Branched-Chain Amino Acids Metabolism and Their Roles in Retinopathy: From Relevance to Mechanism
Abstract
1. Introduction
2. BCAA Metabolism Process
3. The Alterations of BCAAs in Retinopathy
| Species | Samples | Subjects | Platforms | Criteria | Differential Metabolites | References |
|---|---|---|---|---|---|---|
| Human | SRF and vitreous | 46 RRD/7 controls | HPLC | p < 0.05 | ↓ Leucine, Isoleucine, Valine | Yalcinbayir et al. (2014) [36] |
| SRF and vitreous | 20 SRF and 5 vitreous/10 Vitreous controls | HPLC | p < 0.01 | ↓ Leucine, Isoleucine, Valine | Bertram et al. (2008) [37] | |
| Vitreous | 28 PDR/22 controls | GC-TOFMS | p < 0.01 | ↑ L-Leucine, L-alloisoleucine, L-valine | Wang et al. (2020) [38] | |
| Plasma | 105 NDR, 103 NPDR, 103 MNPDR, 113 SNPDR, and 20 PDR | GC-MS, LC-MS | p < 0.05 | ↑ Leucine, Isoleucine, Valine | Xuan et al. (2020) [39] | |
| Plasma | 19 DR/14 controls | NMR | p < 0.05 | ↑ Leucine | Lin et al. (2019) [41] | |
| Plasma | 4 GA/22 controls | Technicon amino acid analyzer | p < 0.05 | ↓ Leucine, Isoleucine, Valine | Valle et al. (1980) [30] | |
| Plasma | 53 AMD/18 controls | UHPLC-MS | p < 0.01 | ↑ Leucine, Isoleucine | Mendez et al. (2021) [40] | |
| Mice | Retina | 10 OIR/10 controls | HPLC-MS/MS | p < 0.01 | P17: ↑ Leucine, Isoleucine, L-Valine | Zhou et al. (2021) [43] |
| Retina | 6 DR mice/9 controls | UHPLC-MS/MS, GC-MS | p < 0.05 | ↑ Leucine, Isoleucine | Wang et al. (2022) [18] | |
| Plasma | DR mice/controls | UHPLC-MS/MS | p < 0.05 | ↑ Leucine, Isoleucine, L-Valine | Gong et al. (2022) [19] | |
| Retina | ||||||
| Retina | 6 AMD mice/6 controls | LC-MS | VIP > 1 | ↑ Leucine, Isoleucine | Natoli et al. (2018) [44] | |
| Plasma | ↑ Leucine, Isoleucine, Valine | |||||
| Retina | GC-MS | p < 0.05 | Leucine, Isoleucine, Valine | Xu et al. (2020) [46] | ||
| RPE/choroid | ||||||
| Rats | Retina | DR rats/controls | HPLC | p < 0.05 | ↑ Leucine, Isoleucine, Valine | Gowda et al. (2011) [42] |
| Retina | DR rats/controls | HPLC | p < 0.01 | ↑ Leucine, Isoleucine, Valine | Ola MS et al. (2019) [20] | |
| Retina | 6 DR rats/4 controls | Beckman Model 121 amino acid analyzer | p < 0.01 | ↑ Leucine, Isoleucine, Valine | Frayser et al. (1978) [29] | |
| Plasma | ||||||
| Retina | 7 AMD rat/6 controls | UHPLC-MS/MS | p < 0.05 | ↑ Leucylleucine, Isoleucylisoleucine | Wei et al. (2022) [45] | |
