The Effect of Sleep on the Health and Dietary Behaviours of GAA Athletes
Abstract
1. Introduction
- To explore the sleep profiles of male and female, elite and sub-elite GAA players;
- To investigate the relationship between sleep and general health in GAA players;
- To investigate the relationship between sleep and food cravings in GAA players.
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Subjective Health Complaints Inventory (SHC)
2.4. Pittsburgh Sleep Quality Index (PSQI)
2.5. Food Cravings Questionnaire-Trait-Reduced (FCQ-T-r)
2.6. Data Analysis
3. Results
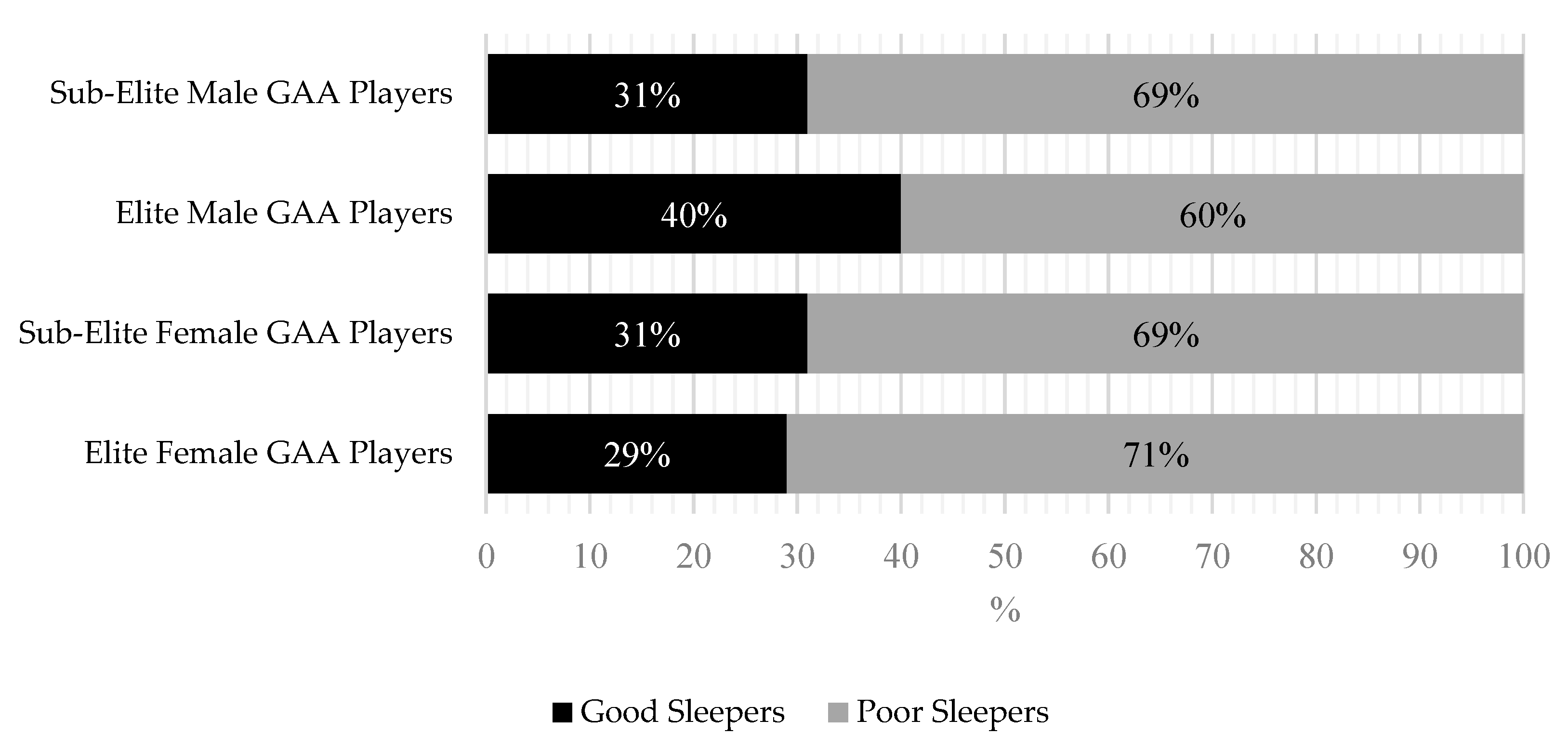
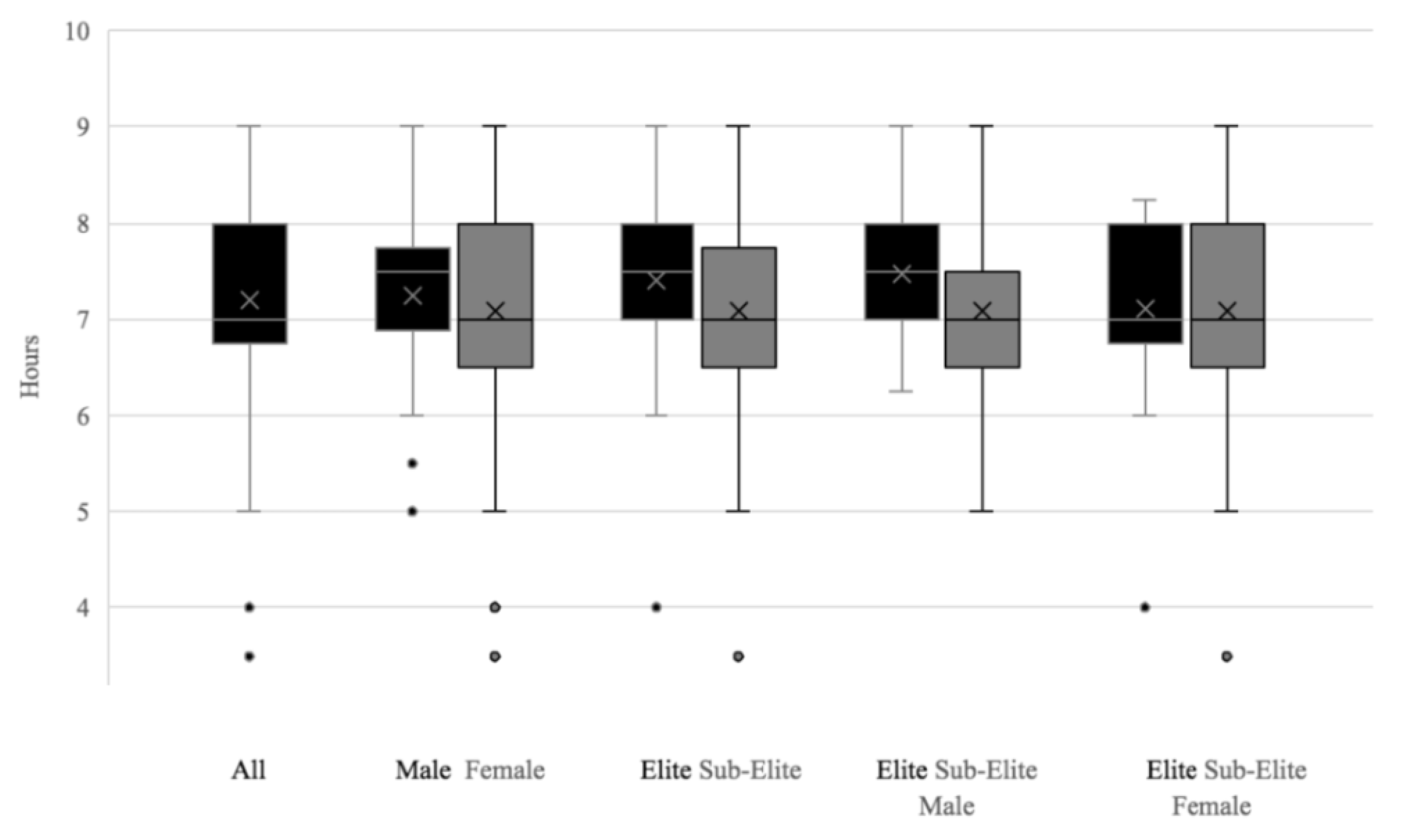
3.1. PSQI Scores
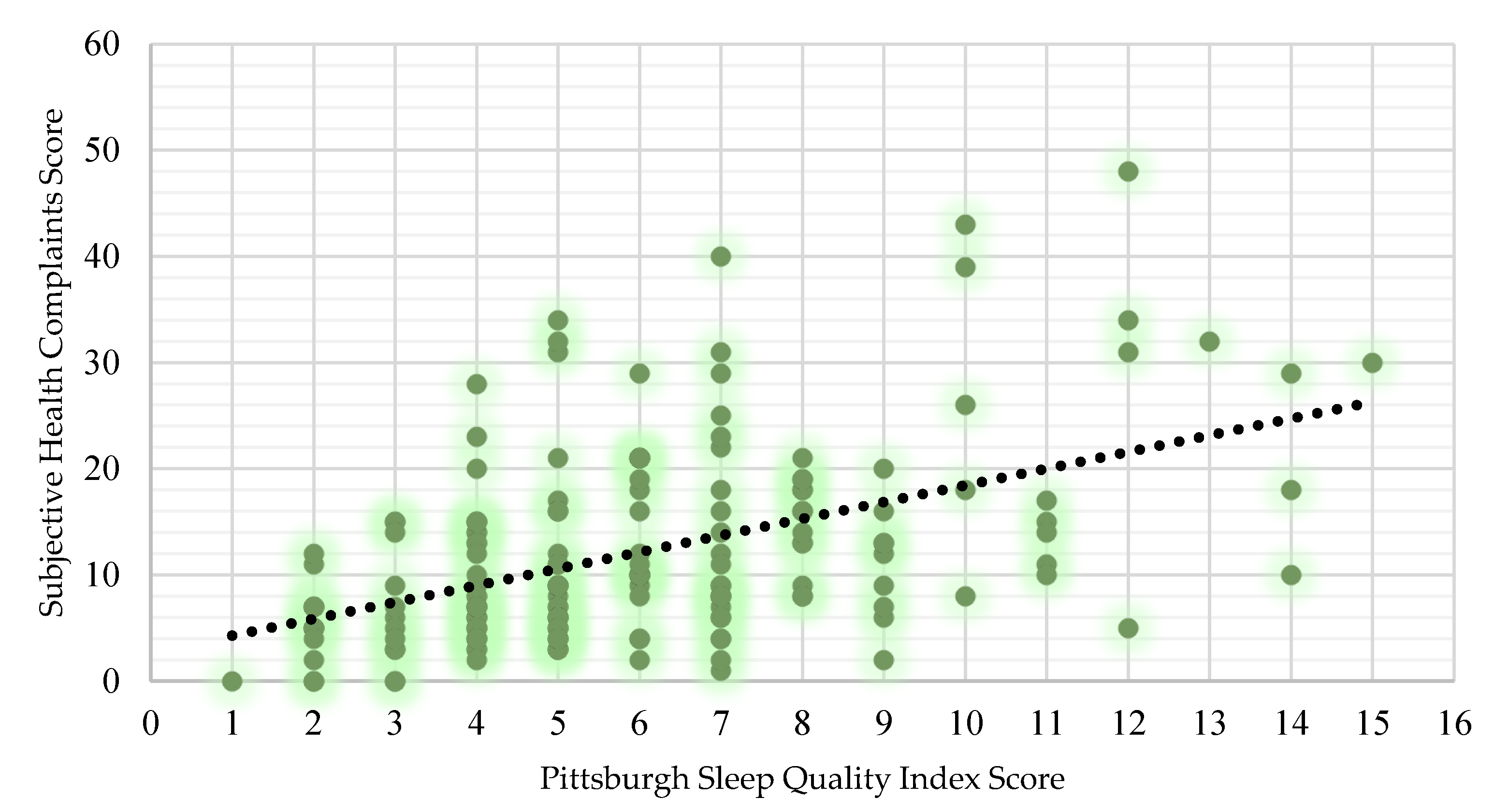
3.1.1. Sleep and General Health
3.1.2. Sleep and Food Cravings
4. Discussion
4.1. Poor Sleep in GAA Athletes
4.2. Poor Sleep and General Health
4.3. Poor Sleep and Food Cravings
4.4. Limitations
4.5. Practical Applications and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, A.M. Sleep and athletic performance. Curr. Sports Med. Rep. 2017, 16, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.H.; Duncan, M.J.; Cistulli, P.A.; Nassar, N.; Hamer, M.; Stamatakis, E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br. J. Sports Med. 2022, 56, 718–724. [Google Scholar] [CrossRef]
- Biggins, M.; Purtill, H.; Fowler, P.; Bender, A.; Sullivan, K.O.; Samuels, C.; Cahalan, R. Sleep in elite multi-sport athletes: Implications for athlete health and wellbeing. Phys. Ther. Sport 2019, 39, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.E.; Vitiello, M.V.; Abdelrahim, D.N.; Cheikh Ismail, L.; Jahrami, H.A.; Khaleel, S.; Khan, M.S.; Shakir, A.Z.; Yusuf, A.M.; Masaad, A.A.; et al. Eating habits are associated with subjective sleep quality outcomes among university students: Findings of a cross-sectional study. Sleep Breath. 