Dietary Survey of Japanese Individuals with Type 2 Diabetes Mellitus on a Low-Carbohydrate Diet: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Exclusion Criteria
2.3. Dietary Assessment
2.4. Dietary Education
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Dietary Characteristics
3.3. Comparison Using Available Carbohydrate–Energy Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF Diabetes Atlas [Internet], 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581934/ (accessed on 5 January 2022).
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S68–S96. [Google Scholar] [CrossRef] [PubMed]
- Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia 2023, 66, 965–985. [Google Scholar] [CrossRef] [PubMed]
- Cowie, C.C.; Casagrande, S.S.; Menke, A.; Cissell, M.A.; Eberhardt, M.S.; Meigs, J.B.; Gregg, E.W.; Knowler, W.C.; Barrett-Connor, E.; Becker, D.J. (Eds.) Diabetes in America, 3rd ed.; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567985/ (accessed on 2 October 2023).
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Al-Mrabeh, A.; Zhyzhneuskaya, S.; Peters, C.; Barnes, A.C.; Aribisala, B.S.; Hollingsworth, K.G.; Mathers, J.C.; Sattar, N.; Lean, M.E.J. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for beta cell recovery. Cell Metab. 2018, 28, 547–556.e3. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Sakakida, H. Food exchange lists for diabetes treatment—Its research and history. In History of Diabetes in Japan; Diabetes Journal Editorial Board, Ed.; Yamanouchi Pharmaceutical: Tokyo, Japan, 1994; pp. 255–261. (In Japanese) [Google Scholar]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese clinical practice guideline for diabetes 2019. Diabetol. Int. 2020, 11, 165–223. [Google Scholar] [CrossRef] [PubMed]
- Japan Diabetes Clinical Data Management Study Group. Basic Tabulation Data. 2021. Available online: http://jddm.jp/public-information/index-2021/ (accessed on 2 October 2023). (In Japanese).
- Menke, A.; Knowler, W.C.; Cowie, C.C. Physical and metabolic characteristics of persons with diabetes and prediabetes. In Diabetes in America, 3rd ed.; Cowie, C.C., Casagrande, S.S., Menke, A., Cissell, M.A., Eberhardt, M.S., Meigs, J.B., Gregg, E.W., Knowler, W.C., Barrett-Connor, E., Becker, D.J., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018. [Google Scholar]
