [Google Scholar] [CrossRef]
- Wang, L.; Tang, L.; Feng, Y.; Zhao, S.; Han, M.; Zhang, C.; Yuan, G.; Zhu, J.; Cao, S.; Wu, Q.; et al. A Purified Membrane Protein from Akkermansia muciniphila or the Pasteurised Bacterium Blunts Colitis Associated Tumourigenesis by Modulation of CD8+ T Cells in Mice. Gut 2020, 69, 1988–1997. [Google Scholar] [CrossRef]
- Yilmaz, O.; Okullu, S.O.; Catakci, M.; Elmas, M.A.; Pinheiro, Y.; Arbak, S.; Demir, E.; Schaefer, K.H.; Kolgazi, M. Akkermansia muciniphila Improves Chronic Colitis-Induced Enteric Neuroinflammation in Mice. Neurogastroenterol. Motil. 2024, 36, e14745. [Google Scholar] [CrossRef] [PubMed]
- Wade, H.; Pan, K.; Duan, Q.; Kaluzny, S.; Pandey, E.; Fatumoju, L.; Saraswathi, V.; Wu, R.; Harris, E.N.; Su, Q. Akkermansia muciniphila and Its Membrane Protein Ameliorates Intestinal Inflammatory Stress and Promotes Epithelial Wound Healing via CREBH and miR-143/145. J. Biomed. Sci. 2023, 30, 38. [Google Scholar] [CrossRef] [PubMed]
- Rath, T.; Atreya, R.; Bodenschatz, J.; Uter, W.; Geppert, C.E.; Vitali, F.; Fischer, S.; Waldner, M.J.; Colombel, J.-F.; Hartmann, A.; et al. Intestinal Barrier Healing Is Superior to Endoscopic and Histologic Remission for Predicting Major Adverse Outcomes in Inflammatory Bowel Disease: The Prospective ERIca Trial. Gastroenterology 2023, 164, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Edogawa, S.; Sundt, W.J.; Dyer, R.B.; Dalenberg, D.A.; Mazzone, A.; Singh, R.J.; Moses, N.; Smyrk, T.C.; Weber, C.; et al. Constipation-Predominant Irritable Bowel Syndrome Females Have Normal Colonic Barrier and Secretory Function. Off. J. Am. Coll. Gastroenterol. ACG 2017, 112, 913. [Google Scholar] [CrossRef] [PubMed]
- Aliu, A.; Bosch, D.H.C.A.; Keszthelyi, D.; Rezazadeh Ardabili, A.; Colombel, J.-F.; Sawyer, R.; Törnblom, H.; Hart, A.; Jonkers, D.M.A.E.; Pierik, M.J.; et al. Review Article: A Practical Approach to Persistent Gastrointestinal Symptoms in Inflammatory Bowel Disease in Remission. Aliment. Pharmacol. Ther. 2024, 59, 1470–1488. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Pu, A.; Jencks, K.; Bishu, S.; Higgins, P.; Chey, W.D.; Rao, K.; Lee, A. Predictors of Irritable Bowel Syndrome-like Symptoms in Quiescent Inflammatory Bowel Disease. Neurogastroenterol. Motil. 2024, 36, e14809. [Google Scholar] [CrossRef]
- Meynier, M.; Daugey, V.; Mallaret, G.; Gervason, S.; Meleine, M.; Barbier, J.; Aissouni, Y.; Lolignier, S.; Bonnet, M.; Ardid, D.; et al. Pasteurized Akkermansia muciniphila Improves Irritable Bowel Syndrome-like Symptoms and Related Behavioral Disorders in Mice. Gut Microbes 2024, 16, 2298026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing Intestinal Barrier Efficiency: A Novel Metabolic Diseases Therapy. Front. Nutr. 2023, 10, 1120168. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.D.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.-H.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2022, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- de Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal Microbiota Composition Differs between Children with β-Cell Autoimmunity and Those Without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut Microbiome Metagenomics Analysis Suggests a Functional Model for the Development of Autoimmunity for Type 1 Diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Derrien, M.; Van Baarlen, P.; Hooiveld, G.; Norin, E.; Müller, M.; de Vos, W.M. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front. Microbiol. 2011, 2, 166. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, C.; Zeng, Q. Gut Microbiota and Immunopathogenesis of Diabetes Mellitus Type 1 and 2. Front. Biosci. (Landmark Ed.) 2016, 21, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, M.; Grys, I.; Kukla, M. Small Intestinal Bacterial Overgrowth and Nonalcoholic Fatty Liver Disease. Clin. Exp. Hepatol. