Abstract
This study examined whey protein’s impact on insulin resistance in a high-fat diet-induced pediatric obesity mouse model. Pregnant mice were fed high-fat diets, and male pups continued this diet until 8 weeks old, then were split into high-fat, whey, and casein diet groups. At 12 weeks old, their body weight, fasting blood glucose (FBG), blood insulin level (IRI), homeostatic model assessment for insulin resistance (HOMA-IR), liver lipid metabolism gene expression, and liver metabolites were compared. The whey group showed significantly lower body weight than the casein group at 12 weeks old (p = 0.034). FBG was lower in the whey group compared to the high-fat diet group (p < 0.01) and casein group (p = 0.058); IRI and HOMA-IR were reduced in the whey group compared to the casein group (p = 0.02, p < 0.01, p < 0.01, respectively). The levels of peroxisome proliferator-activated receptor α and hormone-sensitive lipase were upregulated in the whey group compared to the casein group (p < 0.01, p = 0.03). Metabolomic analysis revealed that the levels of taurine and glycine, both known for their anti-inflammatory and antioxidant properties, were upregulated in the whey group in the liver tissue (p < 0.01, p < 0.01). The intake of whey protein was found to improve insulin resistance in a high-fat diet-induced pediatric obesity mouse model.
1. Introduction
Through epidemiological studies, Barker et al. proposed the concept that birth weight determines the risk of non-communicable disease development in adulthood (Barker theory) [1]. The developmental origins of health and disease (DOHaD) theory, recently proposed, posits that the environment, from fetal to developmental stages, plays a crucial role in establishing risk factors for chronic non-communicable diseases in adulthood and old age [2]. Additionally, infants with low birth weight are more likely to become significantly obese later in life [3].
The incidence of childhood obesity is rising in both developed and developing countries. In Japan, the percentage of overweight children peaked in 2008 but has decreased in recent years. However, this percentage remains high, with slightly less than 10% recorded among upper elementary school students [4].
Childhood obesity is recognized as a risk factor for developing hypertension, abnormalities in glucose and lipid metabolism, hyperuricemia, and nonalcoholic fatty liver disease [4]. These conditions manifest not only in adulthood but also during childhood, underscoring the need for early intervention and management. Treatment for obesity typically involves both exercise and dietary therapies [4]; however, managing exercise in infancy and early childhood is challenging. As a result, dietary interventions have been explored.
Whey protein is a milk-derived protein complex primarily consisting of lactoferrin, β-lactoglobulin, α-lactalbumin, glycomacropeptide, and immunoglobulins. Generally, casein protein, rather than whey protein, is used as feed for mice [5]. Compared to casein protein, which is also derived from milk, whey protein is absorbed more quickly by the digestive tract, resulting in a more rapid increase in blood amino acid concentrations after ingestion [6,7]. Whey protein is renowned for its multiple health benefits [8]. It may improve insulin resistance and hyperglycemia by inhibiting the release of serotonin from high-fat diets and fibroblast growth factor 21 from the liver [9]. Beta-lactoglobulin, a component of whey protein, has been shown to inhibit dipeptidyl peptidase-4 activity, thus increasing incretin levels and enhancing glucose metabolism by reducing postprandial blood glucose levels [10]. Previous research has demonstrated that administering a whey protein-enriched diet to adult males for eight weeks improved the lipid profile of the subjects, indicating enhanced lipid metabolism [11]. Whey protein is also known to exhibit anti-inflammatory effects by reducing levels of interleukin-6 and tumor necrosis factor-α [12] and possess potent antioxidant properties [13].
Oxidative stress, which increases with fat accumulation in obesity [14], notably inhibits glucose uptake in muscles [15] and reduces insulin secretion from pancreatic beta cells [16]. Obesity induces chronic inflammation and insulin resistance due to abnormal cytokine production and macrophage infiltration into adipose tissue [17]. Obesity is thus associated with chronic inflammation and oxidative stress, as previously described.
Previously, we found that administering whey protein to male mice from the embryonic period had a more pronounced effect on visceral fat reduction and insulin resistance than casein protein. Moreover, this improvement was attributed to the stimulation of β-oxidation by whey protein and its superior anti-inflammatory and antioxidative properties compared to casein protein [18]. However, few studies have examined the effects of whey protein using pediatric or young-adult obesity models. In this study, we aimed to elucidate the efficacy and mechanism of whey protein in a high-fat diet-induced pediatric obesity mouse model, where pregnant mice were fed a high-fat diet, and their offspring continued this diet during childhood.
2. Materials and Methods
2.1. Experimental Animals
All experimental plans and procedures were approved by the Animal Experimentation Committee of Nihon University Itabashi Hospital (Approval ID: AP20MED0181, Approval date: 5 June 2020). Pregnant mice from the Institute of Cancer Research (ICR) were purchased from Sankyo Laboratory Services, Inc. (Tokyo, Japan) on day 2 of gestation.
