Investigating the Effectiveness of a Carb-Free Oloproteic Diet in Fibromyalgia Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples-Size Calculation
2.2. Participants
2.3. Clinical and Laboratory Evaluation
2.4. Dietary Intervention and Assessment
2.5. Sample Preparation for NMR Metabolomic Analysis
2.6. NMR Data Acquisition
2.7. NMR Data Processing
2.8. Statistical Analysis
3. Results
3.1. Clinical Analysis
3.2. Multivariate Data Analysis and Enrichment Analysis
4. Discussion
- i.
- Rebalance of amino acid metabolism involved in neurotransmission: The metabolome of FM1 patients at t45 shows decreased serum phenylalanine concentration and increased urinary isoleucine excretion. In keeping with these data, enrichment analysis revealed alteration of biochemical pathways responsible for synthesizing those amino acids that also have roles as neurotransmitters, specifically (i) alanine, aspartate, and glutamate, (ii) D-glutamine and D-glutamate, (iii) phenylalanine and tyrosine, and (iv) taurine and hypotaurine (Table 3). Exploration of the scientific literature in search of a correlation with fibromyalgia through which to interpret these data rapidly revealed studies supporting the association of neuropathic and muscular pain with the dysregulation of amino acid metabolism [2,54].
- ii.
- Rebalancing of inflammatory conditions. FM1 patients at t45 showed a significant decrease in levels of glucuronic acid (Table 3). This is known to be a ligand of toll-like 4 receptors that exacerbate inflammatory conditions and increase pain severity [33]. Accordingly, the oloproteic diet, in FM1 patients, seems to foster a rebalancing of inflammatory conditions that contribute to FM’s etiology. Indeed, previous scientific evidence showed elevated systemic levels of pro-inflammatory cytokines like IL-6 and IL-8 in FM patients compared to healthy individuals [34].
- iii.