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Reichardt, F.; Tabatabavakili, S.; Nezami, B.G.; Chassaing, B.; Mwangi, S.; Vijay-Kumar, M.; Gewirtz, A.; Srinivasan, S. Intestinal Dysbiosis Contributes to the Delayed Gastrointestinal Transit in High-Fat Diet Fed Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, F.; Grander, C.; Effenberger, M.; Adolph, T.E.; Tilg, H. Gut Dysfunction and Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2019, 10, 611. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Microbiota and Diabetes: An Evolving Relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila Inversely Correlates with the Onset of Inflammation, Altered Adipose Tissue Metabolism and Metabolic Disorders during Obesity in Mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef]
- Shih, C.-T.; Yeh, Y.-T.; Lin, C.-C.; Yang, L.-Y.; Chiang, C.-P. Akkermansia muciniphila Is Negatively Correlated with Hemoglobin A1c in Refractory Diabetes. Microorganisms 2020, 8, 1360. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, Q.; Liu, M.; Zhang, X.; He, F.; Wang, G. Akkermansia muciniphila Can Reduce the Damage of Gluco/Lipotoxicity, Oxidative Stress and Inflammation, and Normalize Intestine Microbiota in Streptozotocin-Induced Diabetic Rats. Pathog. Dis. 2018, 76, fty028. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, P.; Hasanzadeh, S.; Tahvildari, A.; Farsi, Y.; Arbabi, M.; Mota, J.F.; Sechi, L.A.; Nasiri, M.J. Is There Any Association between Gut Microbiota and Type 1 Diabetes? A Systematic Review. Gut Pathog. 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Debray-Sachs, M.; Carnaud, C.; Boitard, C.; Cohen, H.; Gresser, I.; Bedossa, P.; Bach, J.F. Prevention of Diabetes in NOD Mice Treated with Antibody to Murine IFN Gamma. J. Autoimmun. 1991, 4, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut Dysbiosis and Detection of “Live Gut Bacteria” in Blood of Japanese Patients with Type 2 Diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, S.; Vanhoutte, P.M.; Woo, C.W.; Xu, A. Akkermansia muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe−/− Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Shen, J.; Tong, X.; Sud, N.; Khound, R.; Song, Y.; Maldonado-Gomez, M.X.; Walter, J.; Su, Q. Low-Density Lipoprotein Receptor Signaling Mediates the Triglyceride-Lowering Action of Akkermansia muciniphila in Genetic-Induced Hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An Increase in the Akkermansia Spp. Population Induced by Metformin Treatment Improves Glucose Homeostasis in Diet-Induced Obese Mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Wu, W.; Kaicen, W.; Bian, X.; Yang, L.; Ding, S.; Li, Y.; Li, S.; Zhuge, A.; Li, L. Akkermansia muciniphila Alleviates High-Fat-Diet-Related Metabolic-Associated Fatty Liver Disease by Modulating Gut Microbiota and Bile Acids. Microb. Biotechnol. 2023, 16, 1924–1939. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Kim, Y.; Seo, Y.; Lee, H.; Ha, J.; Lee, J.; Choi, Y.; Oh, H.; Yoon, Y. Akkermansia muciniphila Prevents Fatty Liver Disease, Decreases Serum Triglycerides, and Maintains Gut Homeostasis. Appl. Environ. Microbiol. 2020, 86, e03004-19. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, W.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Liu, R.; Hong, J. Akkermansia muciniphila Improves Metabolic Profiles by Reducing Inflammation in Chow Diet-Fed Mice. J. Mol. Endocrinol. 2017, 58, 1–14. [Google Scholar] [CrossRef]
- Katiraei, S.; de Vries, M.R.; Costain, A.H.; Thiem, K.; Hoving, L.R.; van Diepen, J.A.; Smits, H.H.; Bouter, K.E.; Rensen, P.C.N.; Quax, P.H.A.; et al. Akkermansia muciniphila Exerts Lipid-Lowering and Immunomodulatory Effects without Affecting Neointima Formation in Hyperlipidemic APOE*3-Leiden.CETP Mice. Mol. Nutr. Food Res. 2020, 64, e1900732. [Google Scholar] [CrossRef] [PubMed]