2.2. Rearing Conditions
Upon arrival, Slc: ICR pregnant mice were fed HFD-60, a high-fat feed with a fat content of 60% [25.6% casein, 0.36% L-cystine, 6% maltodextrin, 16% α-corn starch, 5.5% sucrose, 2% soybean oil, 33% lard, 6.6% cellulose powder, 3.5% minerals, 1.0% vitamins, 0.25% choline tartrate, and 0.18% carbonic acid; calcium: 506 kcal energy; Oriental Yeast Industry Co., Ltd. (Tokyo, Japan)]. According to previous studies, the administration of a high-fat diet with a fat-to-calorie ratio of 40–60% induces obesity, fasting hyperglycemia, hyperinsulinemia, increased insulin resistance, and decreased antioxidant and anti-inflammatory capacity in mice [19,20]. After birth, male pups were administered the same HFD-60 as their mothers until 8 weeks old. Thereafter, mice were divided into three groups: high-fat, casein, or whey groups. All mice were housed at 22 ± 2 °C under 55 ± 5% humidity and 12/12 h light/dark cycle. Male mice are known to be more susceptible to the effects of a high-fat diet than female mice [21]. As male mice were used in our previous experiments [18], male mice were used in this study.
The high-fat group was administered a high-fat diet as is. The casein group was administered AIN-93G (20% casein, 0.3% L-cystine, 39.7486% corn starch, 13.2% α-corn starch, 10.0% sucrose, 7.0% soybean oil, 5.0% cellulose powder, 3.5% minerals, 1.0% vitamins, 0.25% choline tartrate, and 0.0014% tertiary butyl hydroquinone; 359 kcal energy; Oriental Yeast Industry, Ltd., Tokyo, Japan), which is part of the standard rodent diet administered during gestation and development in mouse experiments. The whey group was administered a modified blend diet in which the casein component (AIN-93G) was replaced with whey. Male pups were fed their respective diets until 12 weeks old. Thereafter, physical and biochemical measurements were performed (Figure 1). In present study, a total of 8 mother mice and 36 male pups were used.
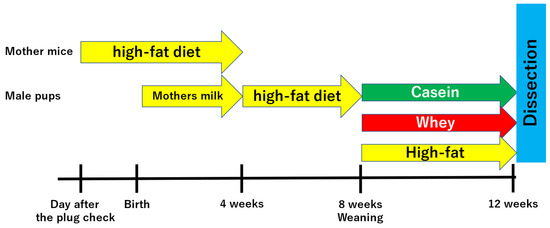
Figure 1.
Experimental procedures.
2.3. Body Weight
Pups were weighed once weekly from birth to 12 weeks old to identify any differences in weight between the three groups each week.
2.4. Fasting Blood Glucose Level, Serum Insulin Level, and Insulin Resistance (HOMA-IR)
Twelve-week-old adult male mice were subjected to a 12 h fast and dissected under isoflurane inhalation anesthesia (5% induction, 2% maintenance). Blood was collected from the heart via a cardiac puncture made through a mid-thoracic incision. Fasting blood glucose levels were measured using a Stat Strip XP2 (Nipro, Osaka, Japan). Serum was separated from whole blood via centrifugation at 3000 rpm for 5 min and stored at −20 °C. Serum immunoreactive insulin levels (IRI) were measured using the mouse/rat total insulin (high sensitivity) assay kit (Immunobiology Laboratories, Inc., Fujioka, Gunma, Japan). Insulin resistance was also measured using the human formula for homeostasis model evaluation of insulin resistance (HOMA-IR) [22].
2.5. Body Composition and Fat Weight
Body composition was measured using a bioimpedance spectroscopy device for laboratory animals (ImpediVETTM: BioResearch Center Corporation, Nagoya, Japan) [23]. To estimate fat mass (FM) and lean body mass (FFM), the difference in bioelectrical impedance for the electrical conductivity of biological tissues was measured, as adipose tissue contains less water per unit volume and is less conductive than muscle and other tissues. Fat weight was measured by removing all observable visceral adipose tissue.
2.6. Serum Lipoprotein Fractionation
The levels of serum lipoproteins, such as cholesterol (Cho) and triglyceride (TG), were determined via gel-permeation high-performance liquid chromatography (HPLC), according to a previously described method (LipoSEARCH®; Skylight Biotech, Akita, Japan) [24,25]. Cho and TG levels were estimated based on the peaks corresponding to different lipoprotein particle sizes in the HPLC elution profiles for 10 major lipoprotein classes [chylomicrons (CM), very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL), high-density lipoproteins (HDL)] [25].
2.7. Oxidative Stress Markers
Derivatives of reactive oxidative metabolites (d-ROM) and biological antioxidant potential (BAP) were measured in the serum of 12-week-old mice using FREE Carrio Duo (WISMERLL, Tokyo, Japan). Furthermore, the oxidant to antioxidant (d-ROM/BAP) ratio was calculated as the oxidative stress index (OSI), as previously described [26].
2.8. Gene Expression Analysis of the Liver Tissue
The expression levels of genes related to lipid metabolism in the liver [peroxisome proliferator-activated receptor (PPAR)α, PPARγ, hormone sensitive lipase (HSL), and lipoprotein lipase (LPL)] were measured using real-time quantitative polymerase chain reaction (RT-qPCR). RNA was isolated from the frozen liver tissue of male mice (n = 5 per group), according to the protocols provided by ReliaPrep RNA Miniprep Systems (Promega Corporation, Madison, WI, USA). RNA was reverse transcribed into complementary DNA using ReverTra Ace qPCR RT Master Mix (Toyobo Co., Ltd., Osaka, Japan) on an ABI Geneamp 9700 PCR-Thermal Cycler (Applied Biosystems, Thermo Fisher Scientific Inc., Tokyo, Japan).