- Feinman, R.D. Fad diets in the treatment of diabetes. Curr. Diabetes Rep. 2011, 11, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.R.; Kossoff, E.H. Dietary treatments for epilepsy: Management guidelines for the general practitioner. Epilepsy Behav. 2011, 21, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A.; Ronco, A.L. Nutrient intake in low-carbohydrate diets in comparison to the 2020–2025 Dietary Guidelines for Americans: A cross-sectional study. Br. J. Nutr. 2022, 129, 1–14. [Google Scholar] [CrossRef]
- Churuangsuk, C.; Griffiths, D.; Lean, M.E.J.; Combet, E. Impacts of carbohydrate-restricted diets on micronutrient intakes and status: A systematic review. Obes. Rev. 2019, 20, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, C.; Yoshimura, Y.; Kamada, C.; Tanaka, S.; Tanaka, S.; Takahashi, A.; Hanyu, O.; Araki, A.; Ito, H.; Tanaka, A.; et al. Dietary intake in Japanese patients with type 2 diabetes: Analysis from Japan Diabetes Complications Study. J. Diabetes Investig. 2014, 5, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Tinguely, D.; Gross, J.; Kosinski, C. Efficacy of ketogenic diets on type 2 diabetes: A systematic review. Curr. Diabetes Rep. 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Zeraattalab-Motlagh, S.; Jabbarzadeh, B.; Hosseini, Y.; Jibril, A.T.; Shahinfar, H.; Mirrafiei, A.; Hosseini, F.; Bidar, S.S. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: A systematic review and dose-response meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 40–56. [Google Scholar] [CrossRef]
- Suzuki, F.; Okamoto, S.; Miyagi, S.; Tsujiguchi, H.; Hara, A.; Nguyen, T.T.T.; Shimizu, Y.; Hayashi, K.; Suzuki, K.; Nakai, S.; et al. Relationship between decreased mineral intake due to oral frailty and bone mineral density: Findings from Shika Study. Nutrients 2021, 13, 1193. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Omura, Y.; Murakami, K.; Matoba, K.; Nishimura, R.; Sasaki, S. Effects of individualized dietary advice compared with conventional dietary advice for adults with type 2 diabetes: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, T.; Sakamoto, R.; Takahashi, K.; Suzuki, J.; Matuura-Shinoda, M.; Takahashi, M.; Shigematsu, E.; Tanaka, S.; Kaneshiro, M.; Asakura, T.; et al. Dietary survey in Japanese patients with type 2 diabetes and the influence of dietary carbohydrate on glycated hemoglobin: The Sleep and Food Registry in Kanagawa study. J. Diabetes Investig. 