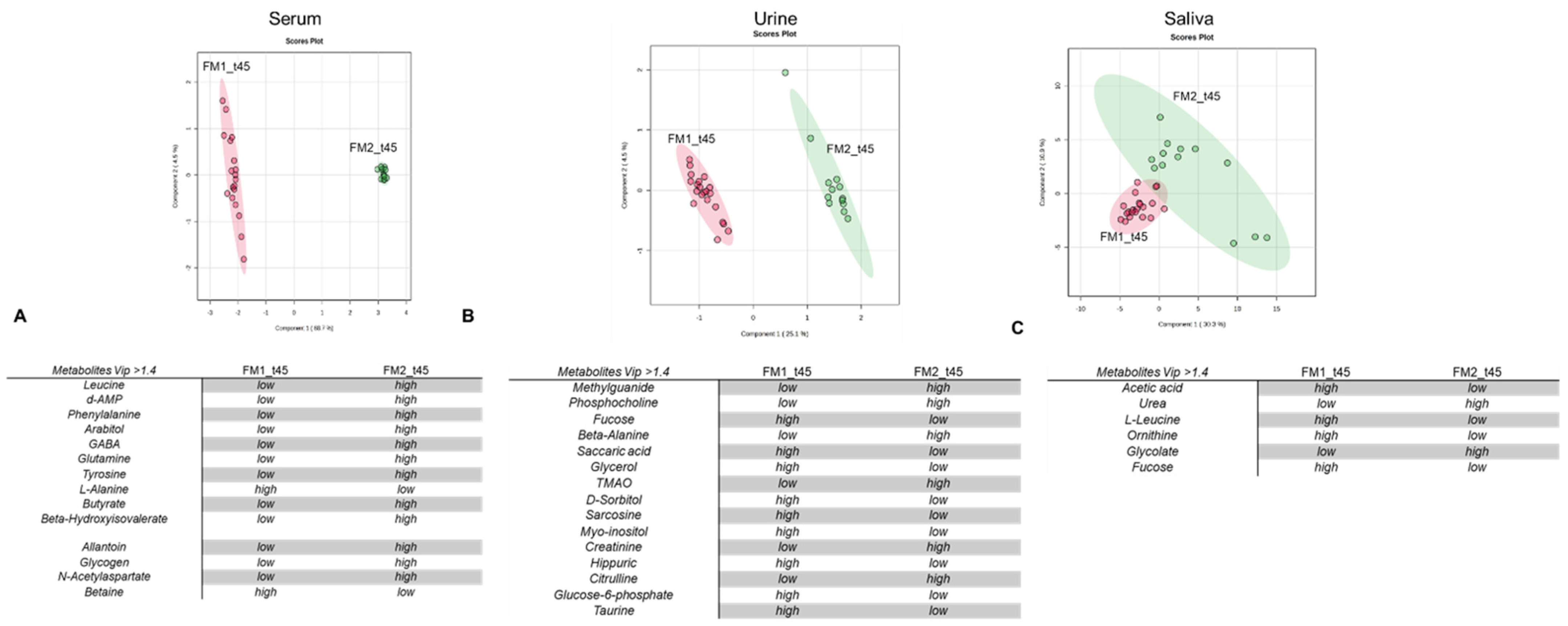
- Based on specific tissue-organelle enrichment, the dysregulation of ketogenic amino acids and energy metabolites has important repercussions at the intestinal level. A significant proportion of our FM patients, exceeding 50%, experience intestinal dysbiosis [55,56], manifesting as symptoms such as dysentery or constipation, recurrent cystitis, and vaginal discharge (Table S2). Confirming the effect of the oloproteic diet on alleviating inflammatory conditions in the gut, treatment with the oloproteic diet leads to notable improvements in these symptoms, with reductions of 59.1% in cystitis and 50.01% in vaginal discharge. Improvement in these symptoms was accompanied by a significant decrease in dysbiosis biomarkers in urine (including hydroxyvalerate, valerate, citrulline, and TMAO) [57,58,59] and saliva (glycolate and urea) [60,61] (Figure 2B,C, Tables S2 and S3).
- iv.
- Increase in dopaminergic transmission: Metabolomic data show that the metabolomic profile of FM1 patients at t45 is characterized by significantly decreased levels of tyrosine and phenylalanine. As tyrosine and phenylalanine are catecholamine precursors, their diminished levels are consistent with increased production of catecholamines [62] and, thus, increased catecholaminergic transmission. Previous scientific inquiries have revealed a link between abnormal pain perception in FM patients and the down-regulation of catecholamine transmission.
- v.
- Modulation of GABAergic transmission: VIP score analysis indicates that the serum metabolome of FM1 patients at t45 exhibits significantly decreased GABA and increased guanidinoacetate (GAA) concentrations. It is well known that fibromyalgia is associated with an alteration of GABAergic neurotransmission [53,63]. Indeed, several pharmacological therapeutic interventions make use of GABA inhibitors [64].
- vi.