RT-qPCR was performed using KOD-Plus-Ver.2 polymerase mix (Toyobo Co., Ltd.) on an ABI Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific Inc.). In this study, the primers [manufactured by Takara Bio Co., Ltd. (Gunma, Japan)] used in a previous study were employed [27]. The sequences are shown in Table 1.

Table 1.
List of DNA primer sets for quantitative RT-qPCR.
2.9. Metabolome Analysis of the Liver Tissue
Briefly, 1500 µL of 50% acetonitrile solution (v/v) (internal standard concentration: 10 µM) was added to a crushing tube containing frozen liver tissue samples (approximately 40 mg, n = 5 per group) from male mice and crushed (1500 rpm, 120 s × 3 times) using a crushing machine under cool conditions. After the crushed tissue was centrifuged (2300× g, 4 °C, 5 min), the supernatant was transferred to an ultrafiltration tube (Ultrafree MC PLHCC, HMT, centrifugal filter unit, 5 kDa), centrifuged (9100× g, 4 °C, 120 min), and subjected to ultrafiltration. The filtrate was dried and dissolved in Milli-Q water.
The solution was subjected to automatic testing using an Agilent CE system [Agilent Technologies, Inc. integrated software (Keio University, Shizuoka, Japan)]. The mass spectrum peak (range: 50–1000 m/z) area, m/z, and migration time were calculated for the detected peaks [28]. The chemical species associated with each peak were identified based on the m/z values and migration times by referencing the HMT metabolite database. The relative amounts of each metabolite were calculated by normalizing the peak areas with internal standards and sample volumes. Principal component analysis and hierarchical cluster analysis were performed as previously described [29].
2.10. Statistical Analysis
Data are presented as mean ± standard error. For comparisons involving three groups, the Steel–Dwass test was used. For comparisons between two groups, the Mann–Whitney U test was used when n was 6 or more in each group (e.g., comparing the experimental group (whey) to the control group (casein)); however, when n was less than 6, the Welch t-test was employed. JMP statistical software (ver.14.0: SAS Institute, Cary, NC, USA) was used for the analysis. p < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Weight Change
Body weights did not significantly differ among all groups of mice from 5 to 9 weeks old. However, at 10 weeks of age, the mice in the whey group weighed significantly less than those in the casein group (51.42 g vs. 57.78 g, p = 0.042) and significantly less than those in the high-fat group (51.42 g vs. 63.23 g, p < 0.01). At 12 weeks, body weight was significantly less in the whey group than in the casein group (53.73 g vs. 59.74 g, p = 0.034) (Figure 2a,b). Weight change from 8 to 12 weeks was also significantly less in the whey group (−0.76 g vs. 3.84 g, p = 0.016) (Figure 2c).
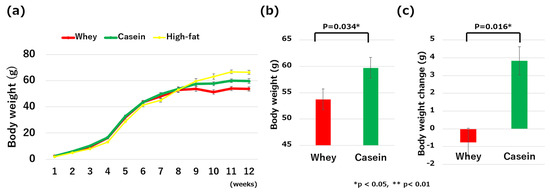
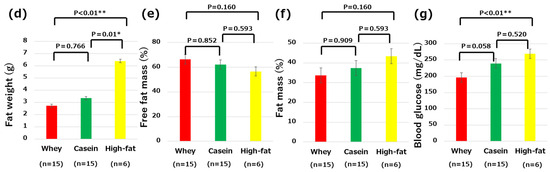
Figure 2.
Comparisons of the clinical and metabolic parameters: (a) changes in body weight from birth to 12 weeks old; (b) body weight on the last day of high-fat-diet intake; (c) body weight changes from 8 to 12 weeks of age; (d) fat weight; (e) free fat mass; (f) fat mass; and (g) fasting blood glucose levels. * p < 0.05, ** p < 0.01.
3.2. Fat Weight and Body Composition
The fat weight did not significantly differ between the whey and casein groups of mice (2.71 g vs. 3.34 g, p = 0.766). However, the fat mass was significantly greater in the high-fat group compared to both the whey group (2.71 g vs. 6.38 g, p < 0.01) and casein group (3.34 g vs. 6.38 g, p = 0.01) (Figure 2d). Body composition, particularly FFM (66.34% in the whey group vs. 62.17% in the casein group vs. 53.67% in the high-fat group) and FM (33.64% in the whey group vs. 37.41% in the casein group vs. 43.36% in the high-fat group), were not significantly different among all groups (Figure 2e,f).
3.3. Fasting Blood Glucose
Fasting blood glucose was significantly lower in the whey group compared to the high-fat group (196.2 mg/dL vs. 268.83 mg/dL, p < 0.01). It also trended lower in the whey group than in the casein group (196.2 mg/dL vs. 239.73 mg/dL, p = 0.058). There was no significant difference in the fasting blood glucose levels of the casein and high-fat groups (239.73 mg/dL vs. 268.83 mg/dL, p = 0.52) (Figure 2g).
3.4. IRI, and HOMA-IR
IRI and HOMA-IR were significantly lower in the whey group than the casein group (IRI: 5.6 μIU/mL vs. 10.62 μIU/mL, p = 0.01; HOMA-IR: 2.65 vs. 6.06, p < 0.01) (Figure 3a,b).