2019, 10, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, R.; Nakatani, E.; Okamoto, K.; Aoshima, S.; Arai, H.; Inoue, T. Effects of sodium-glucose cotransporter 2 inhibitor (dapagliflozin) on food intake and plasma fibroblast growth factor 21 levels in type 2 diabetes patients. Endocr. J. 2019, 66, 6776–6782. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Uchida, J.; Izumi, H.; Tsukamoto, Y.; Inoue, G.; Watanabe, Y.; Irie, J.; Yamada, S. A non-calorie-restricted low-carbohydrate diet is effective as an alternative therapy for patients with type 2 diabetes. Intern. Med. 2014, 53, 13–19. [Google Scholar] [CrossRef]
- Ministry of Health, Labor and Welfare of Japan. National Health and Nutrition Survey in Japan. 2019. Available online: https://www.mhlw.go.jp/content/001066903.pdf (accessed on 2 October 2023). (In Japanese)
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids [Internet]; National Academies Press: Washington, DC, USA, 2005; Available online: https://www.nap.edu/catalog/10490/dietary-reference-intakes-for-energy-carbohydrate-fiber-fat-fatty-acids-cholesterol-protein-and-amino-acids (accessed on 2 October 2023).
- Ministry of Health, Labor and Welfare of Japan. Dietary Reference Intakes for Japanese. 2020. Available online: https://www.mhlw.go.jp/content/10904750/000586553.pdf (accessed on 2 October 2023). (In Japanese)
- Wu, Y.; Qian, Y.; Pan, Y.; Li, P.; Yang, J.; Ye, X.; Xu, G. Association between dietary fiber intake and risk of coronary heart disease: A meta-analysis. Clin. Nutr. 2015, 34, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Je, Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch. Cardiovasc. Dis. 2016, 109, 39–54. [Google Scholar] [CrossRef] [PubMed]
| Normal Limit or Treatment Target * | Total | Male | Female | |
|---|---|---|---|---|
| n | 335 | 222 | 113 | |
| Age (years) | 68 (60–74) | 68 (59–73) | 72 (63–75) | |
| Duration of diabetes (years) | 13 (9–19) | 14 (9–19) | 13 (8–18) | |
| Duration of low-carbohydrate diet (years) | 9 (5–13) | 9 (5–13) | 9 (5–13) | |
| HbA1c (mmol/mol) | <53 | 49 (45–55) | 50 (45–56) | 51 (45–55) |
| (%) | <7.0 | 6.7 (6.3–7.2) | 6.7 (6.3–7.3) | 6.8 (6.3–7.2) |
| Number of <7.0% (n) (%) | 209 (62.4) | 142 (64.0) | 67 (59.3) | |
| Casual plasma glucose (mg/dL) | 131 (114–150) | 132 (116–153) | 128 (110–142) | |
| BMI (kg/m2) | 22~25 | 24.0 (21.8–26.7) | 24.3 (22.3–26.9) | 23.5 (21.5–25.9) |
| SBP (mmHg) | <130 | 125 (117–133) | 124 (117–130) | 128 (117–136) |
