Post-Prandial Cognitive and Blood Pressure Effects of a DHA-Rich Omega-3 Powder in Middle-Aged Males: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Treatments
2.3. Cognitive Assessments
Swinburne University Computerized Cognitive Assessment Battery (SUCCAB)
Simple Reaction Time (SRT)
Choice Reaction Time (CRT)
Immediate Recognition Memory (IRM)
Congruent Stroop (CS)
Incongruent Stroop
Spatial Working Memory (SWM)
Contextual Recognition Memory (CRM)
Delayed Recognition Memory (DRM)
2.4. Cardiovascular Assessments
2.5. Blood Fatty Acid Concentration
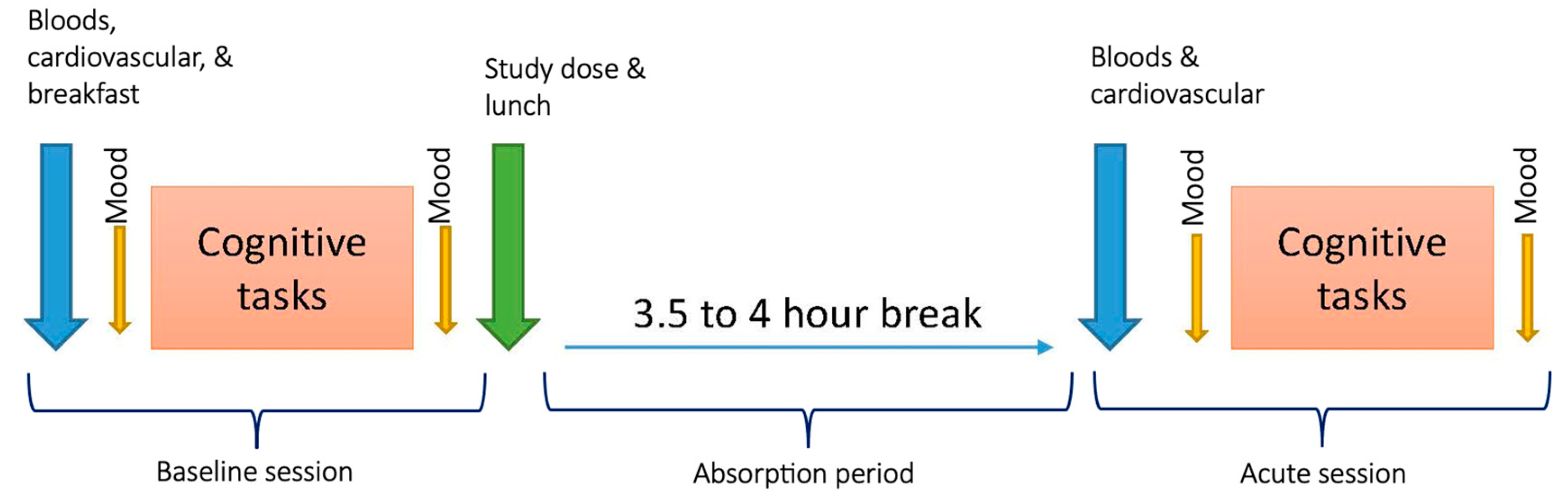
2.6. Procedure
2.7. Statistical Analysis
3. Results
3.1. Participant Demographics
3.2. Primary Analyses
3.3. Secondary Analyses
3.3.1. SUCCAB Response Accuracy and Performance Scores
3.3.2. Cardiovascular Function
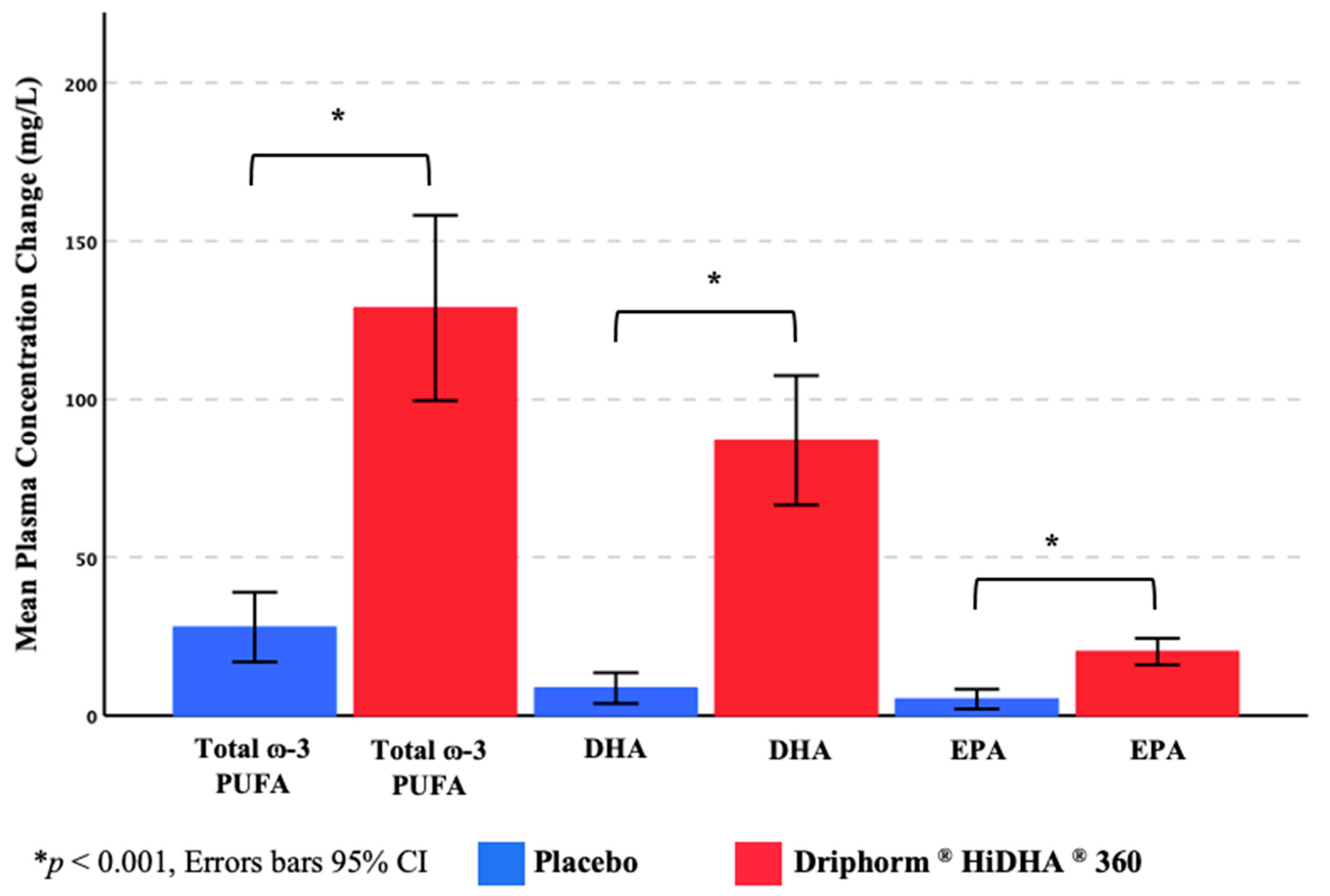
3.3.3. Plasma ω-3 PUFA Concentrations
3.4. Efficacy of Treatment Blinding
3.5. Safety and Tolerance of Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, P.E.; Cooper, K.L.; Relton, C.; Thomas, K.J. Prevalence of complementary and alternative medicine (CAM) use by the general population: A systematic review and update. Int. J. Clin. Pract. 2012, 66, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.E.; McIntyre, E.; Steel, A.; Foley, H.; Sibbritt, D.; Adams, J. Use of complementary medicine products: A nationally representative cross-sectional survey of 2019 Australian adults. BMJ Open 2019, 9, e024198. [Google Scholar] [CrossRef] [PubMed]
- Steel, A.; McIntyre, E.; Harnett, J.; Foley, H.; Adams, J.; Sibbritt, D.; Wardle, J.; Frawley, J. Complementary medicine use in the Australian population: Results of a nationally-representative cross-sectional survey. Sci. Rep. 2018, 8, 17325. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary Supplement Use in the United States, 2003–2006. J. Nutr. 2011, 141, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.E.; Tooze, J.A.; Gahche, J.J.; Eicher-Miller, H.A.; Guenther, P.M.; Dwyer, J.T.; Potischman, N.; Bhadra, A.; Carroll, R.J.; Bailey, R.L. Trends in Overall and Micronutrient-Containing Dietary Supplement Use in US Adults and Children, NHANES 2007–2018. J. Nutr. 2022, 152, 2789–2801. [Google Scholar] [CrossRef]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Data Brief 399: Dietary Supplement Use Among Adults: United States, 2017–2018. 2021. Available online: https://stacks.cdc.gov/view/cdc/101131 (accessed on 14 April 2023). [CrossRef]