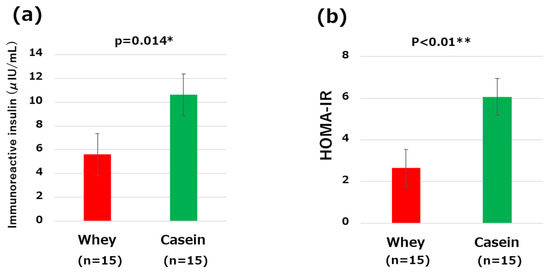
Figure 3.
Comparisons of the clinical and metabolic parameters: (a) serum immunoreactive insulin levels and (b) homeostasis model assessment of insulin resistance (HOMA-IR) levels. * p < 0.05, ** p < 0.01.
3.5. Serum Lipoprotein Fractionation
Cholesterol levels did not significantly differ between the two groups of mice. However, the level of LDL-TG tended to decrease in the whey group (14.29 mg/dL vs. 17.17 mg/dL, p = 0.09) (Table 2).

Table 2.
Serum lipoprotein levels.
3.6. Oxidative Stress, Biological Antioxidant Capacity, and Oxidative Stress Index
The d-ROMs (99.16 vs. 138.83, p = 0.17) and BAP (2939.83 μmol/L vs. 2786.33 μmol/L, p = 0.38) did not significantly differ between the two groups of mice. The OSI tended to be lower in the whey group compared to the casein group (0.03 vs. 0.05 p = 0.09) (Figure 4a–c).
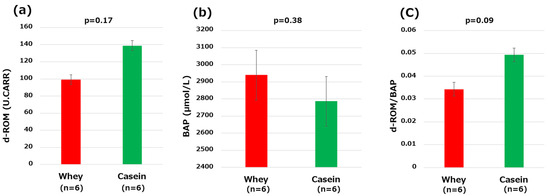
Figure 4.
Oxidative stress markers: (a) derivatives of reactive oxidative metabolites (d-ROMs); (b) biological antioxidant potential (BAP); and (c) oxidative stress index (OSI, d-ROM/BAP).
3.7. Analysis of Genes Related to Lipid Metabolism in the Liver
Based on RT-qPCR, mice in the whey group had significantly higher expression levels of PPARα and HSL in the liver than those in the casein group (PPARα: p < 0.01, HSL: p = 0.03). The expression level of LPL was significantly lower in the whey group than in the casein group (p = 0.03). PPARγ was not found to significantly differ between the two groups of mice (p = 0.72) (Figure 5a–d).
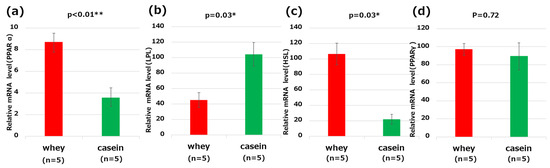
Figure 5.
Genes related to lipid metabolism in the liver: (a) peroxisome proliferator-activated receptor (PPAR)α; (b) lipoprotein lipase (LPL); (c) hormone sensitive lipase (HSL); and (d) peroxisome proliferator-activated receptor (PPAR)γ. * p < 0.05, ** p < 0.01.
3.8. Metabolome Analysis of the Liver
Principal component analysis or hierarchical clustering heat mapping did not reveal any clear differences between the groups; however, individual significant differences were found (Figure 6a,b. Supplementary Tables S1–S3). In particular, the levels of taurine, glycine, and myo-inositol 1-phosphate were significantly elevated in the whey group compared to those in the casein group (taurine: p = 0.02, glycine: p < 0.01, and myoinositol 1-phosphate: p < 0.01) (Figure 6c–e).
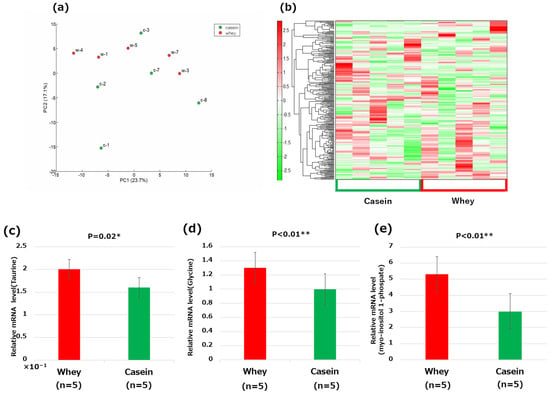
Figure 6.
Metabolome analysis of the liver: (a) principal component (PC) analysis; (b) heat map of the hierarchical cluster analysis; (c) taurine; (d) glycine; and (e) myo-inositol 1-phosphate. * p < 0.05, ** p < 0.01.
4. Discussion
In the present study, conducted on a high-fat diet-induced pediatric obesity mouse model, it was found that intake of whey protein resulted in the suppression of body weight gain, a lower fat mass, and improved fasting blood glucose levels than the casein group when compared to the high-fat group. Furthermore, whey protein intake was observed to improve insulin resistance compared to casein protein. These results suggest that the improvements in insulin resistance associated with whey protein are likely due to enhanced β-oxidation and the promotion of anti-inflammatory and antioxidant effects compared to casein protein.