- Availability of additional energy sources to cope with neuromuscular stress conditions: Our data show that blood sera of FM1 patients at t45 report an increase in N-acetyl aspartate. According to tissue-specific organelle enrichment, an increase in N-acetyl aspartate is consistent with neuromuscular-tissue dysmetabolism. Specifically, N-acetyl aspartate serves as a reservoir for glutamate [52,63] and acts as an energy source for cells during periods of stress when glucose, their primary fuel, is limited.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Tharwat, S.; Mosad, N.R.; Abdelmessih, K.E.; Moatamed, E.; Rihan, M.; Osama, N.; Sallam, N.; Elsayed, Y. Prevalence of fibromyalgia among university students and its impact on their health-related quality of life: A survey-based study from Egypt. BMC Public Health 2023, 23, 2437. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia: An overview. Am. J. Med. 2009, 122, S3–S13. [Google Scholar] [CrossRef]
- Wallace, D.J.; Hallegua, D.S. Fibromyalgia: The gastrointestinal link. Curr. Pain Headache Rep. 2004, 8, 364–368. [Google Scholar] [CrossRef]
- Bhargava, J.; Hurley, J.A. Fibromyalgia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Funovits, J.; Aletaha, D.; Bykerk, V.; Combe, B.; Dougados, M.; Emery, P.; Felson, D.; Hawker, G.; Hazes, J.M.; Huizinga, T.; et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: Methodological report phase I. Ann. Rheum. Dis. 2010, 69, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E.; Frits, M.L.; Iannaccone, C.K.; Weinblatt, M.E.; Shadick, N.A.; Liao, K.P. Clinical characteristics of RA patients with secondary SS and association with joint damage. Rheumatology 2015, 54, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Sánchez, C.M.; Reyes Del Paso, G.A. Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. J. Clin. Med. 2020, 9, 1219. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.C.; Bannwarth, B.; Failde, I.; Abello Carbonell, J.; Blotman, F.; Spaeth, M.; Saraiva, F.; Nacci, F.; Thomas, E.; Caubère, J.P.; et al. Prevalence of fibromyalgia: A survey in five European countries. Semin. Arthritis Rheum. 2010, 39, 448–453. [Google Scholar] [CrossRef]
- Fitzcharles, M.A.; Perrot, S.; Häuser, W. Comorbid fibromyalgia: A qualitative review of prevalence and importance. Eur. J. Pain 2018, 22, 1565–1576. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of diet, lifestyle, chrononutrition and alternative dietary interventions on postprandial glycemia and insulin resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Dąbek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. Implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients 2020, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: Systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Investig. 2019, 42, 1365–1386. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Megna, M.; Cacciapuoti, S.; Frias-Toral, E.; Fabbrocini, G.; Savastano, S.; Colao, A.; Muscogiuri, G. Very low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: An update for dermatologists and nutritionists. Crit. Rev. Food Sci. Nutr. 2022, 62, 398–414. [Google Scholar] [CrossRef]
- Lorenzo, P.M.; Sajoux, I.; Izquierdo, A.G.; Gomez-Arbelaez, D.; Zulet, M.A.; Abete, I.; Castro, A.I.; Baltar, J.; Portillo, M.P.; Tinahones, F.J.; et al. Immunomodulatory effect of a very-low-calorie ketogenic diet compared with bariatric surgery and a low-calorie diet in patients with excessive body weight. Clin. Nutr. 2022, 41, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; Toniolo, L.; Canato, M.; Bianco, A.; Fratter, A. Nutrition and acne: Therapeutic potential of ketogenic diets. Skin Pharmacol. Physiol. 2012, 25, 111–117. [Google Scholar] [CrossRef]