- Lawenius, L.; Scheffler, J.M.; Gustafsson, K.L.; Henning, P.; Nilsson, K.H.; Colldén, H.; Islander, U.; Plovier, H.; Cani, P.D.; de Vos, W.M.; et al. Pasteurized Akkermansia muciniphila Protects from Fat Mass Gain but Not from Bone Loss. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E480–E491. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia muciniphila Predicts Clinical Response to PD-1 Blockade in Patients with Advanced Non-Small-Cell Lung Cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, P.; Du, H.; Chu, W.; Sun, R.; Qin, S.; Tian, Y.; Zhang, Z.; Xu, F. Akkermansia muciniphila Potentiates the Antitumor Efficacy of FOLFOX in Colon Cancer. Front. Pharmacol. 2021, 12, 725583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ji, Q.; Chen, Q.; Wei, Z.; Liu, S.; Zhang, L.; Zhang, Y.; Li, Z.; Liu, H.; Sui, H. Akkermansia muciniphila Inhibits Tryptophan Metabolism via the AhR/β-Catenin Signaling Pathway to Counter the Progression of Colorectal Cancer. Int. J. Biol. Sci. 2023, 19, 4393–4410. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The Commensal Microbiome Is Associated with Anti–PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Galluzzi, L.; Kepp, O.; Smyth, M.J.; Kroemer, G. Type I Interferons in Anticancer Immunity. Nat. Rev. Immunol. 2015, 15, 405–414. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti–PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lurienne, L.; Cervesi, J.; Duhalde, L.; de Gunzburg, J.; Andremont, A.; Zalcman, G.; Buffet, R.; Bandinelli, P.-A. NSCLC Immunotherapy Efficacy and Antibiotic Use: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Xia, K.; Liu, Y.-W.; Liu, J.-H.; Rao, S.-S.; Hu, X.-K.; Chen, C.-Y.; Xu, R.; Wang, Z.-X.; Xie, H. Extracellular Vesicles from Akkermansia muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8+ T Cells and Macrophages. Int. J. Nanomed. 2021, 16, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with Familial Adenomatous Polyposis Harbor Colonic Biofilms Containing Tumorigenic Bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, A.; Carloni, S.; Ravenda, P.S.; Bertalot, G.; Spadoni, I.; Lo Cascio, A.; Gandini, S.; Lizier, M.; Braga, D.; Asnicar, F.; et al. Gut Vascular Barrier Impairment Leads to Intestinal Bacteria Dissemination and Colorectal Cancer Metastasis to Liver. Cancer Cell 2021, 39, 708–724.e11. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium Nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Fan, L.; Xu, C.; Ge, Q.; Lin, Y.; Wong, C.C.; Qi, Y.; Ye, B.; Lian, Q.; Zhuo, W.; Si, J.; et al. A. Muciniphila Suppresses Colorectal Tumorigenesis by Inducing TLR2/NLRP3-Mediated M1-Like TAMs. Cancer Immunol. Res. 2021, 9, 1111–1124. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, D. Potential of Akkermansia muciniphila and Its Outer Membrane Proteins as Therapeutic Targets for Neuropsychological Diseases. Front. Microbiol. 2023, 14, 1191445. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta Amyloid Pathology in APPPS1 Transgenic Mice in the Absence of Gut Microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of Ethanol-Induced Akkermansia muciniphila Depletion Ameliorates Alcoholic Liver Disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Cheng, Y.; Gao, J.; Liu, X.; Shao, L.; Kong, Q.; Zheng, N.; Ling, Z.; Hu, W. Akkermansia muciniphila in Neuropsychiatric Disorders: Friend or Foe? Front. Cell. Infect. Microbiol. 2023, 13, 1224155. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Kang, H.; You, H.J.; Ko, G. Revisiting the Role of Akkermansia muciniphila as a Therapeutic Bacterium. Gut Microbes 2022, 14, 2078619. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L.; Wang, Y.; Ding, W. Early-Life High-Fat Diet-Induced Obesity Programs Hippocampal Development and Cognitive Functions via Regulation of Gut Commensal Akkermansia muciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Wang, A.C.; Parikh, I.; Green, S.J.; Hoffman, J.D.; Chlipala, G.; Murphy, M.P.; Sokola, B.S.; Bauer, B.; Hartz, A.M.S.; et al. Ketogenic Diet Enhances Neurovascular Function with Altered Gut Microbiome in Young Healthy Mice. Sci. Rep. 2018, 8, 6670. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective Effects of Akkermansia muciniphila on Cognitive Deficits and Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Nutr. Diabetes 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut Microbiome Alterations in Alzheimer’s Disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Björklund, T.; Wang, Z.