| DBP (mmHg) | <80 | 73 (67–79) | 74 (69–80) | 70 (65–76) |
| TC (mg/dL) | 183 (164–205) | 180 (160–202) | 191 (174–217) | |
| HDL-C (mg/dL) | ≥40 | 62.6 (51.7–76.4) | 56.3 (48.5–69.0) | 73.3 (60.3–86.6) |
| LDL-C (mg/dL) | <120 | 101.3 (83.9–117.0) | 100.9 (83.9–115.4) | 102.1 (83.0–120.0) |
| TG (mg/dL) | <150 | 105 (73–151) | 109 (76–157) | 97 (72–138) |
| eGFR (mL/min/1.73 m2) | 68.71 (59.21–78.99) | 67.59 (58.33–79.51) | 69.65 (61.35–78.83) | |
| ACR (mg/gCr) | 14.5 (6.4–42.9) | 14.4 (5.9–43.5) | 14.9 (8.1–40.1) | |
| Diabetic retinopathy (%) | 11.0 | 10.8 | 11.5 |
| Total | Male | Female | |
|---|---|---|---|
| n | 318 | 209 | 109 |
| Antidiabetic medication (%) | 86.2 | 85.2 | 88.1 |
| Sulfonylurea (%) | 23.6 | 24.9 | 21.1 |
| Glinide (%) | 0.9 | 1.0 | 0.9 |
| α-glucosidase inhibitor (%) | 4.1 | 4.3 | 3.7 |
| Biguanide (%) | 67.0 | 65.1 | 70.6 |
| SGLT-2 inhibitor (%) | 45.3 | 45.0 | 45.9 |
| DPP-4 inhibitor (%) | 60.4 | 59.3 | 62.4 |
| GLP-1 receptor agonist (%) | 10.1 | 11.0 | 8.3 |
| Thiazolidinediones (%) | 3.8 | 3.8 | 3.7 |
| Antihypertensive medication (%) | 62.0 | 66.0 | 54.1 |
| ACE inhibitor (%) | 0.6 | 1.0 | 0.0 |
| ARB (%) | 56.6 | 60.8 | 48.6 |
| Mineralocorticoid receptor inhibitor (%) | 2.8 | 3.3 | 1.8 |
| Ca antagonist (%) | 40.6 | 42.6 | 36.7 |
| β blocker (%) | 5.0 | 4.3 | 6.4 |
| Thiazide (%) | 6.6 | 7.2 | 5.5 |
| Antilipemic medication (%) | 72.0 | 70.8 | 74.3 |
| Statin (%) | 64.8 | 61.2 | 71.6 |
| Ezetimibe (%) | 7.9 | 8.1 | 7.3 |
| Fibrate (%) | 11.6 | 16.3 | 2.8 |
| Crude | Energy-Adjusted by the Density Method | |||
|---|---|---|---|---|
| Unit | Intake | Unit | Intake | |
| Energy | kcal/day | 1457 (1153–1786) | − | − |
| kJ/day | 6098 (4825–7474) | − | − | |
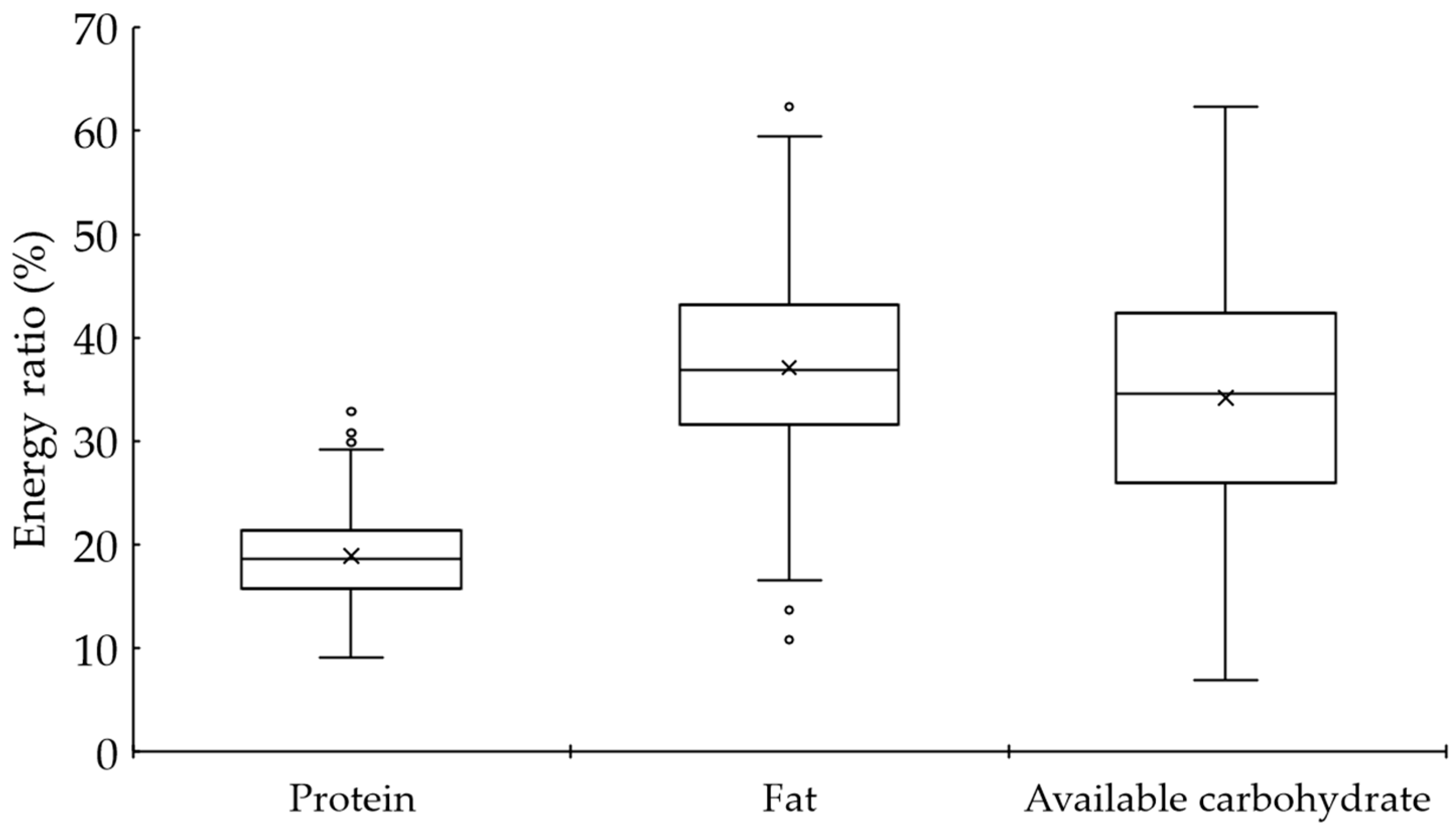
| Protein | g/kg BW/day | 1.0 (0.8–1.3) | % energy | 18.6 (15.7–21.4) |
| Animal protein | g/kg BW/day | 0.6 (0.5–0.9) | % energy | 12.1 (9.5–15.2) |
| Plant protein | g/kg BW/day | 0.3 (0.3–0.4) | % energy | 6.3 (5.3–7.2) |
| Fat | g/day | 56.7 (45.8–75.0) | % energy | 36.8 (31.6–43.2) |
| Saturated fatty acid | g/day | 15.1 (11.6–19.6) | % energy | 9.6 (8.1–11.1) |
| Monounsaturated fatty acid | g/day | 20.9 (16.6–27.7) | % energy | 13.3 (11.1–15.7) |
| Polyunsaturated fatty acid | g/day | 14.1 (11.1–18.3) | % energy | 9.1 (7.4–10.6) |
| Cholesterol | mg/day | 446 (290–590) | mg/1000 kcal | 297 (233–381) |