4.1. Lipid Metabolism-Related Genes
Mice fed whey protein exhibited improved fasting blood glucose levels and insulin resistance compared to those fed casein protein. In terms of body composition and visceral fat mass, the average lean body mass of the mice in the whey group was higher while the average visceral fat mass was lower than those of the mice in the casein group. Furthermore, the mice in the whey group had significantly higher expressions of PPARα and HSL in the liver than those in the casein group. Previously, Sasaki et al. [27] revealed that the intake of whey protein affected the expression of PPARα, PPARγ, and SREBP1c in the gastrocnemius, liver, and epididymis adipose tissue of mice, as well as their downstream enzymes, HSL, LPL, ACCα, and FAS. PPARα is known to promote β-oxidation of fatty acids in intracellular mitochondria to stimulate lipid metabolism [30] and has anti-inflammatory effects [31]. Inflammatory chemokines and cytokines are known to affect insulin resistance [32,33]. HSL breaks down adipocyte TG into free fatty acids and glycerol [34] and may promote β-oxidation [35]. In the present study, we inferred that whey protein intake enhances the reduction of visceral fat and improves insulin resistance by increasing the expression of PPARα and HSL, promoting β-oxidation of fatty acids in the liver, and leveraging the anti-inflammatory effects of PPARα itself (Figure 7a).
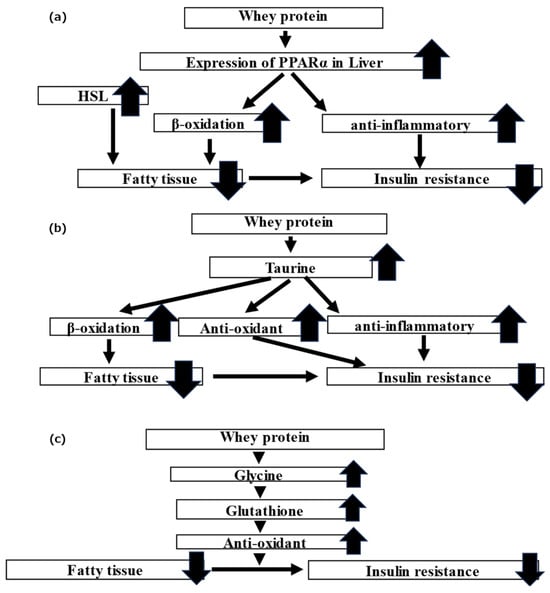
Figure 7.
Schematic of the theory for the mechanism by which whey protein ameliorates the metabolism of lipid and glucose: (a) PPARα, HSL; (b) taurine; and (c) glycine.
4.2. Involvement of Taurine
Metabolomic analysis revealed a significantly higher level of taurine in the whey group relative to the casein group. Taurine is a type of amino acid obtained via diet and is known to be produced from cysteine in the liver and fat cells in vivo [36]. According to a previous study, taurine is more abundant in breast milk than cow’s milk, and powdered milk with more whey protein contains more taurine than powdered milk with the same ratio of casein protein and whey protein as cow’s milk [37]. As whey protein is rich in essential amino acids, including cysteine [38], the elevated level of taurine may occur through endogenous and exogenous paths.
Taurine improves lipid metabolism by promoting bile acid synthesis from Cho and β-oxidation of the fatty acids in the mitochondria in mice administered a high-fat diet [39,40]. Taurine is also reported to exhibit antioxidant effects and inhibit fat oxidation in the liver by improving mitochondrial function [41]. Additionally, taurine exhibits anti-inflammatory effects in adipose tissue [42]. Overall, whey protein intake may elevate taurine endogenously and exogenously and may be involved in the elevation in β-oxidation and the anti-oxidative anti-inflammatory effects (Figure 7b), ultimately leading to improved lipid and glucose metabolism.
4.3. Glycine and Myo-Inositol 1-Phosphate
Previously, we speculated that whey protein improves insulin resistance by increasing glutathione in adipocytes and exhibiting an antioxidant effect [18]. Glutathione is composed of glycine, cysteine, and glutamic acid. Previous research has shown that administering glycine and cysteine to diabetic patients increases glutathione levels and improves oxidative stress [43] and that administration of glycine to model mice with increased insulin resistance and oxidative stress increased glutathione and improved oxidative stress, glucose metabolism, and visceral fat mass [44]. In the present study, glycine levels were elevated in the whey group. We inferred that the administration of whey protein elevated glycine levels, which subsequently raised glutathione levels. This enhancement in glutathione likely contributed to its antioxidant effects, resulting in improved insulin resistance (Figure 7c).
In a previous study, myo-inositol 1-phosphate levels were found to be elevated by whey protein administration [18]. Similar results were obtained in a high-fat diet-induced childhood obesity mouse model. As myo-inositol is known to improve insulin resistance [45] and myo-inositol 1-phosphate belongs to the myo-inositol metabolic pathway [46], myo-inositol 1-phosphate may also play a role in improving glucose metabolism.
4.4. Limitations
In this study, insulin resistance was found to significantly differ between the whey and casein groups; however, no clear differences were found in the blood lipid profile, visceral fat mass, or OSI.
This result may be attributed to the use of a high-fat diet-induced pediatric obesity mouse model, which exhibits elevated fat mass, abnormal lipid metabolism, and heightened oxidative stress, in contrast to mice fed a standard diet [19,20]. In addition, the whey–casein diet was administered for a shorter period (4 weeks) in this study than other studies [9]; therefore, the difference may not be significant.
The present study measured only serum insulin levels and HOMA-IR for assessing insulin resistance, which might not be completely accurate. Future research could more precisely evaluate insulin resistance by employing the glucose clamp technique and 2-deoxyglucose glucose uptake.