-Y.; Roybon, L.; Melki, R.; Li, J.-Y. Direct Evidence of Parkinson Pathology Spread from the Gastrointestinal Tract to the Brain in Rats. Acta Neuropathol. 2014, 128, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Bae, C.-H.; Lee, Y.; Kim, H.-Y.; Kim, S. Korean Red Ginseng Suppresses 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Inflammation in the Substantia Nigra and Colon. Brain Behav. Immun. 2021, 94, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, H.B.; Forsyth, C.B.; Voigt, R.M.; Engen, P.A.; Patel, J.; Shaikh, M.; Green, S.J.; Naqib, A.; Roy, A.; Kordower, J.H.; et al. Chronic Stress-Induced Gut Dysfunction Exacerbates Parkinson’s Disease Phenotype and Pathology in a Rotenone-Induced Mouse Model of Parkinson’s Disease. Neurobiol. Dis. 2020, 135, 104352. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Martinez, G.; Chin, B.; Camarillo, C.; Herrera, G.V.; Yang, B.; Sarosiek, I.; Perez, R.G. A Pilot Microbiota Study in Parkinson’s Disease Patients versus Control Subjects, and Effects of FTY720 and FTY720-Mitoxy Therapies in Parkinsonian and Multiple System Atrophy Mouse Models. J. Park. Dis. 2020, 10, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-F.; Shan, C.; Zhuang, S.-Y.; Zhuang, Q.-Q.; Ghosh, A.; Zhu, K.-C.; Kong, X.-K.; Wang, S.-M.; Gong, Y.-L.; Yang, Y.-Y.; et al. Gut Microbiota-Derived Propionate Mediates the Neuroprotective Effect of Osteocalcin in a Mouse Model of Parkinson’s Disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, A.M.; Preskey, L.; Bakeberg, M.C.; Kenna, J.E.; Gildenhuys, C.; MacDougall, G.; Dunlop, S.A.; Mastaglia, F.L.; Akkari, P.A.; Koengten, F.; et al. Altered Gut Microbiome in Parkinson’s Disease and the Influence of Lipopolysaccharide in a Human α-Synuclein Over-Expressing Mouse Model. Front. Neurosci. 2019, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, H.; Ito, M.; Ishida, T.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov. Disord. 2020, 35, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-Analysis of the Parkinson’s Disease Gut Microbiome Suggests Alterations Linked to Intestinal Inflammation. NPJ Park. Dis. 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yue, Y.; He, T.; Huang, C.; Qu, B.; Lv, W.; Lai, H.-Y. The Association Between the Gut Microbiota and Parkinson’s Disease, a Meta-Analysis. Front. Aging Neurosci. 2021, 13, 636545. [Google Scholar] [CrossRef]
- Pietrucci, D.; Teofani, A.; Unida, V.; Cerroni, R.; Biocca, S.; Stefani, A.; Desideri, A. Can Gut Microbiota Be a Good Predictor for Parkinson’s Disease? A Machine Learning Approach. Brain Sci. 2020, 10, 242. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wüllner, U. Functional Implications of Microbial and Viral Gut Metagenome Changes in Early Stage L-DOPA-Naïve Parkinson’s Disease Patients. Genome Med. 2017, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut Bacteria from Multiple Sclerosis Patients Modulate Human T Cells and Exacerbate Symptoms in Mouse Models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed]
- Pröbstel, A.-K.; Zhou, X.; Baumann, R.; Wischnewski, S.; Kutza, M.; Rojas, O.L.; Sellrie, K.; Bischof, A.; Kim, K.; Ramesh, A.; et al. Gut Microbiota-Specific IgA+ B Cells Traffic to the CNS in Active Multiple Sclerosis. Sci. Immunol. 2020, 5, eabc7191. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.S.; Nagarkatti, M. Combination of Cannabinoids, Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD), Mitigates Experimental Autoimmune Encephalomyelitis (EAE) by Altering the Gut Microbiome. Brain Behav. Immun. 2019, 82, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A Probiotic Modulates the Microbiome and Immunity in Multiple Sclerosis. Ann. Neurol. 2018, 83, 1147–1161. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut Microbiota from Multiple Sclerosis Patients Enables Spontaneous Autoimmune Encephalomyelitis in Mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. Gut Microbiome in Progressive Multiple Sclerosis. Ann. Neurol. 2021, 89, 1195–1211. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzade, M.; Molanouri Shamsi, M.; Abolhasani, M.; Bakhshi, B.; Sahraian, M.A.; Quinn, L.S.; Negaresh, R. Home-Based Exercise Training Influences Gut Bacterial Levels in Multiple Sclerosis. Complement. Ther. Clin. Pract. 2021, 45, 101463. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Gastrointestinal Motility Disorders in Neurologic Disease. J. Clin. Investig. 2021, 131, e143771. [Google Scholar] [CrossRef]