- Laditka, J.N.; Laditka, S.B.; Tait, E.M.; Tsulukidze, M.M. Use of Dietary Supplements for Cognitive Health: Results of a National Survey of Adults in the United States. Am. J. Alzheimer’s Dis. Other Dementiasr 2012, 27, 55–64. [Google Scholar] [CrossRef]
- Parmenter, B.H.; Bumrungpert, A.; Thouas, G.A. Socio-demographic factors, beliefs and health perceptions associated with use of a commercially available Ω-3 fatty acid supplement: A cross-sectional study in Asian countries. PharmaNutrition 2020, 15, 100237. [Google Scholar] [CrossRef]
- Saleh, R.N.M.; Minihane, A.M. Fish, n-3 fatty acids, cognition and dementia risk: Not just a fishy tale. Proc. Nutr. Soc. 2021, 81, 27–40. [Google Scholar] [CrossRef]
- Reddan, J.M.; White, D.J.; Macpherson, H.; Scholey, A.; Pipingas, A. Glycerophospholipid Supplementation as a Potential Intervention for Supporting Cerebral Structure in Older Adults. Front. Aging Neurosci. 2018, 10, 49. [Google Scholar] [CrossRef]
- Andriambelo, B.; Stiffel, M.; Roke, K.; Plourde, M. New perspectives on randomized controlled trials with omega-3 fatty acid supplements and cognition: A scoping review. Ageing Res. Rev. 2023, 85, 101835. [Google Scholar] [CrossRef]
- van der Wurff, I.S.; Meyer, B.J.; de Groot, R.H. Effect of Omega-3 Long Chain Polyunsaturated Fatty Acids (n-3 LCPUFA) Supplementation on Cognition in Children and Adolescents: A Systematic Literature Review with a Focus on n-3 LCPUFA Blood Values and Dose of DHA and EPA. Nutrients 2020, 12, 3115. [Google Scholar] [CrossRef] [PubMed]
- Patan, M.J.; Kennedy, D.O.; Husberg, C.; Hustvedt, S.O.; Calder, P.C.; Khan, J.; Forster, J.; Jackson, P.A. Supplementation with oil rich in eicosapentaenoic acid, but not in docosahexaenoic acid, improves global cognitive function in healthy, young adults: Results from randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Leckie, R.L.; Lehman, D.E.; Gianaros, P.J.; Erickson, K.I.; Sereika, S.M.; Kuan, D.C.H.; Manuck, S.B.; Ryan, C.M.; Yao, J.K.; Muldoon, M.F. The effects of omega-3 fatty acids on neuropsychological functioning and brain morphology in mid-life adults: A randomized clinical trial. Psychol. Med. 2019, 50, 2425–2434. [Google Scholar] [CrossRef]
- Massee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized, controlled trial. Front. Pharmacol. 2015, 6, 93. [Google Scholar] [CrossRef]