2021, 26, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Biggins, M.; Cahalan, R.; Comyns, T.; Purtill, H.; O’Sullivan, K. Poor sleep is related to lower general health, increased stress and increased confusion in elite Gaelic athletes. Physician Sportsmed. 2018, 46, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Juliff, L.E.; Halson, S.L.; Hebert, J.J.; Forsyth, P.L.; Peiffer, J.J. Longer sleep durations are positively associated with finishing place during a national multiday netball competition. J. Strength Cond. Res. 2018, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Halson, S.L. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. 2014, 44 (Suppl. S1), 13–23. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Halson, S.L.; Sargent, C.; Roach, G.D.; Nédélec, M.; Gupta, L.; Leeder, J.; Fullagar, H.H.; Coutts, A.J.; Edwards, B.J.; et al. Sleep and the athlete: Narrative review and 2021 expert consensus recommendations. Br. J. Sports Med. 2021, 55, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, H.P.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef]
- Drew, M.K.; Raysmith, B.P.; Charlton, P.C. Injuries impair the chance of successful performance by sportspeople: A systematic review. Br. J. Sports Med. 2017, 51, 1209–1214. [Google Scholar] [CrossRef]
- Lv, W.; Finlayson, G.; Dando, R. Sleep, food cravings and taste. Appetite 2018, 125, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.; Madigan, S.; Warrington, G.; Ellis, J. Sleep and nutrition interactions: Implications for athletes. Nutrients 2019, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.D.; Roantree, M.T.; McCarren, A.L.; Kelly, D.T.; O’Connor, P.L.; Hughes, S.M.; Daly, P.G.; Moyna, N.M. Physiological profile and activity pattern of minor Gaelic football players. J. Strength Cond. Res. 2017, 31, 1811–1820. [Google Scholar] [CrossRef]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 2021, 17, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Malone, S.; Solan, B.; Collins, K.D.; Doran, D.A. Positional match running performance in elite Gaelic football. J. Strength