- Müller, M.; Hermes, G.D.A.; Canfora, E.E.; Smidt, H.; Masclee, A.A.M.; Zoetendal, E.G.; Blaak, E.E. Distal Colonic Transit Is Linked to Gut Microbiota Diversity and Microbial Fermentation in Humans with Slow Colonic Transit. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G361–G369. [Google Scholar] [CrossRef]
- Mruk-Mazurkiewicz, H.; Kulaszyńska, M.; Jakubczyk, K.; Janda-Milczarek, K.; Czarnecka, W.; Rębacz-Maron, E.; Zacha, S.; Sieńko, J.; Zeair, S.; Dalewski, B.; et al. Clinical Relevance of Gut Microbiota Alterations under the Influence of Selected Drugs—Updated Review. Biomedicines 2023, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Misera, A.; Łoniewski, I.; Palma, J.; Kulaszyńska, M.; Czarnecka, W.; Kaczmarczyk, M.; Liśkiewicz, P.; Samochowiec, J.; Skonieczna-Żydecka, K. Clinical Significance of Microbiota Changes under the Influence of Psychotropic Drugs. An Updated Narrative Review. Front. Microbiol. 2023, 14, 1125022. [Google Scholar] [CrossRef] [PubMed]
- Bruijning, M.; Ayroles, J.F.; Henry, L.P.; Koskella, B.; Meyer, K.M.; Metcalf, C.J.E. Relative Abundance Data Can Misrepresent Heritability of the Microbiome. Microbiome 2023, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Blacher, E.; Bashiardes, S.; Shapiro, H.; Rothschild, D.; Mor, U.; Dori-Bachash, M.; Kleimeyer, C.; Moresi, C.; Harnik, Y.; Zur, M.; et al. Potential Roles of Gut Microbiome and Metabolites in Modulating ALS in Mice. Nature 2019, 572, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Moore, R.J.; Wong, C.H.Y. An Insight into Intestinal Mucosal Microbiota Disruption after Stroke. Sci. Rep. 2018, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Xie, H.; Wang, Y.; Shen, N.; Chen, Q.; Zhao, Y.; Gu, Q.; Zhang, J.; Liu, J.; Sun, J.; et al. Predictive Microbial Feature Analysis in Patients with Depression after Acute Ischemic Stroke. Front. Aging Neurosci. 2023, 15, 1116065. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Lou, Y.; Liu, L.; Liu, Y.; Zhang, W.; Deng, J.; Guan, Y.; She, M.; You, X.; Liu, M.; et al. Gut Microbiotic Features Aiding the Diagnosis of Acute Ischemic Stroke. Front. Cell. Infect. Microbiol. 2020, 10, 587284. [Google Scholar] [CrossRef] [PubMed]
- Ryuk, J.A.; Ko, B.S.; Moon, N.R.; Park, S. Protection against Neurological Symptoms by Consuming Corn Silk Water Extract in Artery-Occluded Gerbils with Reducing Oxidative Stress, Inflammation, and Post-Stroke Hyperglycemia through the Gut-Brain Axis. Antioxidants 2022, 11, 168. [Google Scholar] [CrossRef]
- Stanley, D.; Mason, L.J.; Mackin, K.E.; Srikhanta, Y.N.; Lyras, D.; Prakash, M.D.; Nurgali, K.; Venegas, A.; Hill, M.D.; Moore, R.J.; et al. Translocation and Dissemination of Commensal Bacteria in Post-Stroke Infection. Nat. Med. 2016, 22, 1277–1284. [Google Scholar] [CrossRef]
- Ji, W.; Zhu, Y.; Kan, P.; Cai, Y.; Wang, Z.; Wu, Z.; Yang, P. Analysis of Intestinal Microbial Communities of Cerebral Infarction and Ischemia Patients Based on High Throughput Sequencing Technology and Glucose and Lipid Metabolism. Mol. Med. Rep. 2017, 16, 5413–5417. [Google Scholar] [CrossRef]
- Chang, Y.; Woo, H.G.; Jeong, J.H.; Kim, G.H.; Park, K.D.; Song, T.-J. Microbiota Dysbiosis and Functional Outcome in Acute Ischemic Stroke Patients. Sci. Rep. 2021, 11, 10977. [Google Scholar] [CrossRef] [PubMed]
- Quave, C.B.; Nieto, S.J.; Haile, C.N.; Kosten, T.A. Immune Receptor Toll-like Receptor 4 Contributes to Stress-Induced Affective Responses in a Sex-Specific Manner. Brain Behav. Immun. Health 2021, 14, 100248. [Google Scholar] [CrossRef]