- Scholey, A.B.; French, S.J.; Morris, P.J.; Kennedy, D.O.; Milne, A.L.; Haskell, C.F. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 2009, 24, 1505–1514. [Google Scholar] [CrossRef]
- Field, D.T.; Williams, C.; Butler, L.T. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol. Behav. 2011, 103, 255–260. [Google Scholar] [CrossRef]
- Grassi, D.; Socci, V.; Tempesta, D.; Ferri, C.; De Gennaro, L.; Desideri, G.; Ferrara, M. Flavanol-rich chocolate acutely improves arterial function and working memory performance counteracting the effects of sleep deprivation in healthy individuals. J. Hypertens. 2016, 34, 1298–1308. [Google Scholar] [CrossRef]
- Karabay, A.; Saija, J.D.; Field, D.T.; Akyürek, E.G. The acute effects of cocoa flavanols on temporal and spatial attention. Psychopharmacology 2018, 235, 1497–1511. [Google Scholar] [CrossRef]
- Lamport, D.J.; Christodoulou, E.; Achilleos, C. Beneficial Effects of Dark Chocolate for Episodic Memory in Healthy Young Adults: A Parallel-Groups Acute Intervention with a White Chocolate Control. Nutrients 2020, 12, 483. [Google Scholar] [CrossRef]
- Marsh, C.E.; Carter, H.H.; Guelfi, K.J.; Smith, K.J.; Pike, K.E.; Naylor, L.H.; Green, D.J. Brachial and cerebrovascular functions are enhanced in postmenopausal women after ingestion of chocolate with a high concentration of cocoa. J. Nutr. 2017, 147, 1686–1692. [Google Scholar] [CrossRef]
- Bell, L.; Lamport, D.J.; Butler, L.T.; Williams, C.M. A Review of the Cognitive Effects Observed in Humans Following Acute Supplementation with Flavonoids, and Their Associated Mechanisms of Action. Nutrients 2015, 7, 10290–10306. [Google Scholar] [CrossRef]
- Faridi, Z.; Njike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008, 88, 58–63. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Kroon, P.A.; Philo, M.; Mensink, M.; Kromhout, D.; Hollman, P.C.H. Does epicatechin contribute to the acute vascular function effects of dark chocolate? A randomized, crossover study. Mol. Nutr. Food Res. 2016, 60, 2379–2386. [Google Scholar] [CrossRef]
- Gratton, G.; Weaver, S.R.; Burley, C.V.; Low, K.A.; Maclin, E.L.; Johns, P.W.; Pham, Q.S.; Lucas, S.J.E.; Fabiani, M.; Rendeiro, C. Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults. Sci. Rep. 2020, 10, 19409. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Wang, L.; Lu, X.; Huang, J.; Cao, J.; Li, H.; Gu, D. Effect of omega-3 fatty acids supplementation on endothelial function: A meta-analysis of randomized controlled trials. Atherosclerosis 2012, 221, 536–543. [Google Scholar] [CrossRef]