Cond. Res. 2016, 30, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Schaal, K.; Tafflet, M.; Nassif, H.; Thibault, V.; Pichard, C.; Alcotte, M.; Guillet, T.; El Helou, N.; Berthelot, G.; Simon, S.; et al. Psychological balance in high level athletes: Gender-based differences and sport-specific patterns. PLoS ONE 2011, 6, e19007. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, H.R.; Ihlebæk, C.; Ursin, H. A scoring system for subjective health complaints (SHC). Scand. J. Public Health 1999, 27, 63–72. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Meule, A.; Hermann, T.; Kübler, A. A short version of the Food Cravings Questionnaire—Trait: The FCQ-T-reduced. Front. Psychol. 2014, 5, 190. [Google Scholar] [CrossRef]
- Rosnow, R.L.; Rosenthal, R. Effect sizes for experimenting psychologists. Can. J. Exp. Psychol./Rev. Can. Psychol. Expérimentale 2003, 57, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Benjamin, C.L.; Curtis, R.M.; Huggins, R.A.; Sekiguchi, Y.; Jain, R.K.; McFadden, B.A.; Casa, D.J. Sleep dysfunction and mood in collegiate soccer athletes. Sports Health 2020, 12, 234–240. [Google Scholar] [CrossRef]
- Halson, S.; Appaneal, R.; Peterson, K.; Welvaert, M.; Vlahovich, N.; Hughes, D.; Waddington, G.; Drew, M. High prevalence of poor sleep quality in athletes: Implications to staying healthy and performing. J. Sci. Med. Sport 2017, 20, e80. [Google Scholar] [CrossRef]
- Swinbourne, R.; Gill, N.; Vaile, J.; Smart, D. Prevalence of poor sleep quality, sleepiness and obstructive sleep apnoea risk factors in athletes. Eur. J. Sport Sci. 2016, 16, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Driller, M.W.; Suppiah, H.; Rogerson, D.; Ruddock, A.; James, L.; Virgile, A. Investigating the sleep habits in individual and team-sport athletes using the Athlete Sleep Behavior Questionnaire and the Pittsburgh Sleep Quality Index. Sleep Sci. 2022, 15, 112–117. [Google Scholar] [CrossRef]