- Figueroa-Hall, L.K.; Paulus, M.P.; Savitz, J. Toll-Like Receptor Signaling in Depression. Psychoneuroendocrinology 2020, 121, 104843. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, R.; Duan, Z.; Yuan, X.; Ding, Y.; Feng, Z.; Bu, F.; Liu, L.; Wang, Q.; Zhou, J.; et al. Akkermansia muciniphila Protects Against Psychological Disorder-Induced Gut Microbiota-Mediated Colonic Mucosal Barrier Damage and Aggravation of Colitis. Front. Cell. Infect. Microbiol. 2021, 11, 723856. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A Next-Generation Probiotic: Akkermansia muciniphila Ameliorates Chronic Stress–Induced Depressive-like Behavior in Mice by Regulating Gut Microbiota and Metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, H.; Cheng, R.; Tang, Z.; Zhang, M. Outer Membrane Protein Amuc_1100 of Akkermansia muciniphila Alleviates Antibiotic-Induced Anxiety and Depression-like Behavior in Mice. Physiol. Behav. 2023, 258, 114023. [Google Scholar] [CrossRef]
- McGaughey, K.D.; Yilmaz-Swenson, T.; Elsayed, N.M.; Cruz, D.A.; Rodriguiz, R.M.; Kritzer, M.D.; Peterchev, A.V.; Roach, J.; Wetsel, W.C.; Williamson, D.E. Relative Abundance of Akkermansia Spp. and Other Bacterial Phylotypes Correlates with Anxiety- and Depressive-like Behavior Following Social Defeat in Mice. Sci. Rep. 2019, 9, 3281. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, S.H.; Park, J.W.; Kho, Y.; Seok, P.R.; Shin, J.-H.; Choi, Y.J.; Jun, J.-H.; Jung, H.C.; Kim, E.K. Melatonin in the Colon Modulates Intestinal Microbiota in Response to Stress and Sleep Deprivation. Intest. Res. 2020, 18, 325–336. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Chen, T.; Wang, X.; Zhang, X. Akkermansia muciniphila Improves Depressive-Like Symptoms by Modulating the Level of 5-HT Neurotransmitters in the Gut and Brain of Mice. Mol. Neurobiol. 2023, 61, 821–834. [Google Scholar] [CrossRef]
- Zou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 1614–1625. [Google Scholar] [CrossRef]
- Newell, C.; Bomhof, M.R.; Reimer, R.A.; Hittel, D.S.; Rho, J.M.; Shearer, J. Ketogenic Diet Modifies the Gut Microbiota in a Murine Model of Autism Spectrum Disorder. Mol. Autism 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, Y.; Zhang, Y.; Xiang, G.; Yu, M.; Wang, X.; Qiu, B.; Li, X.; Liu, W.; Zhang, D. Rescue of Social Deficits by Early-Life Melatonin Supplementation through Modulation of Gut Microbiota in a Murine Model of Autism. Biomed. Pharmacother. 2022, 156, 113949. [Google Scholar] [CrossRef] [PubMed]
- Suriano, F.; Bindels, L.B.; Verspreet, J.; Courtin, C.M.; Verbeke, K.; Cani, P.D.; Neyrinck, A.M.; Delzenne, N.M. Fat Binding Capacity and Modulation of the Gut Microbiota Both Determine the Effect of Wheat Bran Fractions on Adiposity. Sci. Rep. 2017, 7, 5621. [Google Scholar] [CrossRef]
- Ramnani, P.; Gaudier, E.; Bingham, M.; van Bruggen, P.; Tuohy, K.M.; Gibson, G.R. Prebiotic Effect of Fruit and Vegetable Shots Containing Jerusalem Artichoke Inulin: A Human Intervention Study. Br. J. Nutr. 2010, 104, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban, J.F.; Davis, C.D.; Solano-Aguilar, G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs1234. J. Nutr. 2016, 146, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Coelho, O.G.L.; Cândido, F.G.; Alfenas, R.d.C.G. Dietary Fat and Gut Microbiota: Mechanisms Involved in Obesity Control. Crit. Rev. Food Sci. Nutr. 2019, 59, 3045–3053. [Google Scholar] [CrossRef]
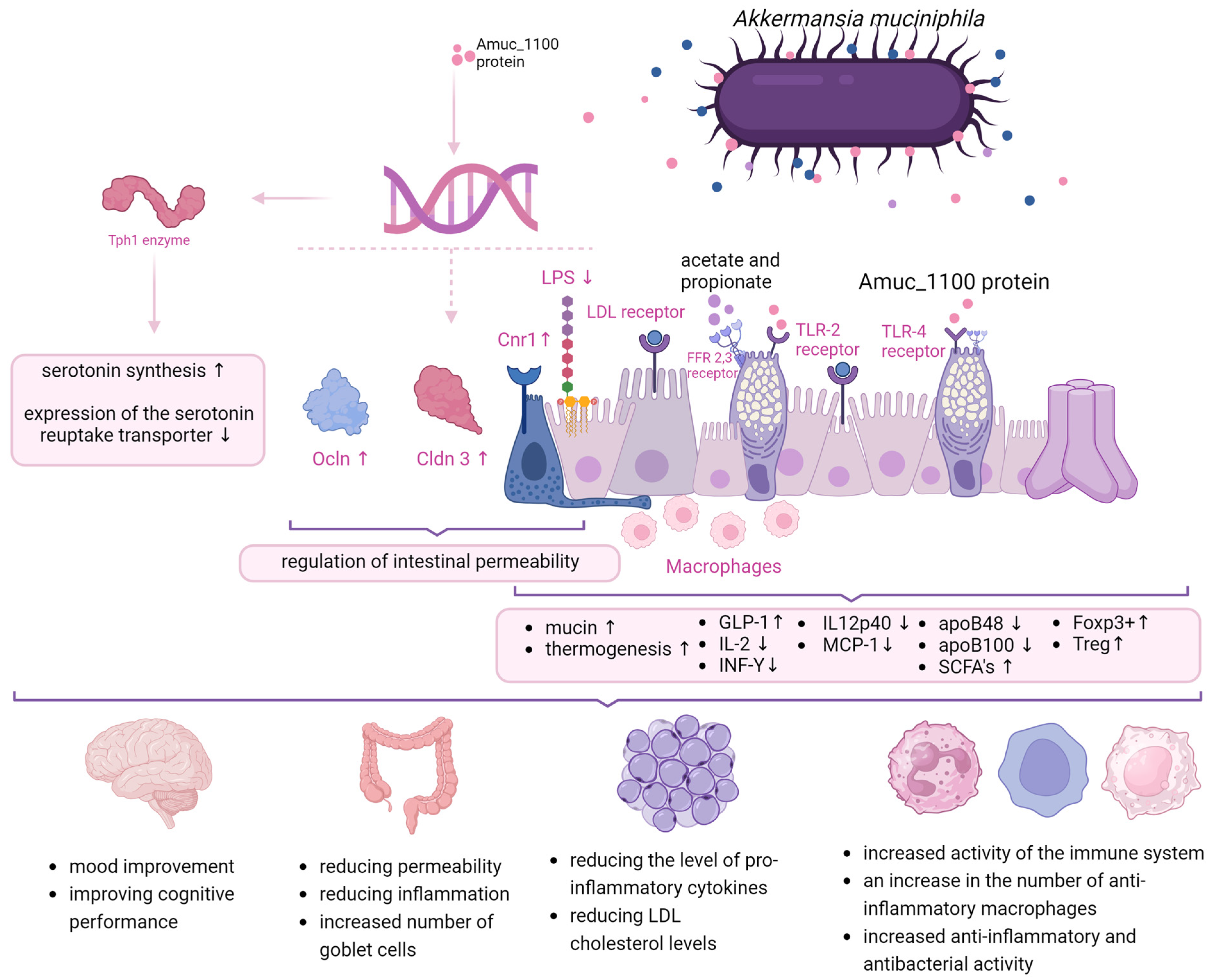


| PROS | CONS |
|---|---|
| Improves Gut Health: Supplementation with A. muciniphila can enhance gut health by maintaining gut barrier integrity and reducing permeability. | Reduced Levels in Disease: In patients with IBD, A. muciniphila levels are significantly reduced, indicating a potential challenge in naturally maintaining its beneficial effects. |