| Available carbohydrate | g/day | 119.0 (85.6–162.9) | % energy | 34.6 (26.0–42.4) |
| Total dietary fibre | g/day | 9.8 (7.8–13.0) | g/1000 kcal | 7.1 (5.6–8.4) |
| Salt | g/day | 9.4 (7.5–11.7) | g/1000 kcal | 6.5 (5.6–7.5) |
| Alcohol | g/day | 2.5 (0.0–22.6) | g/1000 kcal | 1.6 (0.0–13.7) |
| Q1 | Q2 | Q3 | Q4 | ||
|---|---|---|---|---|---|
| Available carbohydrate (%E) | 21.2 (16.6–24.3) | 30.2 (28.6–32.2) | 38.5 (36.4–40.1) | 46.9 (44.2–50.9) | |
| (g/1000 kcal) | 53.0 (41.4–60.7) | 75.5 (71.5–80.6) | 96.2 (91.2–100.2) | 117.3 (110.5–127.2) | p-value |
| n (male) | 83 (60) | 84 (56) | 84 (56) | 84 (50) | |
| Age (years) | 68 (57–73) | 66 (58–74) | 68 (60–74) | 72 (65–78) | 0.002 ‡|| |
| Duration of diabetes (years) | 11 (6–17) | 13 (6–18) | 14 (10–19) | 15 (11–20) | 0.005 ‡|| |
| Duration of low-carbohydrate diet (years) | 7 (3–10) | 9 (3–13) | 10 (6–13) | 11 (6–13) | <0.001 †‡ |
| Protein (%E) | 22.2 (19.2–25.9) | 20.1 (17.3–21.6) | 18.0 (15.2–20.2) | 15.9 (14.1–17.6) | <0.001 *†‡§||¶ |
| Animal protein (%E) | 15.9 (13.6–18.9) | 13.6 (11.1–15.5) | 11.1 (9.0–13.4) | 9.5 (7.4–11.3) | <0.001 *†‡§||¶ |
| Plant protein (%E) | 6.1 (4.6–7.9) | 5.8 (5.0–6.8) | 6.3 (5.5–7.2) | 6.5 (6.1–7.2) | 0.005 || |
| Fat (%E) | 45.5 (39.2–50.1) | 41.5 (34.7–44.3) | 35.4 (32.7–38.2) | 30.1 (26.9–33.0) | <0.001 *†‡§||¶ |
| Saturated fatty acid (%E) | 10.6 (9.1–12.3) | 10.3 (8.5–12.0) | 9.4 (7.9–10.4) | 8.5 (7.0–9.6) | <0.001 †‡§||¶ |
| Monounsaturated fatty acid (%E) | 16.7 (14.1–19.0) | 15.1 (13.0–16.4) | 12.9 (11.0–14.3) | 10.8 (9.7–11.9) | <0.001 *†‡§||¶ |
| Polyunsaturated fatty acid (%E) | 11.7 (9.9–13.0) | 9.8 (8.5–11.0) | 8.5 (7.2–9.5) | 7.2 (6.3–7.9) | <0.001 *†‡§||¶ |
| Cholesterol (mg/1000 kcal) | 406 (293–490) | 330 (271–393) | 275 (229–335) | 218 (172–279) | <0.001 *†‡§||¶ |
| Total dietary fibre (g/1000 kcal) | 7.4 (5.5–9.5) | 7.0 (5.5–8.3) | 7.3 (5.9–8.3) | 6.8 (5.6–8.0) | 0.380 |
| Salt (g/1000 kcal) | 7.2 (6.3–7.9) | 6.5 (5.7–7.2) | 6.4 (5.6–7.4) | 6.0 (5.4–7.1) | <0.001 *†‡ |
| Alcohol (g/1000 kcal) | 7.3 (0.0–22.1) | 1.9 (0.0–21.4) | 2.4 (0.0–12.1) | 0.0 (0.0–3.9) | <0.001 ‡||¶ |
| HbA1c (mmol/mol) | 47 (44–55) | 48 (45–54) | 52 (47–59) | 51 (46–55) | 0.002 †§ |
| (%) | 6.5 (6.2–7.2) | 6.6 (6.3–7.1) | 7.0 (6.5–7.6) | 6.9 (6.4–7.3) | 0.002 †§ |
| Casual plasma glucose (mg/dL) | 130 (120–146) | 133 (114–154) | 133 (114–152) | 125 (110–144) | 0.431 |
| BMI (kg/m2) | 23.6 (21.8–26.5) | 23.8 (21.0–26.0) | 24.6 (22.9–27.8) | 24.2 (22.2–26.0) | 0.041 § |