- Yeomans, C.; Comyns, T.M.; Cahalan, R.; Hayes, K.; Costello, V.; Warrington, G.D.; Harrison, A.J.; Lyons, M.; Campbell, M.J.; Glynn, L.G.; et al. The relationship between physical and wellness measures and injury in amateur rugby union players. Phys. Ther. Sport 2019, 40, 59–65. [Google Scholar] [CrossRef]
- Sargent, C.; Rogalski, B.; Montero, A.; Roach, G.D. The sleep behaviors of elite Australian rules footballers before and after games during an entire season. Int. J. Sports Physiol. Perform. 2022, 17, 932–942. [Google Scholar] [CrossRef]
- Paul, R.W.; Sonnier, J.H.; Johnson, E.E.; Hall, A.T.; Osman, A.; Connors, G.M.; Freedman, K.B.; Bishop, M.E. Inequalities in the evaluation of male versus female athletes in sports medicine research: A systematic review. Am. J. Sports Med. 2023, 51, 3335–3342. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.G.; Ayas, N.T. The impact of daily sleep duration on health: A review of the literature. Prog. Cardiovasc. Nurs. 2004, 19, 56–59. [Google Scholar] [CrossRef]
- Kripke, D.F.; Garfinkel, L.; Wingard, D.L.; Klauber, M.R.; Marler, M.R. Mortality associated with sleep duration and insomnia. Arch. Gen. Psychiatry 2002, 59, 131–136. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflügers Arch.-Eur. J. Physiol. 2012, 463, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Janicki-Deverts, D.; Hall, M.H.; Cohen, S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep 2015, 38, 1353–1359. [Google Scholar] [CrossRef]
- Nobari, H.; Banihashemi, M.; Saedmocheshi, S.; Prieto-González, P.; Oliveira, R. Overview of the impact of sleep monitoring on optimal performance, immune system function and injury risk reduction in athletes: A narrative review. Sci. Prog. 2023, 106, 00368504231206265. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.; Cahalan, R.; Bonnett, L.; Maguire, M.; Glasgow, P.; Madigan, S.; O’Sullivan, K.; Comyns, T. General health complaints and sleep associated with new injury within an endurance sporting population: A prospective study. J. Sci. Med. Sport 2020, 23, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Duggan, J.D.; Collins, K.; Keane, K. Factors influencing performance and injury risk in elite female Gaelic team sport players and future research directions: A narrative review. BMC Sports Sci. Med. Rehabil. 2022, 14, 164. [Google Scholar] [CrossRef]
- Holtzman, B.; Ackerman, K.E. Recommendations and nutritional considerations for female athletes: Health and performance. Sports Med. 2021, 51 (Suppl. S1), 43–57. [Google Scholar] [CrossRef]
- Soltanieh, S.; Solgi, S.; Ansari, M.; Santos, H.O.; Abbasi, B. Effect of sleep duration on dietary intake, desire to eat, measures of food intake and metabolic hormones: A systematic review of clinical trials. Clin. Nutr. ESPEN 2021, 45, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Paxman, J.; Dalton, C.; Winter, E.; Broom, D.R. Effects of a 12-week aerobic exercise intervention on eating behaviour, food cravings, and 7-day energy intake and energy expenditure in inactive men. Appl. Physiol. Nutr. Metab. 2016, 41, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Caruso, L.; Zauli, E.; Vaccarezza, M. Physical Exercise and Appetite Regulation: New Insights. Biomolecules 2023, 13, 1170. [Google Scholar] [CrossRef]
- Hallam, J.; Boswell, R.G.; DeVito, E.E.; Kober, H. Focus: Sex and gender health: Gender-related differences in food craving and obesity. Yale J. Biol. Med. 2016, 89, 161–173. [Google Scholar]
- Wells, K.R.; Jeacocke, N.A.; Appaneal, R.; Smith, H.D.; Vlahovich, N.; Burke, L.M.; Hughes, D. The Australian Institute of Sport (AIS) and National Eating Disorders Collaboration (NEDC) position statement on disordered eating in high performance sport. Br. J. Sports Med. 2020, 54, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.I.; Janelle, K.C.; Prior, J.C. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am. J. Clin. Nutr. 1995, 61, 39–43. [Google Scholar] [CrossRef]