The expression level of PPARα elevated while that of LPL reduced in the liver of mice in the whey group compared to those of mice in the casein group. According to a previous study, LPL is upregulated in fat and muscle and downregulated in the liver of mice administered a high-fat diet [47]. In this study, metabolomic analysis was only performed on the liver; therefore, other organs must assessed to determine LPL levels. In addition, this study evaluated only the gene expression levels of lipid metabolism-related genes. In future research, we will also assess the protein expression of these genes using Western blotting and other techniques.
4.5. Future Prospects
Fetal administration of whey protein [18] and administration of whey protein to mice models of high-fat diet-induced childhood obesity led to improvements in fasting blood glucose, insulin resistance, visceral fat loss, and blood lipid profiles. This study suggests that whey protein promotes β-oxidation and enhances anti-inflammatory and antioxidant effects. Low birthweight infants are at increased risk of developing type 2 diabetes in adulthood due to the accumulation of visceral fat and insufficient muscle mass [48]. In addition, oxidative stress has been found to be higher in low-birth-weight infants [49]. Whey protein may have a preventive effect on the development of type 2 diabetes in infants with a low birth weight. Feeding usually transitions from breast milk or artificial baby milk to cow’s milk. Mature milk and powdered milk have a whey and casein protein ratio of 60% to 40%, whereas cow’s milk has a reduced whey protein content of 20% and 80% casein protein [50]. The addition of whey protein during this transition period may reduce the risk of diabetes development in infants with low birth weight. However, although some reports suggest that a high-protein diet has protective effects against diabetes and obesity in adults [51], other studies indicate that a high-protein diet in early infancy may induce an early adiposity rebound [52]. Therefore, merely adding whey protein to regular nutrition could increase protein intake and potentially be counterproductive concerning future risks of diabetes, obesity, and other diseases. We plan to conduct whey protein intervention experiments using the low-birth-weight, non-obese hyperglycemic mouse model developed by Katayama et al. [53] to assess the potential of whey protein in enhancing glycolipid metabolism. In our previous studies, we administered only whey protein or casein protein. However, both breast milk and powdered milk contain a mixture of whey and casein proteins. For future clinical applications, it is necessary to conduct studies using feed that includes a mixture of whey and casein proteins in varying ratios.
5. Conclusions
Compared to casein protein, whey protein has been shown to improve insulin resistance in a high-fat diet-induced pediatric obesity mouse model. This improvement in insulin resistance is believed to result from enhanced beta oxidation, which is facilitated by elevated expressions of HSL and PPARα, as well as the augmented anti-inflammatory and antioxidant effects due to elevated levels of taurine and glycine.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16111622/s1, Table S1: principal component score; Table S2: metabolites and principal component score; and Table S3: Results of comparative analysis.
Author Contributions
Conceptualization: K.M., N.N. and I.M.; methodology: K.M., N.N., S.S. and D.K.; formal analysis: K.M., N.N. and I.M.; investigation: K.M., N.N., K.N., D.K., S.S., W.T., R.A., K.F. (Kazumasa Fuwa), K.O., K.S. and K.F. (Kazumichi Fujioka); data curation: N.N. and I.M.; writing—original draft preparation: K.M., N.N. and I.M.; writing—review and editing: S.S., D.K., K.N., W.T., K.O., R.A., K.F. (Kazumasa Fuwa), K.S. and K.F. (Kazumichi Fujioka); visualization: K.M., N.N. and I.M.; supervision: I.M.; funding acquisition: N.N. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Nihon University Research Grant (2021), Nihon University School of Medicine Alumni Association’s 60th anniversary fund research grant (2020), the Grants-in-Aid for Young Scientists (grant number: 19K20194, 22K15908, 22K15446, and 22K17839) and Scientific Research (C) (grant numbers: 21K11582 and 23K07258) of JSPS KAKENHI, the Japanese Society for Pediatric Endocrinology Future Development Grant supported by Novo Nordisk Pharma Ltd., and Kawano Masanori Memorial Public Interest Incorporated Foundation for Promotion of Pediatrics (2022).