- Newens, K.J.; Thompson, A.K.; Jackson, K.G.; Wright, J.; Williams, C.M. DHA-rich fish oil reverses the detrimental effects of saturated fatty acids on postprandial vascular reactivity. Am. J. Clin. Nutr. 2011, 94, 742–748. [Google Scholar] [CrossRef]
- Fahs, C.A.; Yan, H.; Ranadive, S.; Rossow, L.M.; Agiovlasitis, S.; Wilund, K.R.; Fernhall, B. The effect of acute fish-oil supplementation on endothelial function and arterial stiffness following a high-fat meal. Appl. Physiol. Nutr. Metab. 2010, 35, 294–302. [Google Scholar] [CrossRef]
- Armah, C.K.; Jackson, K.G.; Doman, I.; James, L.; Cheghani, F.; Minihane, A.M. Fish oil fatty acids improve postprandial vascular reactivity in healthy men. Clin. Sci. 2008, 114, 679–686. [Google Scholar] [CrossRef]
- West, S.G.; Hecker, K.D.; Mustad, V.A.; Nicholson, S.; Schoemer, S.L.; Wagner, P.; Hinderliter, A.L.; Ulbrecht, J.; Ruey, P.; Kris-Etherton, P.M. Acute effects of monounsaturated fatty acids with and without omega-3 fatty acids on vascular reactivity in individuals with type 2 diabetes. Diabetologia 2004, 48, 113–122. [Google Scholar] [CrossRef]
- Jackson, K.G.; Armah, C.K.; Doman, I.; James, L.; Cheghani, F.; Minihane, A.M. The impact of age on the postprandial vascular response to a fish oil-enriched meal. Br. J. Nutr. 2009, 102, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Sanders, K.A.; Sanders, T.A.B.; Chowienczyk, P.J. A High-Fat Meal Enriched with Eicosapentaenoic Acid Reduces Postprandial Arterial Stiffness Measured by Digital Volume Pulse Analysis in Healthy Men. J. Nutr. 2008, 138, 287–291. [Google Scholar] [CrossRef] [PubMed]
- McManus, S.; Tejera, N.; Awwad, K.; Vauzour, D.; Rigby, N.; Fleming, I.; Cassidy, A.; Minihane, A.M. Differential effects of EPA versus DHA on postprandial vascular function and the plasma oxylipin profile in men. J. Lipid Res. 2016, 57, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Kheloui, S.; Brouillard, A.; Rossi, M.; Marin, M.-F.; Mendrek, A.; Paquette, D.; Juster, R.-P. Exploring the sex and gender correlates of cognitive sex differences. Acta Psychol. 2021, 221, 103452. [Google Scholar] [CrossRef] [PubMed]
- Lejbak, L.; Crossley, M.; Vrbancic, M. A male advantage for spatial and object but not verbal working memory using the n-back task. Brain Cogn. 2011, 76, 191–196. [Google Scholar] [CrossRef]