- Wells, J.; Swaminathan, A.; Paseka, J.; Hanson, C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy—A review. Nutrients 2020, 12, 1809. [Google Scholar] [CrossRef]
- Klement, R.J.; Brehm, N.; Sweeney, R.A. Ketogenic diets in medical oncology: A systematic review with focus on clinical outcomes. Med. Oncol. 2020, 37, 14. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, G.; Pagano, I.; Grimaldi, M.; Marino, C.; Molettieri, P.; Santoro, A.; Stillitano, I.; Romano, R.; Montoro, P.; D’Ursi, A.M.; et al. Effect of Very-Low-Calorie Ketogenic Diet on Psoriasis Patients: A Nuclear Magnetic Resonance-Based Metabolomic Study. J. Proteome Res. 2021, 20, 1509–1521. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Castaldo, G.; Monaco, L.; Castaldo, L.; Galdo, G.; Cereda, E. An observational study of sequential protein-sparing, very low-calorie ketogenic diet (Oloproteic diet) and hypocaloric Mediterranean-like diet for the treatment of obesity. Int. J. Food Sci. Nutr. 2016, 67, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Pincus, T. The American College of Rheumatology (ACR) Core Data Set and derivative “patient only” indices to assess rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23, S109. [Google Scholar]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef]
- Lanzola, E.; Santini, P. Body temperature and weight. Quad. Nutr. 1959, 19, 157–164. [Google Scholar]
- Marino, C.; Grimaldi, M.; Sabatini, P.; Amato, P.; Pallavicino, A.; Ricciardelli, C.; D’Ursi, A.M. Fibromyalgia and depression in women: An 1h-nmr metabolomic study. Metabolites 2021, 11, 429. [Google Scholar] [CrossRef]
- Bakhach, M.; Shah, V.; Harwood, T.; Lappe, S.; Bhesania, N.; Mansoor, S.; Alkhouri, N. The Protein-Sparing Modified Fast Diet: An Effective and Safe Approach to Induce Rapid Weight Loss in Severely Obese Adolescents. Glob. Pediatr. Health 2016, 3, 2333794x15623245. [Google Scholar] [CrossRef]
- Ni, C.; Jia, Q.; Ding, G.; Wu, X.; Yang, M. Low-Glycemic Index Diets as an Intervention in Metabolic Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 307. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2007, 2, 2692. [Google Scholar] [CrossRef] [PubMed]
- Palisi, A.; Grimaldi, M.; Sabatini, P.; Montoro, P.; Scrima, M.; Rodriquez, M.; D’Ursi, A.M. A serum nuclear magnetic resonance-based metabolomic signature of antiphospholipid syndrome. J. Pharm. Biomed. Anal. 2017, 133, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Bernini, P.; Bertini, I.; Luchinat, C.; Nincheri, P.; Staderini, S.; Turano, P. Standard operating procedures for pre-analytical handling of blood and urine for metabolomic studies and biobanks. J. Biomol. NMR 2011, 49, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Mckay, R.T. How the 1D-NOESY suppresses solvent signal in metabonomics NMR spectroscopy: An examination of the pulse sequence components and evolution. Concepts Magn. Reson. Part A 2011, 38, 197–220. [Google Scholar] [CrossRef]