Institutional Review Board Statement
This study was carried out in accordance with the ARRIVE guidelines and was approved by the Nihon University Institutional Animal Care and Use Committee (protocol nos. AP20MED018-1 [5 June 2020]).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Tomoo Okada and Hajime Sasaki (Department of Nutrition and Life Sciences, Kanagawa Institute of Technology) for their technical support in conducting the experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barker, D.J. Obesity and early life. Obes. Rev. 2007, 8 (Suppl. 1), 45–49. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jin, Z.; Yang, Y.; Jiang, F.; Huang, H.; Liu, S.; Jin, X. Association of low birth weight with thinness and severe obesity in children aged 3-12 years: A large-scale population-based cross-sectional study in Shanghai, China. BMJ Open 2019, 9, e028738. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T. Common approach to childhood obesity in Japan. J. Pediatr. Endocrinol. Metab. 2014, 27, 581–592. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Toffolon, A.; de Rocco-Ponce, M.; Vettore, M.; Iori, E.; Lante, A.; Tessari, P. Effect of Reversal of Whey-Protein to Casein Ratio of Cow Milk, on Insulin, Incretin, and Amino Acid Responses in Humans. Mol. Nutr. Food Res. 2021, 65, e2100069. [Google Scholar] [CrossRef] [PubMed]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Marshall, K. Therapeutic applications of whey protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar]
- Nonogaki, K.; Kaji, T. Whey protein isolate inhibits hepatic FGF21 production, which precedes weight gain, hyperinsulinemia and hyperglycemia in mice fed a high-fat diet. Sci. Rep. 2020, 10, 15784. [Google Scholar] [CrossRef]
- Tulipano, G.; Sibilia, V.; Caroli, A.M.; Cocchi, D. Whey proteins as a source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 2011, 32, 835–838. [Google Scholar] [CrossRef]
- Kawase, M.; Hashimoto, H.; Hosoda, M.; Morita, H.; Hosono, A. Effect of administration of fermented milk containing whey protein concentrate to rats on serum lipids and blood pressure. J. Dairy Sci. 2000, 83, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Mazidi, M.; Sankaranarayanan, R.; Tajik, B.; McArdle, A.; Isanejad, M. Effects of whey and soy protein supplementation on inflammatory cytokines in older adults: A systematic review and meta-analysis. Br. J. Nutr. 2023, 129, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Veskoukis, A.S.; Kerasioti, E.; Skaperda, Z.; Papapostolou, P.A.; Nepka, C.; Spandidos, D.A.; Asprodini, E.; Taitzoglou, I.; Kouretas, D. Whey protein boosts the antioxidant profile of rats by enhancing the activities of crucial antioxidant enzymes in a tissue-specific manner. Food Chem. Toxicol. 2020, 142, 111508. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Maddux, B.A.; See, W.; Lawrence, J.C., Jr.; Goldfine, A.L.; Goldfine, I.D.; Evans, J.L. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by mircomolar concentrations of alpha-lipoic acid. Diabetes 2001, 50, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, T.; Kajimoto, Y.; Watada, H.; Kaneto, H.; Kishimoto, M.; Umayahara, Y.; Fujitani, Y.; Kamada, T.; Kawamori, R.; Yamasaki, Y. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J. Clin. Investig. 1997, 99, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, T.; Perez-Tilve, D.; Ogawa, D.; Gizard, F.; Zhao, Y.; Heywood, E.B.; Jones, K.L.; Kawamori, R.; Cassis, L.A.; Tschöp, M.H.; et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Investig. 2007, 117, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Nakazaki, K.; Nagano, N.; Katayama, D.; Shimizu, S.; Matsuda, K.; Tokunaga, W.; Aoki, R.; Fuwa, K.; Morioka, I. Body Fat-Reducing Effects of Whey. Nutrients 2023, 15, 2263. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedűs, C.; Kovács, D.; Peitl, B.; Gál, F.; Stündl, L.; Szilvássy, Z.; Remenyik, J. Effect of Anthocyanin-Rich Tart Cherry Extract on Inflammatory Mediators and Adipokines Involved in Type 2 Diabetes in a High Fat Diet Induced Obesity Mouse Model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.; Einwallner, E. Study of In Vivo Glucose Metabolism in High-fat Diet-fed Mice Using Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT). J. Vis. Exp. 2018, 131, 56672. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Krull, J.E.; Douglass, J.D.; Fasnacht, R.; Lara-Lince, F.; Meek, T.H.; Shi, X.; Damian, V.; Nguyen, H.T.; Matsen, M.E.; et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 2017, 8, 14556. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of fat-free mass using bioelectrical impedance measurements of the human. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Yamashita, S. Recent Advances in Analytical Methods on Lipoprotein Subclasses: Calculation of Particle Numbers from Lipid Levels by Gel Permeation HPLC Using “Spherical Particle Model”. J. Oleo Sci. 2016, 65, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Usui, S.; Hara, Y.; Hosaki, S.; Okazaki, M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J. Lipid Res. 2002, 43, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxid. Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Sasaki, H. Whey Protein Intake Modulates Lipid Metabolism by Transcriptionally Affecting PPARs and SREBP1c and Their Downstream Enzymes in Mice. Food Nutr. Sci. 2019, 10, 1045–1055. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Fujimori, T.; Sato, H.; Ishikawa, G.; Kami, K.; Ohashi, Y. Statistical hypothesis testing of factor loading in principal component analysis and its application to metabolite set enrichment analysis. BMC Bioinform. 2014, 15, 51. [Google Scholar] [CrossRef]
