Diet-Wide Association Study for the Incidence of Type 2 Diabetes Mellitus in Community-Dwelling Adults Using the UK Biobank Data
Abstract
:1. Introduction
2. Method
2.1. Ascertainment of T2DM
2.2. Dietary and Nutrient Intake
2.3. Covariates
2.4. Statistical Analysis
2.5. DWAS
3. Results
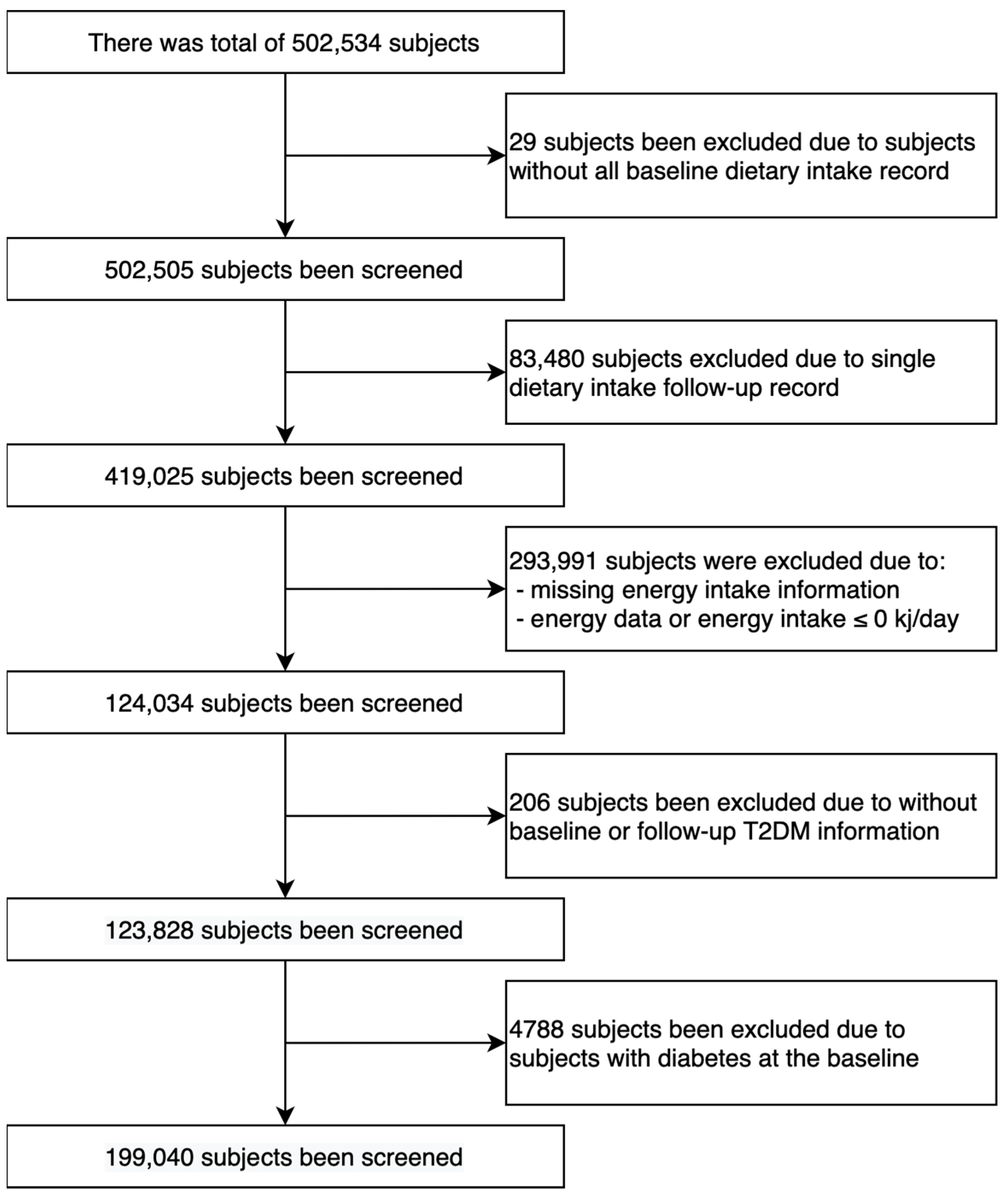
3.1. Population Selection
3.2. Incidence of T2DM
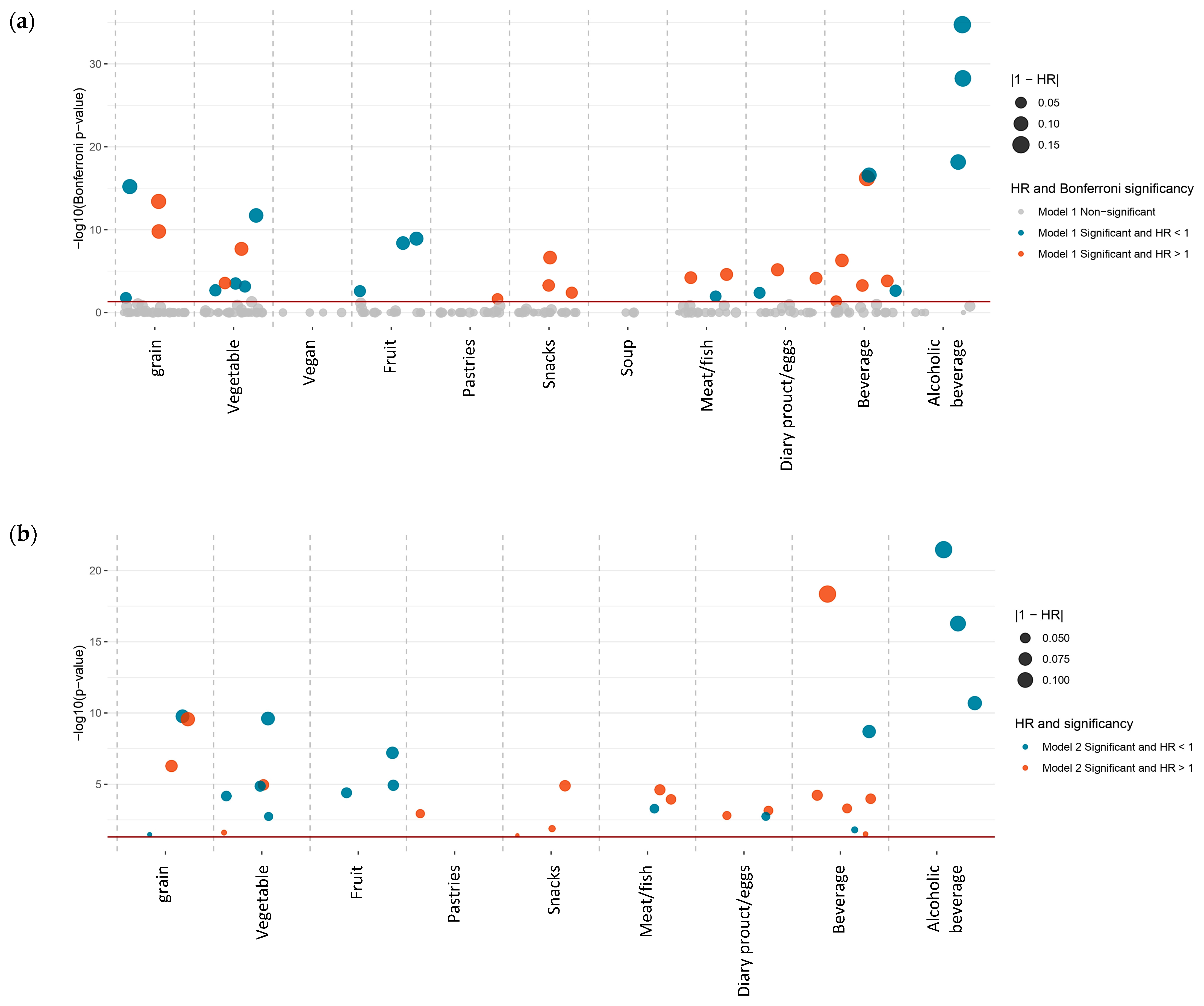
3.3. DWAS
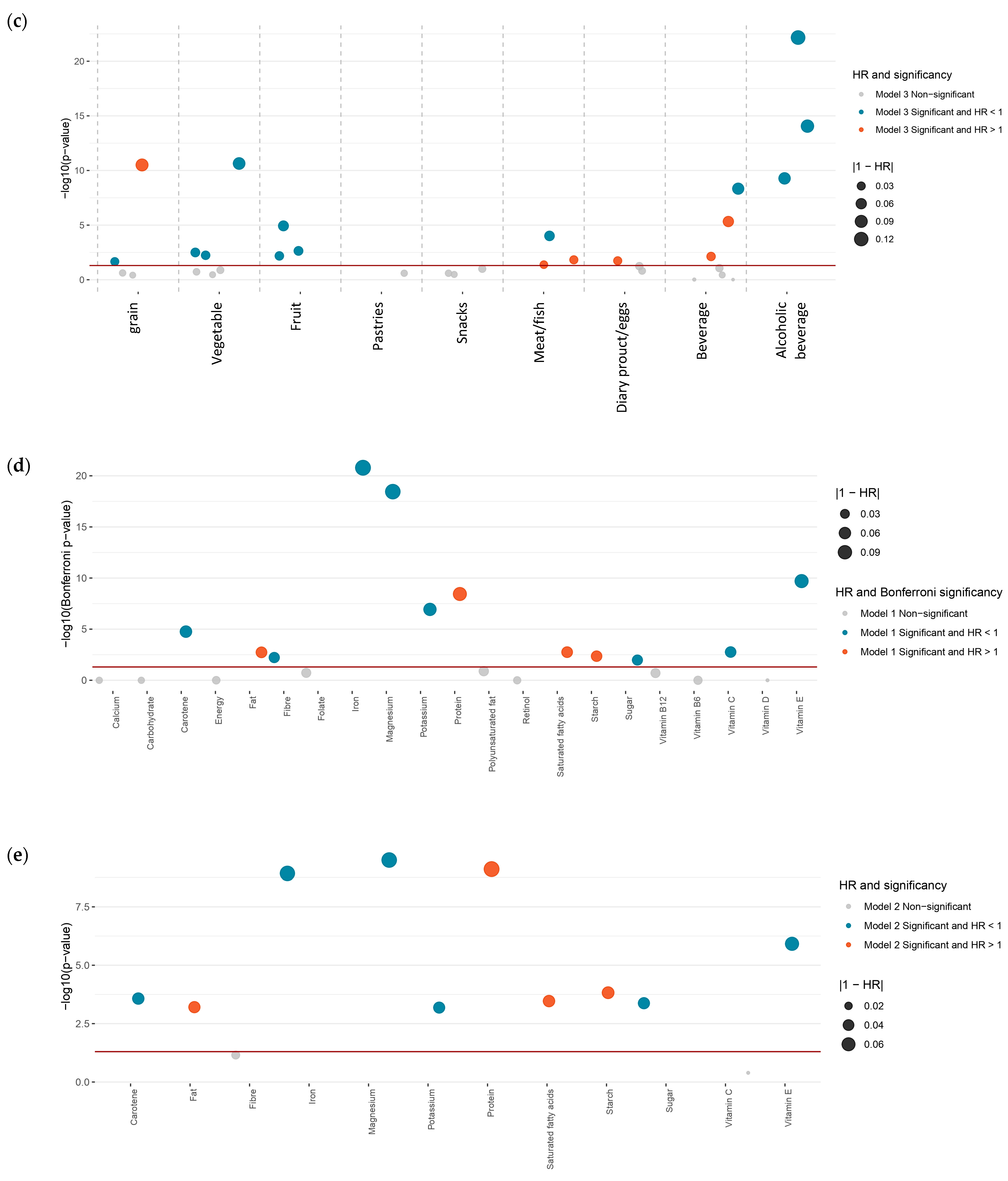
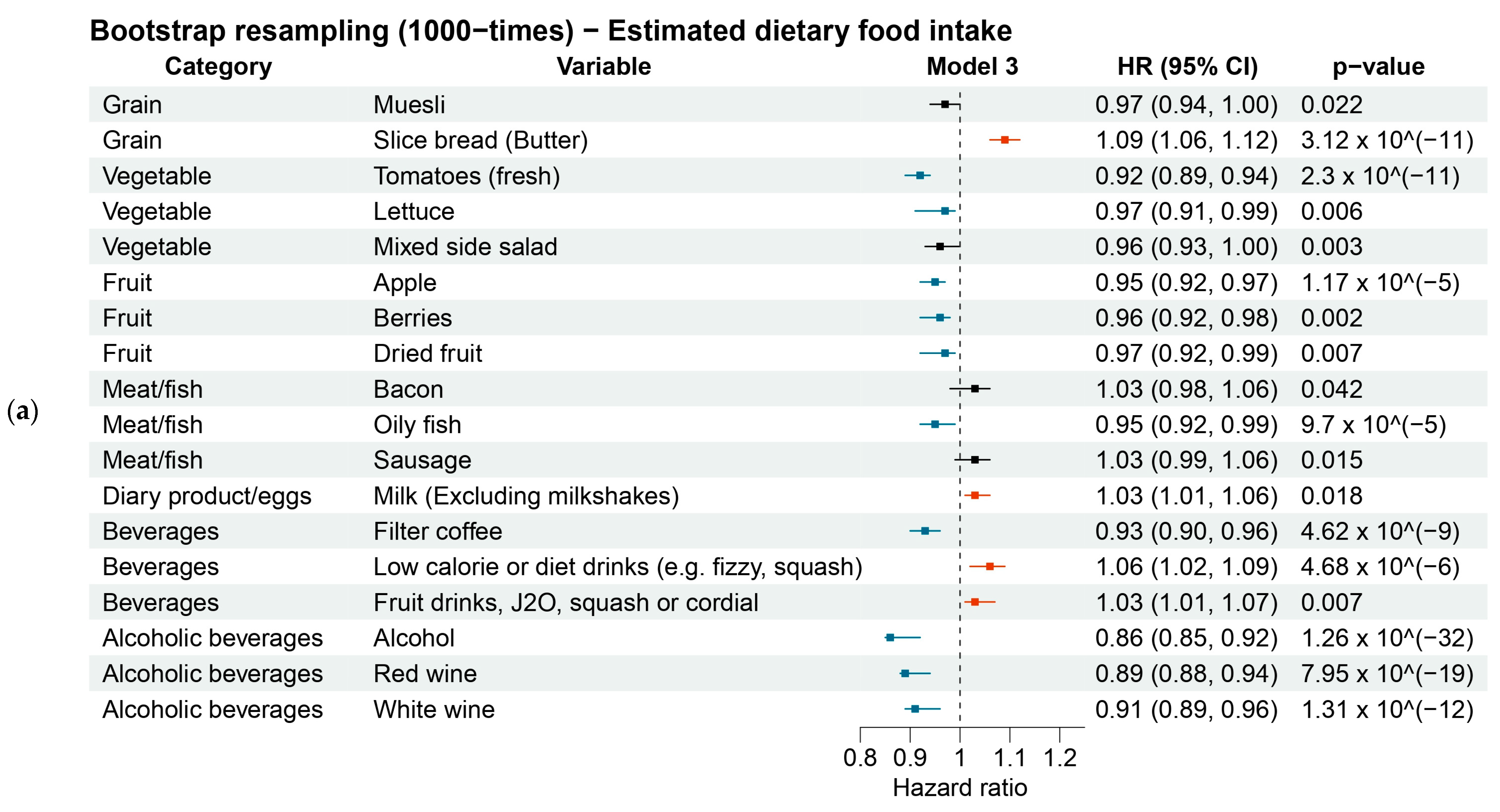
3.3.1. Estimated Dietary Foods
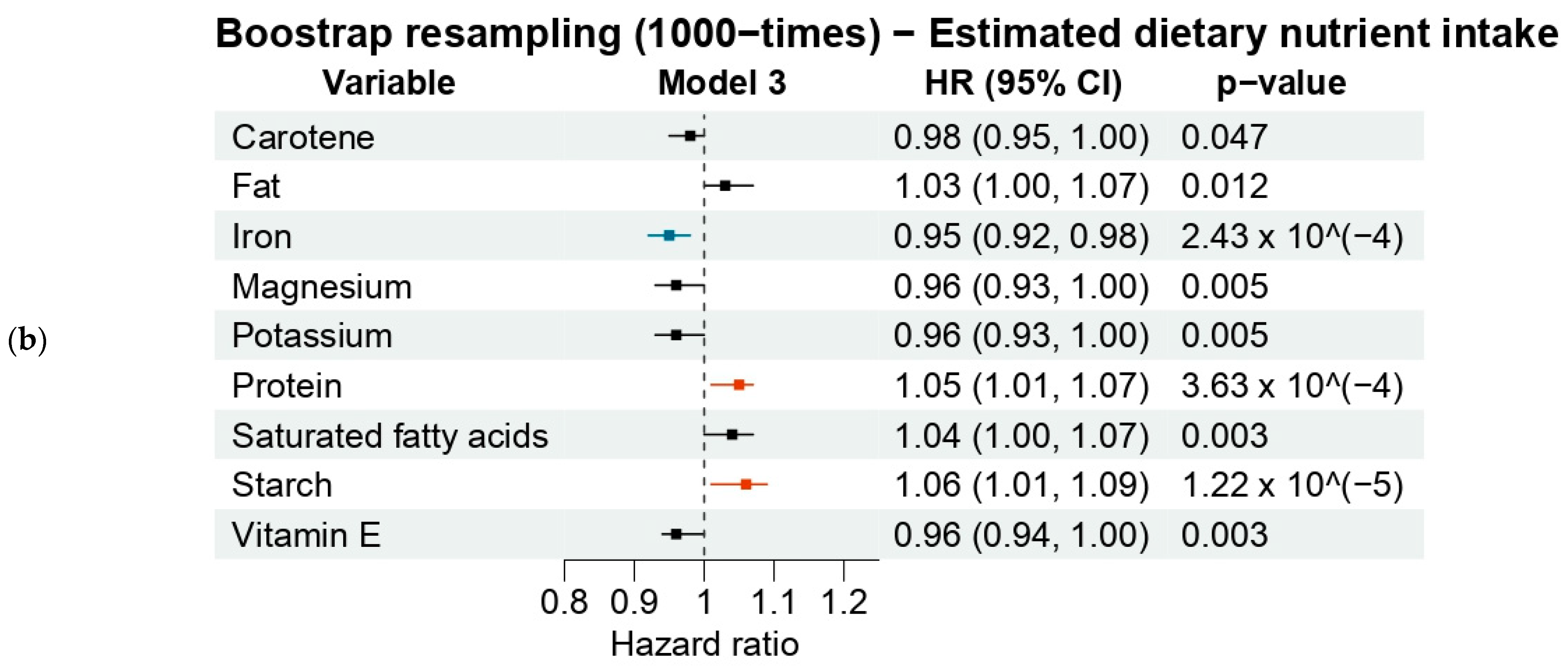
3.3.2. Estimated Dietary Nutrients
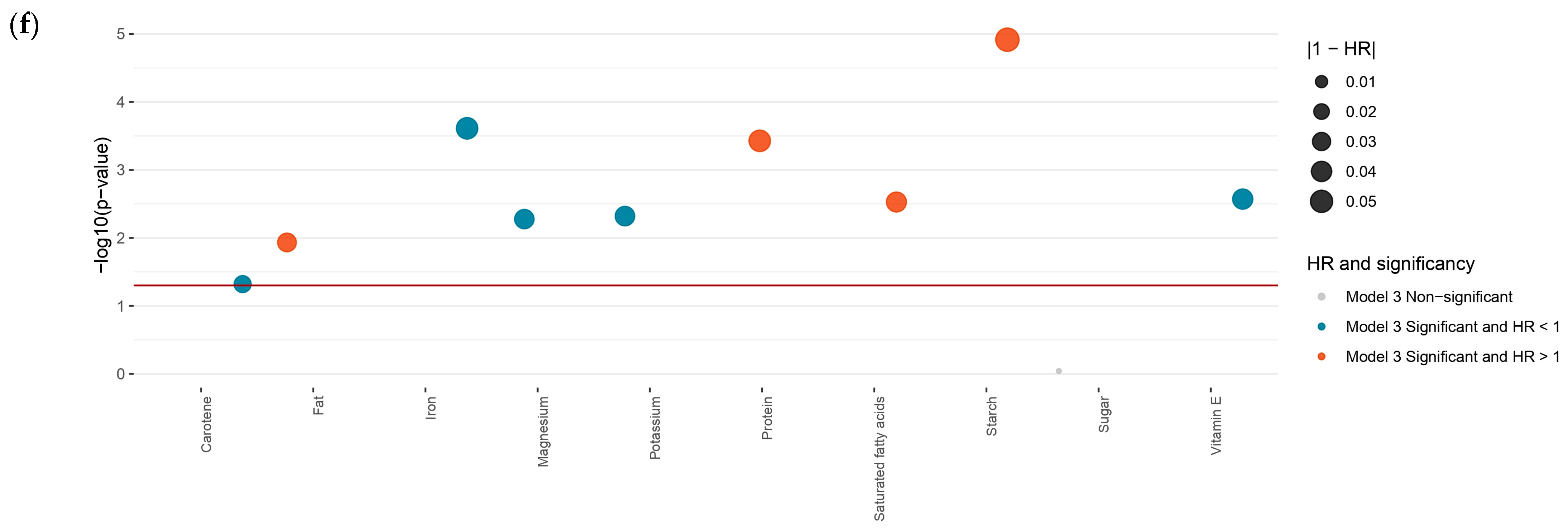
3.4. Sensitivity Analysis
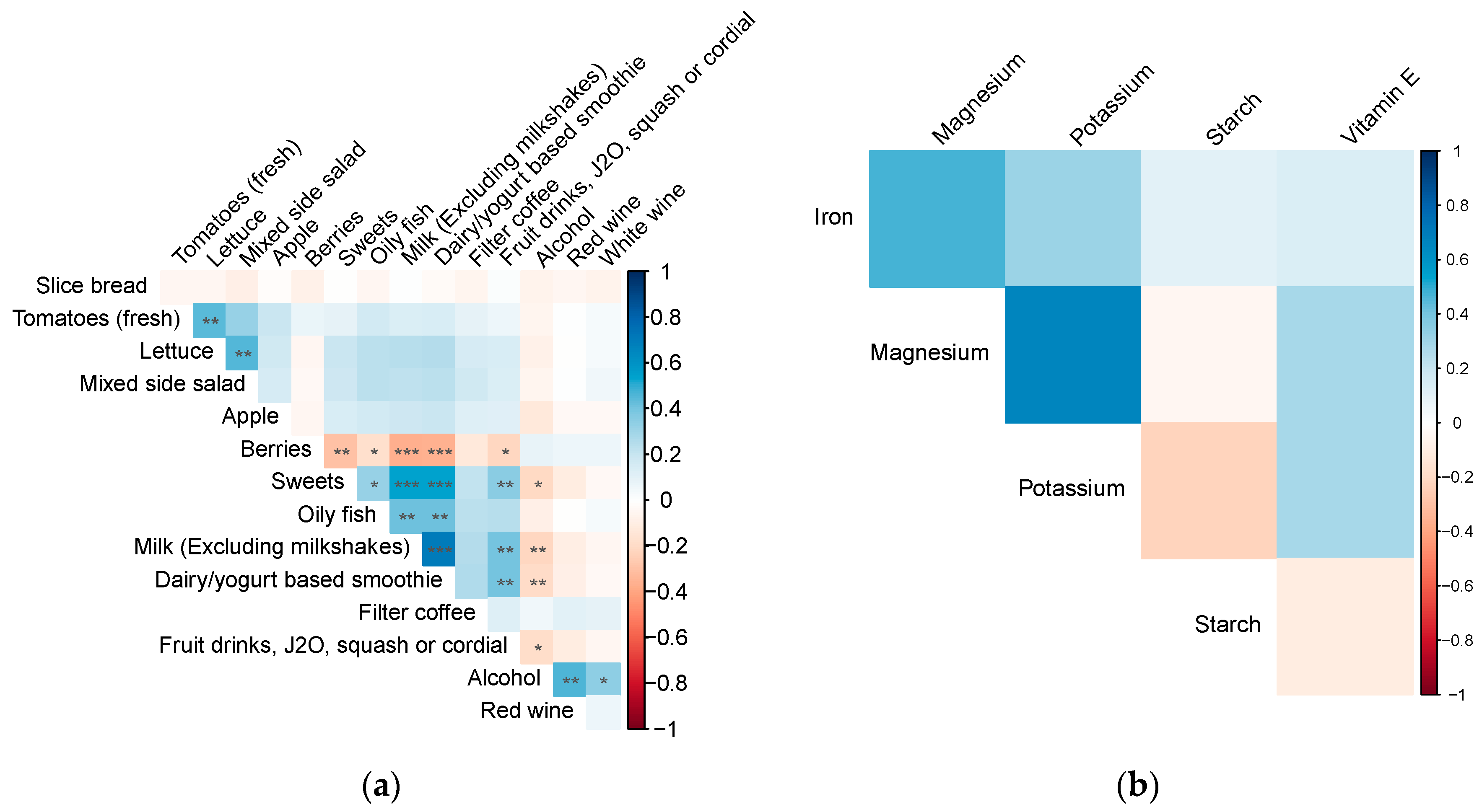
3.5. Dietary Factors Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Noncommunicable Dis. 2016, 1, 3. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Barbaresko, J.; Neuenschwander, M.; Schwingshackl, L.; Schlesinger, S. Dietary factors and diabetes-related health outcomes in patients with type 2 diabetes: Protocol for a systematic review and meta-analysis of prospective observational studies. BMJ Open 2019, 9, e027298. [Google Scholar] [CrossRef]