- Marino, C.; Grimaldi, M.; Sommella, E.M.; Ciaglia, T.; Santoro, A.; Buonocore, M.; Salviati, E.; Trojsi, F.; Polverino, A.; Sorrentino, P.; et al. The Metabolomic Profile in Amyotrophic Lateral Sclerosis Changes According to the Progression of the Disease: An Exploratory Study. Metabolites 2022, 12, 837. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Marino, C.; Pagano, I.; Castaldo, G.; Grimaldi, M.; D’Elia, M.; Santoro, A.; Conte, A.; Molettieri, P.; Parisella, C.; Buonocore, M. Supplementing Low-Sodium Bicarbonate–Calcic (Lete)® Water: Effects in Women on Bone and Systemic Metabolism. Metabolites 2023, 13, 1109. [Google Scholar] [CrossRef]
- Lefort, G.; Liaubet, L.; Canlet, C.; Tardivel, P.; Père, M.-C.; Quesnel, H.; Paris, A.; Iannuccelli, N.; Vialaneix, N.; Servien, R. ASICS: An R package for a whole analysis workflow of 1D 1H NMR spectra. Bioinformatics 2019, 35, 4356–4363. [Google Scholar] [CrossRef]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef]
- Kim, T.K. T test as a parametric statistic. Kor. J. Anesthesiol. 2015, 68, 540–546. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef]
- Grimaldi, M.; Palisi, A.; Marino, C.; Montoro, P.; Capasso, A.; Novi, S.; Tecce, M.F.; D’Ursi, A.M. NMR-based metabolomic profile of hypercholesterolemic human sera: Relationship with in vitro gene expression? PLoS ONE 2020, 15, e0231506. [Google Scholar] [CrossRef]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. “Circlize” implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Shapiro, J.R.; Anderson, D.A.; Danoff-Burg, S. A pilot study of the effects of behavioral weight loss treatment on fibromyalgia symptoms. J. Psychosom. Res. 2005, 59, 275–282. [Google Scholar] [CrossRef]
- Senna, M.K.; Sallam, R.A.; Ashour, H.S.; Elarman, M. Effect of weight reduction on the quality of life in obese patients with fibromyalgia syndrome: A randomized controlled trial. Clin. Rheumatol. 2012, 31, 1591–1597. [Google Scholar] [CrossRef]
- Donaldson, M.S.; Speight, N.; Loomis, S. Fibromyalgia syndrome improved using a mostly raw vegetarian diet: An observational study. BMC Complement. Altern. Med. 2001, 1, 7. [Google Scholar] [CrossRef]
- Kaartinen, K.; Lammi, K.; Hypen, M.; Nenonen, M.; Hanninen, O.; Rauma, A.L. Vegan diet alleviates fibromyalgia symptoms. Scand. J. Rheumatol. 2000, 29, 308–313. [Google Scholar] [CrossRef]
- Marum, A.P.; Moreira, C.; Saraiva, F.; Tomas-Carus, P.; Sousa-Guerreiro, C. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand. J. Pain 2016, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Cordero, M.D.; de Miguel, M.; Carmona-López, I.; Bonal, P.; Campa, F.; Moreno-Fernández, A.M. Oxidative stress and mitochondrial dysfunction in fibromyalgia. Neuro Endocrinol. Lett. 2010, 31, 169–173. [Google Scholar] [PubMed]
- Neu, A.; Neuhoff, H.; Trube, G.; Fehr, S.; Ullrich, K.; Roeper, J.; Isbrandt, D. Activation of GABA(A) receptors by guanidinoacetate: A novel pathophysiological mechanism. Neurobiol. Dis. 2002, 11, 298–307. [Google Scholar] [CrossRef]
- Mathar, D.; Erfanian Abdoust, M.; Marrenbach, T.; Tuzsus, D.; Peters, J. The catecholamine precursor Tyrosine reduces autonomic arousal and decreases decision thresholds in reinforcement learning and temporal discounting. PLOS Comput. Biol. 2022, 18, e1010785. [Google Scholar] [CrossRef]
- Woolfson, A.M. Amino acids—Their role as an energy source. Proc. Nutr. Soc. 1983, 42, 489–495. [Google Scholar] [CrossRef]
- Bains, A.; Kohrman, S.; Punko, D.; Fricchione, G. A Link Between Inflammatory Mechanisms and Fibromyalgia. Adv. Exp. Med. Biol. 2023, 1411, 357–378. [Google Scholar] [CrossRef]