- Greendale, G.A.; Wight, R.G.; Huang, M.-H.; Avis, N.; Gold, E.B.; Joffe, H.; Seeman, T.; Vuge, M.; Karlamangla, A.S. Menopause-associated Symptoms and Cognitive Performance: Results From the Study of Women’s Health Across the Nation. Am. J. Epidemiology 2010, 171, 1214–1224. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Pipingas, A.; Harris, E.; Tournier, E.; King, R.; Kras, M.; Stough, C.K. Assessing the efficacy of nutraceutical interventions on cognitive functioning in the elderly. Curr. Top. Nutraceuticals Res. 2010, 8, 79–88. [Google Scholar]
- Young, L.M.; Gauci, S.; Arnoldy, L.; Martin, L.; Perry, N.; White, D.J.; Meyer, D.; Lassemillante, A.-C.; Ogden, E.; Silber, B.; et al. Investigating the Effects of a Multinutrient Supplement on Cognition, Mood and Biochemical Markers in Middle-Aged Adults with ‘Optimal’ and ‘Sub-Optimal’ Diets: A Randomized Double Blind Placebo Controlled Trial. Nutrients 2022, 14, 5079. [Google Scholar] [CrossRef]
- Macpherson, H.; Ellis, K.; Sali, A.; Pipingas, A. Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement. Psychopharmacology 2011, 220, 351–365. [Google Scholar] [CrossRef]
- Heitz, R.P. The speed-accuracy tradeoff: History, physiology, methodology, and behavior. Front. Neurosci. 2014, 8, 150. [Google Scholar] [CrossRef]
- Kennedy, G.; Meyer, D.; Hardman, R.J.; Macpherson, H.; Scholey, A.B.; Pipingas, A. Physical Fitness and Aortic Stiffness Explain the Reduced Cognitive Performance Associated with Increasing Age in Older People. J. Alzheimers Dis. 2018, 63, 1307–1316. [Google Scholar] [CrossRef]
- Pase, M.P.; Stough, C.; Grima, N.A.; Harris, E.; MacPherson, H.; Scholey, A.B.; Pipingas, A. Blood Pressure and Cognitive Function: The role of central aortic and brachial pressures. Psychol. Sci. 2013, 24, 2173–2181. [Google Scholar] [CrossRef]
- The Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Young, L.M.; Gauci, S.; Scholey, A.; White, D.J.; Lassemillante, A.-C.; Meyer, D.; Pipingas, A. Self-Reported Diet Quality Differentiates Nutrient Intake, Blood Nutrient Status, Mood, and Cognition: Implications for Identifying Nutritional Neurocognitive Risk Factors in Middle Age. Nutrients 2020, 12, 2964. [Google Scholar] [CrossRef]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2019, 30, 160–164. [Google Scholar] [CrossRef]
- Harris, W.S.; von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-Chain Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200. [Google Scholar] [CrossRef]