- Golay, A.; Ybarra, J. Link between obesity and type 2 diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 649–663. [Google Scholar] [CrossRef]
- Brayner, B.; Kaur, G.; Keske, M.A.; Perez-Cornago, A.; Piernas, C.; Livingstone, K.M. Dietary Patterns Characterized by Fat Type in Association with Obesity and Type 2 Diabetes: A Longitudinal Study of UK Biobank Participants. J. Nutr. 2021, 151, 3570–3578. [Google Scholar] [CrossRef]
- Moriconi, E.; Camajani, E.; Fabbri, A.; Lenzi, A.; Caprio, M. Very-Low-Calorie Ketogenic Diet as a Safe and Valuable Tool for Long-Term Glycemic Management in Patients with Obesity and Type 2 Diabetes. Nutrients 2021, 13, 758. [Google Scholar] [CrossRef]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. Mediterranean dietary pattern and the risk of type 2 diabetes: A systematic review and dose–response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2022, 61, 1735–1748. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Frayling, T.M. Genome–wide association studies provide new insights into type 2 diabetes aetiology. Nat. Rev. Genet. 2007, 8, 657–662. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wang, L.; Li, X.; Jiang, F.; Chen, X.; Mantzoros, C.S. Circulating lipoprotein(a) levels and health outcomes: Phenome-wide Mendelian randomization and disease-trajectory analyses. Metabolism 2022, 137, 155347. [Google Scholar] [CrossRef]
- Biobank, U. UK Biobank: Protocol for a Large-Scale Prospective Epidemiological Resource; UK Biobank Coordinating Centre: Cheshire, UK, 2021. [Google Scholar]
- Eastwood, S.V.; Mathur, R.; Atkinson, M.; Brophy, S.; Sudlow, C.; Flaig, R.; de Lusignan, S.; Allen, N.; Chaturvedi, N. Algorithms for the Capture and Adjudication of Prevalent and Incident Diabetes in UK Biobank. PLoS ONE 2016, 11, e0162388. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65 (Suppl. S4), 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; Bradbury, K.E.; Cross, A.J.; Gunter, M.J.; Murphy, N. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br. J. Cancer 2018, 118, 920–929. [Google Scholar] [CrossRef] [PubMed]
- UK Biobank. Available online: https://www.ukbiobank.ac.uk/ (accessed on 1 April 2021).