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. eBioMedicine 2019, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Minerbi, A.; Gonzalez, E.; Brereton, N.J.; Anjarkouchian, A.; Dewar, K.; Fitzcharles, M.-A.; Chevalier, S.; Shir, Y. Altered microbiome composition in individuals with fibromyalgia. Pain 2019, 160, 2589–2602. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S; discussion 1548S. [Google Scholar] [CrossRef]
- Goudet, C.; Magnaghi, V.; Landry, M.; Nagy, F.; Gereau, R.W.t.; Pin, J.P. Metabotropic receptors for glutamate and GABA in pain. Brain Res. Rev. 2009, 60, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Sekito, T.; Wada, K.; Ishii, A.; Iwata, T.; Matsubara, T.; Tomida, S.; Watanabe, M.; Araki, M.; Sadahira, T. Etiology of recurrent cystitis in postmenopausal women based on vaginal microbiota and the role of Lactobacillus vaginal suppository. Front. Microbiol. 2023, 14, 1187479. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Kuo, Y.L.; Tsai, M.H.; Chiu, C.C.; Lin, G. Gut microbial-derived butyrate is inversely associated with IgE responses to allergens in childhood asthma. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2019, 30, 689–697. [Google Scholar] [CrossRef]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Harnett, J.E. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: A systematic review. BMC Musculoskelet Disord. 2020, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Thurm, T.; Ablin, J.N.; Buskila, D.; Maharshak, N. Fecal microbiota transplantation for fibromyalgia: A case report and review of the literature. Open J. Gastroenterol. 2017, 7, 131–139. [Google Scholar] [CrossRef]



| Parameters | Fibromyalgia First Group (N = 22) | Fibromyalgia Second Group (N = 19) |
|---|---|---|
| Sex (male/female) | 0/22 | 0/19 |
| Age (mean ± SD, year) | 42.66 ± 8 | 40.22 ± 3 |
| BMI (kg/m2) | 31.82 ± 2.66 | 27.36 ± 7.12 |
| Weight | 78.00 ± 2.02 | 76.55 ± 1.52 |
| Smokers (YES/NO) | 5/17 | 3/16 |
| f Value | p Value | FDR | Fisher’s LSD | |
|---|---|---|---|---|
| WPI | 30.555 | 2.33 × 10−9 | 9.31 × 10−9 | FM1_t0–FM1_t45; FM1_t0–FM2_t45; FM2_t0–FM1_t45; FM2_t45–FM1_t45; FM2_t0–FM2_t45 |
| SSS | 23.568 | 4.27 × 10−7 | 7.44 × 10−7 | FM1_t0–FM1_t45; FM1_t0–FM2_t45; FM2_t0–FM1_t45; FM2_t45–FM1_t45; FM2_t0–FM2_t46 |
| HAM-A | 23.231 | 5.58 × 10−7 | 7.44 × 10−7 | FM1_t0–FM1_t45; FM1_t0–FM2_t45; FM2_t0–FM1_t45; FM2_t45–FM1_t45; FM2_t0–FM2_t47 |
| HAM-D | 13.414 | 3.33 × 10−3 | 3.33 × 10−3 | FM1_t0–FM1_t45; FM1_t0–FM2_t45; FM2_t0–FM1_t45; FM2_t45–FM1_t45; FM2_t0–FM2_t48 |
| Serum | FM1 | FM2 | ||
|---|---|---|---|---|
| Metabolites VIP > 1.7 | t0 | t45 | t0 | t45 |
| guanidoacetate | low | high | / | / |
| ATP | high | low | / | / |
| N-acetylaspartate | high | low | / | / |
| d-glucoronic acid | high | low | / | / |
| ascorbic acid | low | high | / | / |
| ornithine | low | high | / | / |
| saccaric acid | low | high | / | / |
| phenylalanine | high | low | / | / |
| malic acid | low | high | / | / |
| β-hydroxyisovalerate | low | high | / | / |
| glycogen | high | low | / | / |
| l-leucine | low | high | low | high |
| acetoacetate | / | / | low | high |
| glutamine | / | / | low | high |
| alanine | / | / | high | low |
| valine | / | / | high | low |
| Urine | FM1 | FM2 | ||
| Metabolites VIP > 1.7 | t0 | t45 | t0 | t45 |
| valerate | high | low | / | / |
| β-hydroxyisovalerate | high | low | / | / |
| isoleucine | high | low | / | / |
| serine | high | low | ||
| fucose | low | high | / | / |
| citrulline | high | low | / | / |
| TMAO | low | high | / | / |
| malate | / | / | high | low |
| succinate | / | / | low | high |