- Pal, S.; Ellis, V. The Chronic Effects of Whey Proteins on Blood Pressure, Vascular Function, and Inflammatory Markers in Overweight Individuals. Obesity 2010, 18, 1354–1359. [Google Scholar] [CrossRef]
- Arnberg, K.; Larnkjær, A.; Michaelsen, K.F.; Jensen, S.M.; Hoppe, C.; Mølgaard, C. Casein improves brachial and central aortic diastolic blood pressure in overweight adolescents: A randomised, controlled trial. J. Nutr. Sci. 2013, 2, e43. [Google Scholar] [CrossRef]
- Fekete, Á.A.; Giromini, C.; Chatzidiakou, Y.; Givens, D.I.; Lovegrove, J.A. Whey protein lowers blood pressure and improves endothelial function and lipid biomarkers in adults with prehypertension and mild hypertension: Results from the chronic Whey2Go randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Á.A.; Givens, D.I.; Lovegrove, J.A. The impact of milk proteins and peptides on blood pressure and vascular function: A review of evidence from human intervention studies. Nutr. Res. Rev. 2013, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ellis, V. Acute effects of whey protein isolate on blood pressure, vascular function and inflammatory markers in overweight postmenopausal women. Br. J. Nutr. 2011, 105, 1512–1519. [Google Scholar] [CrossRef] [PubMed]

| M (%) | SD | Range | |
|---|---|---|---|
| Age (years) | 52.79 | 5.29 | 42.00–60.00 |
| Education (years) | 16.78 | 2.19 | 11.00–21.00 |
| Highest education achieved, n (%) | |||
| Secondary | 2 (6.90) | ||
| Tertiary | 19 (65.50) | ||
| Postgraduate | 8 (27.60) | ||
| Current employment status, n (%) | |||
| Student | 1 (3.40) | ||
| Part time/casual | 3 (10.30) | ||
| Full time | 18 (62.10) | ||
| Retired | 4 (13.80) | ||
| Unemployed | 3 (10.30) | ||
| Height (cm) | 178.03 | 7.13 | 162.20–194.00 |
| Body weight (kg) | 87.12 | 14.21 | 66.50–130.70 |
| Body mass index (kg/m2) | 27.44 | 3.79 | 21.10–38.60 |
| Systolic blood pressure (mmHg) * | 127.17 | 9.46 | 112–153 |
| Diastolic blood pressure (mmHg) * | 77.86 | 9.74 | 43–90 |
| RBC ω-3 PUFA Content (as %) | |||
| Total ω-3 PUFA † | 8.51 | 1.52 | 4.33–10.71 |
| DHA | 4.54 | 1.17 | 2.13–6.68 |
| EPA | 0.94 | 0.24 | 0.47–1.43 |
| ω-3 Index | 5.48 | 1.29 | 2.60–7.82 |
| Placebo | ω-3 PUFA * | Interaction 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SUCCAB Outcome | Pre-Dose | Post-Dose | Pre-Dose | Post-Dose | ||||||
| M | SD | M | SD | M | SD | M | SD | F | p | |
| Response Time (ms) | ||||||||||
| SRT | 267.56 | 30.60 | 274.59 | 38.95 | 270.82 | 32.18 | 277.99 | 36.50 | <0.01 | 0.982 |