| Reduces Inflammation: Oral supplementation of A. muciniphila or its protein Amuc_1100 reduces macrophage and cytotoxic T lymphocyte infiltration, thereby decreasing inflammation in colitis models. | Research is Limited: More research is needed to fully understand the efficacy and mechanisms of A. muciniphila in treating IBD and IBS. |
| Protects Intestinal Barrier: Increased colonization of A. muciniphila in the intestine elevates CREBH expression, which alleviates ER stress and reduces gut barrier permeability and blood endotoxemia. | Variability in Abundance: A. muciniphila abundance varies significantly among individuals and disease states, complicating its use as a reliable therapeutic agent. |
| Potential in IBS Treatment: Supplementation has shown potential in reducing visceral hypersensitivity, anxiety-like behavior, and pain sensations in IBS models. | Effectiveness in Humans: Although animal studies show promise, the effectiveness and safety of A. muciniphila supplementation in humans need further validation through clinical trials. |
| Therapeutic Role in IBD: Transplantation of intestinal microbiota increases A. muciniphila levels, correlating with improved clinical outcomes in IBD patients. | Intestinal Mucosal Barrier Damage: In cases of severe mucosal barrier damage due to pathogenic bacteria, the reduced levels of A. muciniphila pose a challenge in restoring its benefits. |
| Inverse Relationship with Inflammation: Studies confirm an inverse relationship between A. muciniphila abundance and inflammation, suggesting its protective role in gut diseases. | Need for Targeted Therapies: There is a need for targeted therapies to specifically enhance A. muciniphila colonization and activity in the gut for consistent therapeutic benefits. |
| References | Model | Study Groups | Intervention | Survey Results |
|---|---|---|---|---|
| Wu et al. (2020) [83] | Animal | 10-week-old male C57BL/6J mice orally fed | 2 × 108 per day; live or pasteurized A. muciniphila; 4 weeks | Induction of metabolism, reduction of body weight and improvement of body composition, reduction of insulin resistance, reduction of adipose tissue mass, induction of adipocytes, reduction of serum glucose after its oral administration, restoration of fat layer thickness after a high-fat diet. |
| Li et al. (2016) [79] | Animal | Apoe−/− mice fed orally | 5 × 109 per day; live or pasteurized A. muciniphila; 9 weeks | Pasteurized A. mucniphila. Reduced fat gain, insulin resistance, and dyslipidaemia compared to mice supplemented with live A. muciniphila. Glucose tolerance comparable in both groups. |
| Zhao et al. (2017) [85] | Animal | Six-week-old pathogen-free mice on a low-carbohydrate diet | 1 × 109 for day, live A. muciniphila; 14 weeks; | Reduced weight gain and body fat, improved glucose tolerance and insulin sensitivity, reduced fatty-acid-related gene expression, reduced chronic low-grade inflammation, increased anti-inflammatory factors. |
| Chelakkot et al. (2018) [31] | Animal | 6–8-week-old male mice C57BL/6 | 10 μg EV per day, 2 weeks | Reduced intestinal permeability, weight reduction; improved glucose tolerance. |
| Ashrafian et al. (2019) [32] | Animal | 8-week-old male C57BL mice on a low-carbohydrate or high-fat diet | 1 × 109 live A. muciniphila per day; 10 mg protein/200 µL EV, 5 weeks | Significant weight loss in mice on a fat-rich diet, improved intestinal barrier integrity, improved glucose tolerance and lipid profile. |
| Everard et al. (2019) [78] | Animal | 10-week-old male mice on a low-carbohydrate or high-fat diet | 2 × 108 per day; live A. muciniphila; 4 weeks | Reduction in diet-induced obesity; reduced appetite, body weight, and fat mass; reduced hyperglycemia and hyperinsulinemia. |
| Katiraei et al. (2019) [86] | Animal | 9–13-week-old male E3L. CETP mice with an increased lipid profile | 2 × 108 per day; live A. muciniphila, 4 weeks | Reduction in body weight; reduction in plasma triglyceride and cholesterol levels. |