- Hirschberg, A.L. Sex hormones, appetite and eating behaviour in women. Maturitas 2012, 71, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Pfarr, S.; Schaaf, L.; Reinert, J.K.; Paul, E.; Herrmannsdorfer, F.; Rossmanith, M.; Kuner, T.; Hansson, A.C.; Spanagel, R.; Korber, C. Choice for Drug or Natural Reward Engages Largely Overlapping Neuronal Ensembles in the Infralimbic Prefrontal Cortex. J. Neurosci. 2018, 38, 3507–3519. [Google Scholar] [CrossRef] [PubMed]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Donnell SJ, M.; Corish, C.A. Low energy availability in athletes 2020: An updated narrative review of prevalence, risk, within-day energy balance, knowledge, and impact on sports performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Logue, D.M.; Madigan, S.M.; Heinen, M.; McDonnell, S.J.; Delahunt, E.; Corish, C.A. Screening for risk of low energy availability in athletic and recreationally active females in Ireland. Eur. J. Sport Sci. 2019, 19, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.; Madigan, S.M.; Nevill, A.; Warrington, G.; Ellis, J.G. The sleep and recovery practices of athletes. Nutrients 2021, 13, 1330. [Google Scholar] [CrossRef]
- Miles, K.H.; Clark, B.; Fowler, P.M.; Miller, J.; Pumpa, K.L. What are the sleep characteristics of elite female athletes? A systematic review with meta-analysis. Biol. Sport 2022, 39, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Beracci, A.; Martoni, M.; Meneo, D.; Tonetti, L.; Natale, V. Measuring subjective sleep quality: A review. Int. J. Environ. Res. Public Health 2021, 18, 1082. [Google Scholar] [CrossRef]
- Colbey, C.; Cox, A.J.; Pyne, D.B.; Zhang, P.; Cripps, A.W.; West, N.P. Upper respiratory symptoms, gut health and mucosal immunity in athletes. Sports Med. 2018, 48, 65–77. [Google Scholar] [CrossRef]
- Capling, L.; Beck, K.L.; Gifford, J.A.; Slater, G.; Flood, V.M.; O’Connor, H. Validity of dietary assessment in athletes: A systematic review. Nutrients 2017, 9, 1313. [Google Scholar] [CrossRef] [PubMed]