| CRT | 462.59 | 73.54 | 457.77 | 71.82 | 468.71 | 61.31 | 459.35 | 75.26 | 0.19 | 0.667 |
| IRM | 1085.31 | 276.22 | 962.64 | 155.89 | 1106.11 | 220.71 | 979.62 | 176.49 | 0.01 | 0.929 |
| CS | 726.32 | 88.65 | 674.45 | 110.13 | 706.73 | 85.40 | 668.10 | 89.76 | 0.87 | 0.359 |
| ICS | 862.97 | 131.10 | 808.63 | 111.05 | 863.14 | 158.62 | 797.14 | 145.71 | 0.31 | 0.584 |
| Stroop Effect | 136.65 | 89.64 | 134.18 | 92.32 | 156.41 | 107.33 | 129.04 | 92.85 | 1.29 | 0.267 |
| SWM | 946.34 | 258.96 | 912.45 | 192.60 | 996.82 | 296.02 | 926.34 | 200.52 | 0.65 | 0.426 |
| CRM | 1024.55 | 219.03 | 1007.73 | 217.05 | 1063.74 | 274.45 | 1008.89 | 196.42 | 1.19 | 0.285 |
| DRM | 1087.32 | 190.06 | 1089.76 | 190.96 | 1124.48 | 283.93 | 1108.86 | 213.40 | 0.14 | 0.710 |
| Response Accuracy (%) | ||||||||||
| CRT | 98.28 | 2.76 | 96.72 | 4.07 | 97.93 | 2.84 | 98.10 | 2.81 | 2.64 | 0.115 |
| IRM | 84.25 | 8.54 | 85.98 | 7.89 | 82.41 | 9.96 | 84.83 | 9.28 | 0.10 | 0.756 |
| CS | 99.14 | 1.67 | 98.70 | 2.19 | 98.71 | 1.72 | 98.62 | 2.07 | 0.23 | 0.634 |
| ICS | 98.19 | 2.30 | 98.02 | 3.50 | 98.45 | 2.35 | 97.33 | 2.49 | 1.14 | 0.296 |
| SWM | 85.45 | 10.71 | 85.90 | 9.11 | 86.02 | 9.33 | 84.46 | 10.33 | 1.18 | 0.287 |
| CRW | 81.38 | 20.04 | 86.03 | 12.42 | 86.90 | 13.12 | 86.03 | 12.91 | 2.04 | 0.164 |
| DRM | 72.53 | 12.07 | 73.47 | 9.93 | 77.20 | 11.81 | 74.53 | 13.67 | 1.58 | 0.221 |
| Performance Score | ||||||||||
| CRT | 217.24 | 32.23 | 215.59 | 30.28 | 212.32 | 28.16 | 217.92 | 29.20 | 2.39 | 0.133 |
| IRM | 81.48 | 18.64 | 91.19 | 15.04 | 77.31 | 17.75 | 89.04 | 17.48 | 0.38 | 0.545 |
| CS | 138.64 | 18.81 | 149.50 | 20.65 | 141.61 | 16.98 | 150.19 | 20.42 | 0.61 | 0.440 |
| ICS | 116.48 | 18.82 | 123.60 | 18.65 | 117.72 | 21.24 | 125.78 | 21.38 | 0.08 | 0.775 |
| SWM | 97.12 | 28.70 | 98.89 | 26.78 | 93.41 | 27.54 | 96.00 | 26.85 | 0.03 | 0.856 |
| CRW | 83.34 | 27.05 | 89.07 | 23.12 | 86.15 | 23.21 | 88.32 | 21.44 | 0.87 | 0.358 |
| DRM | 68.65 | 15.97 | 69.02 | 13.36 | 72.08 | 17.91 | 69.27 | 17.21 | 0.88 | 0.357 |
| Placebo | ω-3 PUFA * | Interaction 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Pre-Dose | Post-Dose | Pre-Dose | Post-Dose | ||||||
| M | SD | M | SD | M | SD | M | SD | F | p | |
| Brachial Pressures (mmHg) | ||||||||||
| SBP | 115.18 | 10.86 | 113.82 | 8.97 | 115.57 | 10.33 | 111.79 | 7.27 | 3.25 | 0.082 |
| DBP | 71.89 | 6.78 | 69.71 | 6.53 | 71.50 | 6.45 | 68.18 | 4.57 | 1.48 | 0.235 |
| PP | 43.29 | 6.42 | 44.11 | 5.32 | 44.07 | 5.54 | 43.61 | 5.97 | 0.72 | 0.404 |