| Shin et al. (2019) [81] | Animal | 8-week-old female C57BL/6 mice on a high-fat or low-carbohydrate diet treated with Akk growing on medium with (+) or without (−) mucilage addition | 1 × 108 live A. muciniphila per day, 4 weeks | A. muciniphila (−) attenuated the changes induced by the high-fat diet, reduced adipocyte hypertrophy and increased the proportion of small adipocytes, improved glucose levels and increased insulin tolerance, reduced LPS levels and inhibited diet-induced progressive intestinal inflammation. |
| Wu et al. (2020) [83] | Animal | 8-week-old female C57BL/6 mice on a low-carbohydrate, high-fat diet | 1 × 109 live A. muciniphila per day, 10 months | Decreased weight gain, decreased appetite, increased expression of the anti-inflammatory factor IL-10. |
| Kim et al. (2020) [84] | Animal | 5-week-old C57BL/6N mice on a high-fat, low-carbohydrate diet | 1 × 108–1 × 109 live A. muciniphila per day, 10 weeks | No difference in weight gain, reduced TG and ALT levels in obese mice, reduced hepatic IL-6 expression in obese mice. |
| Lawenius et al. (2020) [87] | Animal | 12-week-old female mice C57BL/6 | Pasteurized A. muciniphila 2 × 108 per day, 4 weeks | Reduced weight and fat gain, reduced incidence of T lymphocytes in the bone marrow. |
| Depommier et al. (2019) [37] | Human | 32 people with overweight, insulin resistance, metabolic diseases | Pasteurized or live A. muciniphila 1 × 1010 per day, 3 months | Reduced plasma insulin levels and increased insulin sensitivity; reduced cholesterol, GGT, AST, LPS, LDH and serum creatine kinase; slight weight loss. |
| Advantage | Proof |
|---|---|
| Enhancing gut barrier integrity | A. muciniphila improves gut barrier function, reducing permeability and endotoxemia. |
| Reducing inflammation | It decreases the levels of pro-inflammatory cytokines and markers, thereby alleviating systemic and gut inflammation. |
| Improving metabolic parameters | Supplementation has been shown to reduce insulin resistance, improve glucose tolerance, and lower plasma lipid levels. |
| Weight management | A. muciniphila has been linked to reduced body weight and fat mass, making it beneficial in obesity management. |
| Protecting against liver damage | It reduces liver enzymes (AST, ALT) and protects against liver fibrosis and NAFLD. |
| Supporting immune function | It enhances immune signaling, promoting a balanced immune response and reducing systemic inflammation. |
| Improving lipid profiles | Supplementation reduces cholesterol, LDL, and triglycerides levels, benefiting cardiovascular health. |
| Alleviating symptoms of metabolic syndrome | It has shown potential in reversing metabolic disorders like metabolic endotoxemia and associated conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mruk-Mazurkiewicz, H.; Kulaszyńska, M.; Czarnecka, W.; Podkówka, A.; Ekstedt, N.; Zawodny, P.; Wierzbicka-Woś, A.; Marlicz, W.; Skupin, B.; Stachowska, E.; et al. Insights into the Mechanisms of Action of Akkermansia muciniphila in the Treatment of Non-Communicable Diseases. Nutrients 2024, 16, 1695. https://doi.org/10.3390/nu16111695
Mruk-Mazurkiewicz H, Kulaszyńska M, Czarnecka W, Podkówka A, Ekstedt N, Zawodny P, Wierzbicka-Woś A, Marlicz W, Skupin B, Stachowska E, et al. Insights into the Mechanisms of Action of Akkermansia muciniphila in the Treatment of Non-Communicable Diseases. Nutrients. 2024; 16(11):1695. https://doi.org/10.3390/nu16111695
Chicago/Turabian StyleMruk-Mazurkiewicz, Honorata, Monika Kulaszyńska, Wiktoria Czarnecka, Albert Podkówka, Natalia Ekstedt, Piotr Zawodny, Anna Wierzbicka-Woś, Wojciech Marlicz, Błażej Skupin, Ewa Stachowska, and et al. 2024. "Insights into the Mechanisms of Action of Akkermansia muciniphila in the Treatment of Non-Communicable Diseases" Nutrients 16, no. 11: 1695. https://doi.org/10.3390/nu16111695
APA StyleMruk-Mazurkiewicz, H., Kulaszyńska, M., Czarnecka, W., Podkówka, A., Ekstedt, N., Zawodny, P., Wierzbicka-Woś, A., Marlicz, W., Skupin, B., Stachowska, E., Łoniewski, I., & Skonieczna-Żydecka, K. (2024). Insights into the Mechanisms of Action of Akkermansia muciniphila in the Treatment of Non-Communicable Diseases. Nutrients, 16(11), 1695. https://doi.org/10.3390/nu16111695