- Berger, J.; Leibowitz, M.D.; Doebber, T.W.; Elbrecht, A.; Zhang, B.; Zhou, G.; Biswas, C.; Cullinan, C.A.; Hayes, N.S.; Li, Y.; et al. Novel peroxisome proliferator-activated receptor (PPAR) gamma and PPARdelta ligands produce distinct biological effects. J. Biol. Chem. 1999, 274, 6718–6725. [Google Scholar] [CrossRef]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef]
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of obesity-induced inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Ota, T. Chemokine systems link obesity to insulin resistance. Diabetes Metab. J. 2013, 37, 165–172. [Google Scholar] [CrossRef]
- Althaher, A.R. An Overview of Hormone-Sensitive Lipase (HSL). ScientificWorldJournal 2022, 2022, 1964684. [Google Scholar] [CrossRef]
- Reid, B.N.; Ables, G.P.; Otlivanchik, O.A.; Schoiswohl, G.; Zechner, R.; Blaner, W.S.; Goldberg, I.J.; Schwabe, R.F.; Chua, S.C., Jr.; Huang, L.S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J. Biol. Chem. 2008, 283, 13087–13099. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Hosokawa, Y. Cysteine dioxygenase. Methods Enzymol. 1987, 143, 395–403. [Google Scholar] [CrossRef]
- Erbersdobler, H.F.; Trautwein, E.; Greulich, H.G. Determinations of taurine in milk and infant formula diets. Eur. J. Pediatr. 1984, 142, 133–134. [Google Scholar] [CrossRef]
- Chitapanarux, T.; Tienboon, P.; Pojchamarnwiputh, S.; Leelarungrayub, D. Open-labeled pilot study of cysteine-rich whey protein isolate supplementation for nonalcoholic steatohepatitis patients. J. Gastroenterol. Hepatol. 2009, 24, 1045–1050. [Google Scholar] [CrossRef]
- Tsuboyama-Kasaoka, N.; Shozawa, C.; Sano, K.; Kamei, Y.; Kasaoka, S.; Hosokawa, Y.; Ezaki, O. Taurine (2-aminoethanesulfonic acid) deficiency. Endocrinology 2006, 147, 3276–3284. [Google Scholar] [CrossRef]
- Murakami, S.; Fujita, M.; Nakamura, M.; Sakono, M.; Nishizono, S.; Sato, M.; Imaizumi, K.; Mori, M.; Fukuda, N. Taurine ameliorates cholesterol metabolism by stimulating bile acid production in high-cholesterol-fed rats. Clin. Exp. Pharmacol. Physiol. 2016, 43, 372–378. [Google Scholar] [CrossRef]
- Fukuda, N.; Yoshitama, A.; Sugita, S.; Fujita, M.; Murakami, S. Dietary taurine reduces hepatic secretion of cholesteryl ester and enhances fatty acid oxidation in rats fed a high-cholesterol diet. J. Nutr. Sci. Vitaminol. 2011, 57, 144–149. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Brandao, C.F.C.; Batitucci, G.; Souza, A.O.; Ferrari, G.D.; Alberici, L.C.; Muñoz, V.R.; Pauli, J.R.; De Moura, L.P.; Ropelle, E.R.; et al. Taurine supplementation associated with exercise increases mitochondrial activity and fatty acid oxidation gene expression in subcutaneous white adipose tissue of obese women. Clin. Nutr. 2021, 40, 2180–2187. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, A.; Ortiz-Balderas, E.; Cardozo-Saldaña, G.; Diaz-Diaz, E.; El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. 2014, 126, 19–29. [Google Scholar] [CrossRef]
- El-Hafidi, M.; Franco, M.; Ramírez, A.R.; Sosa, J.S.; Flores, J.A.P.; Acosta, O.L.; Salgado, M.C.; Cardoso-Saldaña, G. Glycine Increases Insulin Sensitivity and Glutathione Biosynthesis and Protects against Oxidative Stress in a Model of Sucrose-Induced Insulin Resistance. Oxidative Med. Cell. Longev. 2018, 2018, 2101562. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Tu-Sekine, B.; Kim, S.F. The Inositol Phosphate System-A Coordinator of Metabolic Adaptability. Int. J. Mol. Sci. 2022, 23, 6747. [Google Scholar] [CrossRef]
- Tang, X.; Xu, G.; Pan, Y.-X.; Chen, H. Epigenetic Regulations of Genes Related to Lipid Metabolism by MicroRNA in Mice Fed High Fat Diet. FASEB J. 2018, 32, 648.20. [Google Scholar] [CrossRef]
- Kuwabara, R.; Urakami, T.; Yoshida, K.; Morioka, I. Case of type 2 diabetes possibly caused by excessive accumulation of visceral fat in a child born small-for-gestational age. J. Diabetes Investig. 2020, 11, 1366–1369. [Google Scholar] [CrossRef]
- Matsubasa, T.; Uchino, T.; Karashima, S.; Kondo, Y.; Maruyama, K.; Tanimura, M.; Endo, F. Oxidative stress in very low birth weight infants as measured by urinary 8-OHdG. Free Radic. Res. 2002, 36, 189–193. [Google Scholar] [CrossRef]
- Kunz, C.; Lönnerdal, B. Re-evaluation of the whey protein/casein ratio of human milk. Acta Paediatr. 1992, 81, 107–112. [Google Scholar] [CrossRef]
- Sluik, D.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; Mikkilä, V.; Poppitt, S.D.; Silvestre, M.P.; Tremblay, A.; Pérusse, L.; Bouchard, C.; Raben, R.A.; et al. Protein intake and the incidence of pre-diabetes and diabetes in four population-based studies: The PREVIEW project. Am. J. Clin. Nutr. 2019, 109, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Pietrobelli, A.; Agosti, M. Nutrition in the First 1000 Days: Ten Practices to Minimize Obesity Emerging from Published Science. Int. J. Environ. Res. Public Health 2017, 14, 1491. [Google Scholar] [CrossRef]
- Katayama, D.; Nagano, N.; Shimizu, S.; Nakazaki, K.; Matsuda, K.; Tokunaga, W.; Fuwa, K.; Aoki, R.; Morioka, I. A Non-Obese Hyperglycemic Mouse Model that Develops after Birth with Low Birthweight. Biomedicines 2022, 10, 1642. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).