| MAP | 86.32 | 7.79 | 84.42 | 7.00 | 86.19 | 7.52 | 82.71 | 4.86 | 3.61 | 0.068 |
| Aortic Pressures (mmHg) | ||||||||||
| SBP | 105.89 | 9.60 | 104.50 | 8.20 | 106.96 | 9.95 | 102.86 | 6.52 | 5.95 | 0.022 |
| DBP | 72.68 | 6.79 | 70.57 | 6.59 | 72.46 | 6.61 | 69.21 | 4.57 | 1.57 | 0.221 |
| PP | 33.21 | 4.79 | 34.50 | 4.76 | 33.93 | 4.59 | 33.64 | 4.51 | 1.77 | 0.195 |
| MAP | 83.75 | 7.51 | 81.88 | 6.83 | 83.96 | 7.56 | 80.43 | 4.86 | 4.23 | 0.050 |
| Arterial Stiffness | ||||||||||
| AP (mmHg) | 7.75 | 3.22 | 7.57 | 3.69 | 8.50 | 4.41 | 8.25 | 3.98 | 0.01 | 0.931 |
| AiX@75 (%) | 14.50 | 10.33 | 14.50 | 12.49 | 15.14 | 12.71 | 16.04 | 12.46 | 0.17 | 0.682 |
| CF-PWV (m/s) | 10.00 | 0.99 | 9.86 | 0.95 | 9.77 | 0.89 | 9.65 | 0.73 | 0.01 | 0.928 |
| Plasma ω-3 Concentration | ||||||||||
| Total ω-3 PUFA † (mg/L) | 135.59 | 37.78 | 158.40 | 49.49 | 126.41 | 29.48 | 263.72 | 86.34 | - | - |
| DHA (mg/L) | 59.93 | 16.51 | 65.70 | 19.34 | 56.18 | 15.66 | 150.32 | 52.39 | - | - |
| EPA (mg/L) | 30.69 | 12.12 | 34.44 | 16.56 | 26.71 | 8.39 | 48.32 | 12.62 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipingas, A.; Reddan, J.M.; Gauci, S.; Young, L.M.; Kennedy, G.; Rowsell, R.; King, R.; Spiteri, S.; Minihane, A.M.; Scholey, A. Post-Prandial Cognitive and Blood Pressure Effects of a DHA-Rich Omega-3 Powder in Middle-Aged Males: A Pilot Study. Nutrients 2023, 15, 2198. https://doi.org/10.3390/nu15092198
Pipingas A, Reddan JM, Gauci S, Young LM, Kennedy G, Rowsell R, King R, Spiteri S, Minihane AM, Scholey A. Post-Prandial Cognitive and Blood Pressure Effects of a DHA-Rich Omega-3 Powder in Middle-Aged Males: A Pilot Study. Nutrients. 2023; 15(9):2198. https://doi.org/10.3390/nu15092198
Chicago/Turabian StylePipingas, Andrew, Jeffery Michael Reddan, Sarah Gauci, Lauren M. Young, Greg Kennedy, Renee Rowsell, Rebecca King, Sam Spiteri, Anne Marie Minihane, and Andrew Scholey. 2023. "Post-Prandial Cognitive and Blood Pressure Effects of a DHA-Rich Omega-3 Powder in Middle-Aged Males: A Pilot Study" Nutrients 15, no. 9: 2198. https://doi.org/10.3390/nu15092198
APA StylePipingas, A., Reddan, J. M., Gauci, S., Young, L. M., Kennedy, G., Rowsell, R., King, R., Spiteri, S., Minihane, A. M., & Scholey, A. (2023). Post-Prandial Cognitive and Blood Pressure Effects of a DHA-Rich Omega-3 Powder in Middle-Aged Males: A Pilot Study. Nutrients, 15(9), 2198. https://doi.org/10.3390/nu15092198