| SBP (mmHg) | 122 (115–130) | 124 (115–130) | 126 (117–135) | 128 (119–135) | 0.208 |
| DBP (mmHg) | 74 (69–79) | 72 (66–79) | 74 (67–80) | 71 (66–76) | 0.296 |
| TC (mg/dL) | 186 (167–215) | 196 (167–216) | 179 (162–192) | 180 (155–201) | 0.021 |
| HDL-C (mg/dL) | 65.9 (51.6–82.0) | 64.1 (54.4–82.4) | 58.9 (51.6–74.8) | 58.8 (49.8–73.8) | 0.078 |
| LDL-C (mg/dL) | 106.7 (88.9–121.1) | 103.8 (89.3–120.9) | 97.9 (82.1–111.1) | 99.6 (80.1–118.7) | 0.151 |
| TG (mg/dL) | 95 (61–147) | 105 (76–139) | 108 (73–154) | 124 (77–159) | 0.139 |
| eGFR (mL/min/1.73 m2) | 71.13 (59.21–87.14) | 72.78 (62.10–83.47) | 68.39 (59.22–77.06) | 62.51 (55.23–74.53) | 0.002 ‡|| |
| ACR (mg/gCr) | 15.4 (6.6–42.3) | 14.6 (5.6–34.6) | 19.7 (8.2–43.6) | 13.1 (6.5–46.2) | 0.374 |
| Diabetic retinopathy (%) | 12.0 | 9.5 | 10.7 | 11.9 | 0.953 |
| Antidiabetic medication (%) | 84.8 | 80.5 | 88.9 | 90.1 | 0.306 |
| Antihypertensive medication (%) | 58.2 | 61.0 | 60.5 | 67.9 | 0.621 |
| Antilipemic medication (%) | 60.8 | 75.3 | 72.8 | 79.0 | 0.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inaba, S.; Shirai, T.; Sanada, M.; Miyashita, H.; Inoue, G.; Nagahisa, T.; Wakana, N.; Homma, K.; Fukuyama, N.; Yamada, S. Dietary Survey of Japanese Individuals with Type 2 Diabetes Mellitus on a Low-Carbohydrate Diet: An Observational Study. Nutrients 2024, 16, 1658. https://doi.org/10.3390/nu16111658
Inaba S, Shirai T, Sanada M, Miyashita H, Inoue G, Nagahisa T, Wakana N, Homma K, Fukuyama N, Yamada S. Dietary Survey of Japanese Individuals with Type 2 Diabetes Mellitus on a Low-Carbohydrate Diet: An Observational Study. Nutrients. 2024; 16(11):1658. https://doi.org/10.3390/nu16111658
Chicago/Turabian StyleInaba, Sakiko, Tomomi Shirai, Mariko Sanada, Hiroyuki Miyashita, Gaku Inoue, Taichi Nagahisa, Noriaki Wakana, Kazuhiro Homma, Naoto Fukuyama, and Satoru Yamada. 2024. "Dietary Survey of Japanese Individuals with Type 2 Diabetes Mellitus on a Low-Carbohydrate Diet: An Observational Study" Nutrients 16, no. 11: 1658. https://doi.org/10.3390/nu16111658
APA StyleInaba, S., Shirai, T., Sanada, M., Miyashita, H., Inoue, G., Nagahisa, T., Wakana, N., Homma, K., Fukuyama, N., & Yamada, S. (2024). Dietary Survey of Japanese Individuals with Type 2 Diabetes Mellitus on a Low-Carbohydrate Diet: An Observational Study. Nutrients, 16(11), 1658. https://doi.org/10.3390/nu16111658