- Gnagnarella, P.; Maisonneuve, P.; Bellomi, M.; Rampinelli, C.; Bertolotti, R.; Spaggiari, L.; Palli, D.; Veronesi, G. Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann. Oncol. 2013, 24, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Hommel, G. A Stagewise Rejective Multiple Test Procedure Based on a Modified Bonferroni Test. Biometrika 1988, 75, 383. [Google Scholar] [CrossRef]
- Myers, L.; Sirois, M.J. Spearman correlation coefficients, differences between. In Encyclopedia of Statistical Sciences; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; p. 1. [Google Scholar]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association between plant-based dietary patterns and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Upritchard, J.E.; Sutherland, W.H.; Mann, J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. Br. J. Sport. Med. 2016, 50, 496–504. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Becerra-Tomás, N.; Ruiz-Canela, M.; Corella, D.; Schröder, H.; Estruch, R.; Ros, E.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevención con Dieta Mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 2017, 105, 723–735. [Google Scholar] [CrossRef]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 fatty acids for nutrition and medicine: Considering microalgae oil as a vegetarian source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Arthur, R.; Qin, L.-Q.; Chen, L.-H.; Mei, Z.; Zheng, Y.; Li, Y.; Wang, T.; Rohan, T.E.; Qi, Q. Association of oily and nonoily fish consumption and fish oil supplements with incident type 2 diabetes: A large population-based prospective study. Diabetes Care 2021, 44, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Khan, T.A.; McGlynn, N.; Malik, V.S.; Hill, J.O.; Leiter, L.A.; Jeppesen, P.B.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; et al. Relation of Change or Substitution of Low- and No-Calorie Sweetened Beverages with Cardiometabolic Outcomes: A Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, J.; Huang, T. Coffee types and type 2 diabetes mellitus: Large-scale cross-phenotype association study and mendelian randomization analysis. Front. Endocrinol. 2022, 13, 818831. [Google Scholar] [CrossRef]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, A. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef]