| Elite (n = 40) | Sub-Elite (n = 65) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||
| Male (n = 105) | Age | 25.48 | 6.28 | 18–41 | 24.03 | 4.39 | 18–36 |
| Height (cm) | 180.25 | 8.17 | 155–206 | 181.65 | 7.53 | 145–200 | |
| Weight (kg) | 83.18 | 7.35 | 72–102 | 83.54 | 12.01 | 68–145 | |
| BMI (kg/m2) | 25.65 | 2.03 | 23–31 | 25.32 | 3.08 | 21–40 | |
| Global PSQI Score | 4.95 | 1.88 | 1–9 | 6 | 2.52 | 2–12 | |
| SHC | 8.82 | 5.98 | 0–23 | 10.91 | 9.21 | 0–48 | |
| FCQ-T-r | 30.13 | 12.02 | 15–67 | 31.83 | 11.85 | 15–62 | |
| Elite (n = 17) | Sub-Elite (n = 48) | ||||||
| Mean | SD | Range | Mean | SD | Range | ||
| Female (n = 65) | Age | 25.71 | 6.04 | 19–36 | 23.42 | 5.06 | 18–37 |
| Height (cm) | 167.69 | 6.02 | 151–178 | 165.47 | 5.15 | 155–179 | |
| Weight (kg) | 68 | 8.44 | 55–85 | 63.37 | 7.84 | 47–92 | |
| BMI (kg/m2) | 24.13 | 3.61 | 20–30 | 23.1 | 2.7 | 19–34 | |
| Global PSQI Score | 6.59 | 3.18 | 3–14 | 6.75 | 3.34 | 2–15 | |
| SHC | 16.88 | 8.62 | 3–32 | 15.02 | 10.18 | 0–43 | |
| FCQ-T-r | 39 | 14.88 | 17–70 | 35.81 | 14.28 | 18–84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moran, M.; Ryan, L.; Doherty, R.; Biggins, M.; Keane, K.M. The Effect of Sleep on the Health and Dietary Behaviours of GAA Athletes. Nutrients 2024, 16, 1660. https://doi.org/10.3390/nu16111660
Moran M, Ryan L, Doherty R, Biggins M, Keane KM. The Effect of Sleep on the Health and Dietary Behaviours of GAA Athletes. Nutrients. 2024; 16(11):1660. https://doi.org/10.3390/nu16111660
Chicago/Turabian StyleMoran, Matt, Lisa Ryan, Rónán Doherty, Michelle Biggins, and Karen M. Keane. 2024. "The Effect of Sleep on the Health and Dietary Behaviours of GAA Athletes" Nutrients 16, no. 11: 1660. https://doi.org/10.3390/nu16111660
APA StyleMoran, M., Ryan, L., Doherty, R., Biggins, M., & Keane, K. M. (2024). The Effect of Sleep on the Health and Dietary Behaviours of GAA Athletes. Nutrients, 16(11), 1660. https://doi.org/10.3390/nu16111660