| mannose | / | / | low | high |
| glucose | / | / | low | high |
| 7-methylxanthine | / | / | high | low |
| citrate | / | / | high | low |
| Saliva | FM1 | FM2 | ||
| Metabolites Vip > 1.7 | t0 | t45 | t0 | t45 |
| d-galactose | low | high | / | / |
| glycolate | high | low | / | / |
| acetoacetate | high | low | / | / |
| valine | high | low | / | / |
| sorbitol | low | high | / | / |
| d-glucoronic | low | high | high | low |
| ornithine | / | / | high | low |
| Serum FM1 t0_t45 | Hits | Raw p | Holm Adjust | Impact |
|---|---|---|---|---|
| alanine. aspartate and glutamate metabolism | 11 | 6.68 × 10−6 | 3.21 × 10−5 | 0.78 |
| Arginine biosynthesis | 8 | 8.36 × 10−5 | 3.57 × 10−3 | 0.6 |
| D-glutamine and D-glutamate metabolism | 3 | 8.53 × 10−4 | 2.39 × 10−2 | 0.5 |
| Phenyalanine and tyrosine metabolism | 3 | 1.39 × 10−3 | 3.20 × 10−2 | 1 |
| taurine and hypotaurine metabolism | 3 | 1.31 × 10−2 | 2.24 × 10−2 | 0.65 |
| TCA | 4 | 1.22 × 10−2 | 2.20 × 10−2 | 0.47 |
| Synthesis and degradation of ketone bodies | 4 | 1.99 × 10−2 | 1.08 × 10−2 | 0.60 |
| Urine FM1 t0_t45 | Hits | Raw p | Holm adjust | Impact |
| Aminoacyl-tRNA biosynthesis | 14 | 3.90 × 10−18 | 1.8790 × 10−17 | 0.52 |
| valine. leucine and isoleucine biosynthesis | 5 | 1.4290 × 10−14 | 6.5290 × 10−13 | 0.75 |
| Arginine biosynthesis | 6 | 1.0390 × 10−10 | 4.1190 × 10−9 | 0.56 |
| alanine. aspartate and glutamate metabolism | 6 | 1.73 × 10−4 | 4.1590 × 10−3 | 0.53 |
| taurine and hypotaurine metabolism | 3 | 1.05 × 10−3 | 0.0285 | 0.71 |
| Saliva FM1 t0_t45 | Hits | Raw p | Holm adjust | Impact |
| alanine. aspartate and glutamate metabolism | 6 | 3.67 × 10−3 | 1.3690 × 10−1 | 0.45 |
| glycine. serine and threonine metabolism | 9 | 5.73 × 10−4 | 0.0143 | 0.68 |
| Serum FM2 t0_t45 | Hits | Raw p | Holm adjust | Impact |
| Synthesis and degradation of ketone bodies | 4 | 9.3490 × 10−11 | 0.00224 | 0.6 |
| TCA | 4 | 3.2290 × 10−10 | 2.0190 × 10−11 | 0.49 |
| beta-alanine metabolism | 3 | 0.005 | 0.00454 | 0.45 |
| Urine FM2 t0_t45 | Hits | Raw p | Holm adjust | Impact |
| Synthesis and degradation of ketone bodies | 4 | 3.04 × 10−4 | 1.25 × 10−2 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldo, G.; Marino, C.; Atteno, M.; D’Elia, M.; Pagano, I.; Grimaldi, M.; Conte, A.; Molettieri, P.; Santoro, A.; Napolitano, E.; et al. Investigating the Effectiveness of a Carb-Free Oloproteic Diet in Fibromyalgia Treatment. Nutrients 2024, 16, 1620. https://doi.org/10.3390/nu16111620
Castaldo G, Marino C, Atteno M, D’Elia M, Pagano I, Grimaldi M, Conte A, Molettieri P, Santoro A, Napolitano E, et al. Investigating the Effectiveness of a Carb-Free Oloproteic Diet in Fibromyalgia Treatment. Nutrients. 2024; 16(11):1620. https://doi.org/10.3390/nu16111620
Chicago/Turabian StyleCastaldo, Giuseppe, Carmen Marino, Mariangela Atteno, Maria D’Elia, Imma Pagano, Manuela Grimaldi, Aurelio Conte, Paola Molettieri, Angelo Santoro, Enza Napolitano, and et al. 2024. "Investigating the Effectiveness of a Carb-Free Oloproteic Diet in Fibromyalgia Treatment" Nutrients 16, no. 11: 1620. https://doi.org/10.3390/nu16111620
APA StyleCastaldo, G., Marino, C., Atteno, M., D’Elia, M., Pagano, I., Grimaldi, M., Conte, A., Molettieri, P., Santoro, A., Napolitano, E., Puca, I., Raimondo, M., Parisella, C., D’Ursi, A. M., & Rastrelli, L. (2024). Investigating the Effectiveness of a Carb-Free Oloproteic Diet in Fibromyalgia Treatment. Nutrients, 16(11), 1620. https://doi.org/10.3390/nu16111620