| Overall | Non-T2DM | T2DM Onsets | p-Value | |
|---|---|---|---|---|
| N | 119,040 | 115,799 | 3241 | |
| Gender (%) | <0.001 | |||
| Female | 67,364 (56.6) | 66,009 (57.0) | 1355 (41.8) | |
| Male | 51,676 (43.4) | 49,790 (43.0) | 1886 (58.2) | |
| Age at recruitment (mean (SD)) | 55.97 (7.85) | 55.90 (7.85) | 58.61 (7.19) | <0.001 |
| Ethnic background (%) | <0.001 | |||
| White | 115,183 (96.8) | 112,126 (96.8) | 3057 (94.3) | |
| White and other mixed background | 443 (0.4) | 429 (0.4) | 14 (0.4) | |
| Asian and Asian mixed background | 1664 (1.4) | 1579 (1.4) | 85 (2.6) | |
| Other ethnic background | 1750 (1.5) | 1665 (1.4) | 85 (2.6) | |
| Smoking status (%) | <0.001 | |||
| Never smoke | 68,527 (57.6) | 67,076 (57.9) | 1451 (44.8) | |
| Previous smoker | 42,201 (35.5) | 40,760 (35.2) | 1441 (44.5) | |
| Current smoker | 8312 (7.0) | 7963 (6.9) | 349 (10.8) | |
| Alcohol intake status (%) | <0.001 | |||
| Never drink | 3339 (2.8) | 3183 (2.7) | 156 (4.8) | |
| Previous drinker | 3364 (2.8) | 3185 (2.8) | 179 (5.5) | |
| Current drinker | 112,337 (94.4) | 109,431 (94.5) | 2906 (89.7) | |
| Education level (%) | <0.001 | |||
| Low level | 7894 (6.6) | 7460 (6.4) | 434 (13.4) | |
| Intermediate level | 56,210 (47.2) | 55,119 (47.6) | 1091 (33.7) | |
| High level | 54,936 (46.1) | 53,220 (46.0) | 1716 (52.9) | |
| Physical activity (MET-min/week) (Mean [SD]) | 2427.62 (2356.48) | 2432.42 (2352.23) | 2256.11 (2497.81) | <0.001 |
| Townsend Index (mean (SD)) | −1.63 (2.84) | −1.65 (2.83) | −1.08 (3.07) | <0.001 |
| BMI (kg/m2) (Mean [SD]) | 26.56 (4.45) | 26.44 (4.36) | 30.65 (5.67) | <0.001 |
| HbA1c (mmol/L) (Mean [SD]) | 34.81 (4.25) | 34.64 (3.91) | 40.98 (8.81) | <0.001 |
| Blood cholesterol (mmol/L) (mean (SD)) | 5.76 (1.06) | 5.76 (1.06) | 5.59 (1.18) | <0.001 |
| Medicine for blood pressure (%) | 18,745 (15.7) | 17,472 (15.1) | 1273 (39.3) | <0.001 |
| Medicine for exogenous hormone (%) | 7101 (6.0) | 6968 (6.0) | 133 (4.1) | <0.001 |
| Hypertension (%) | 26,164 (22.0) | 24,686 (21.3) | 1478 (45.6) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Shang, X.; Chen, Y.; Tang, W.; Yusufu, M.; Chen, Z.; Chen, R.; Hu, W.; Jan, C.; Li, L.; et al. Diet-Wide Association Study for the Incidence of Type 2 Diabetes Mellitus in Community-Dwelling Adults Using the UK Biobank Data. Nutrients 2024, 16, 103. https://doi.org/10.3390/nu16010103
Liu J, Shang X, Chen Y, Tang W, Yusufu M, Chen Z, Chen R, Hu W, Jan C, Li L, et al. Diet-Wide Association Study for the Incidence of Type 2 Diabetes Mellitus in Community-Dwelling Adults Using the UK Biobank Data. Nutrients. 2024; 16(1):103. https://doi.org/10.3390/nu16010103
Chicago/Turabian StyleLiu, Jiahao, Xianwen Shang, Yutong Chen, Wentao Tang, Mayinuer Yusufu, Ziqi Chen, Ruiye Chen, Wenyi Hu, Catherine Jan, Li Li, and et al. 2024. "Diet-Wide Association Study for the Incidence of Type 2 Diabetes Mellitus in Community-Dwelling Adults Using the UK Biobank Data" Nutrients 16, no. 1: 103. https://doi.org/10.3390/nu16010103
APA StyleLiu, J., Shang, X., Chen, Y., Tang, W., Yusufu, M., Chen, Z., Chen, R., Hu, W., Jan, C., Li, L., He, M., Zhu, Z., & Zhang, L. (2024). Diet-Wide Association Study for the Incidence of Type 2 Diabetes Mellitus in Community-Dwelling Adults Using the UK Biobank Data. Nutrients, 16(1), 103. https://doi.org/10.3390/nu16010103