| Vitreous | ↓ L-Leucine, Isoleucine | |||||
| RPE/choroid | ↓ L-Leucine, Leucylleucine, Isoleucylisoleucine | |||||
| Serum | DR rats/controls | Spectrophotometric | p < 0.05 | ↑ Leucine, Isoleucine, Valine | Masser et al. (2014) [31] | |
| Serum | DR rats/controls | LC | p < 0.05 | ↑ Leucine, Isoleucine, Valine | Gibson et al. (1988) [47] | |
| Pig | VH | 13 treated with IAA/17 controls | 1H NMR | p < 0.01 | ↓ Leucine, Isoleucine, Valine | Elmi et al. (2019) [48] |
| Human cell | Human retinal Müller cell | Müller cell with HG or NG | UHPLC-MS/MS | p < 0.05 | ↑ Leucine, Isoleucine, Valine | Gong et al. (2022) [19] |
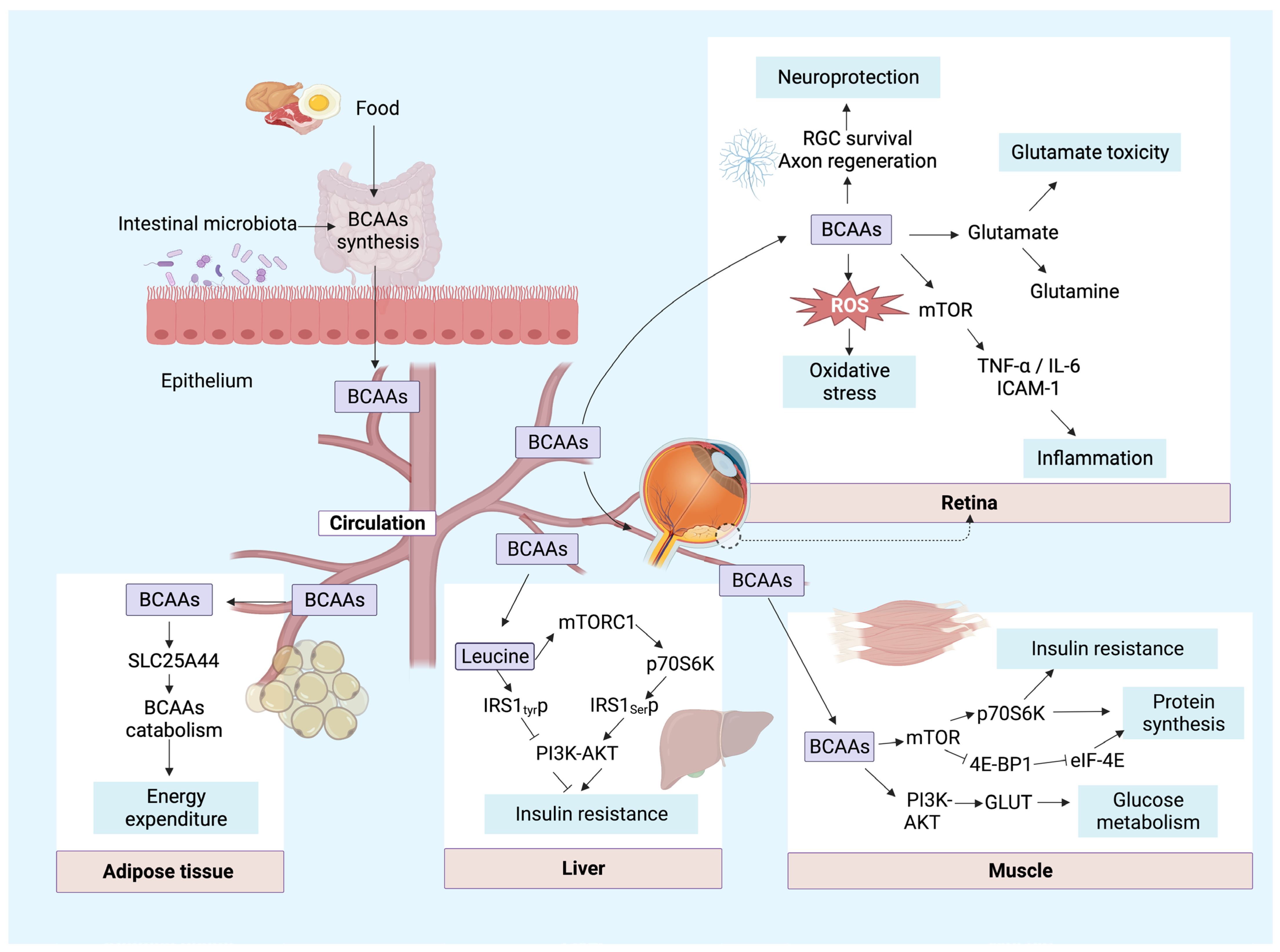
4. Biological Functions of BCAAs in the Retina and Other Major Tissues
4.1. Regulation of the Excitatory Toxicity of Glutamate
4.2. Neuroprotection
4.3. Oxidative Stress
4.4. Inflammation
4.5. Other Functions
5. Mechanisms Responsible for Regulation of BCAA Catabolism and BCAA Function
5.1. Mechanisms Responsible for Regulation of BCAA Catabolism
5.1.1. BCKDK
5.1.2. PPM1K
5.1.3. AMPK
5.1.4. SGLT2 Inhibitor and L-Type Amino Acid Transporter (LAT1)
5.2. Mechanisms of BCAA Function
5.2.1. PI3K-Akt Pathway
5.2.2. mTOR Pathway
5.2.3. Other Pathways
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- DCCT/EDIC Research Group; Nathan, D.M.; Bebu, I.; Hainsworth, D.; Klein, R.; Tamborlane, W.; Lorenzi, G.; Gubitosi-Klug, R.; Lachin, J.M. Frequency of Evidence-Based Screening for Retinopathy in Type 1 Diabetes. N. Engl. J. Med. 2017, 376, 1507–1516. [Google Scholar]
- Fu, Z.; Usui-Ouchi, A.; Allen, W.; Tomita, Y. Retinal Disease and Metabolism. Life 2022, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Simó-Servat, O.; Hernández, C.; Simó, R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic. Res. 2019, 62, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simó, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Jian, Q.; Wu, Y.; Zhang, F. Metabolomics in Diabetic Retinopathy: From Potential Biomarkers to Molecular Basis of Oxidative Stress. Cells 2022, 11, 3005. [Google Scholar] [CrossRef]
- Grauslund, J. Vascular endothelial growth factor inhibition for proliferative diabetic retinopathy: Et tu, Brute? Acta Ophthalmol. 2017, 95, 757–758. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Casten, R.J.; Rovner, B.W. Update on depression and age-related macular degeneration. Curr. Opin. Ophthalmol. 2013, 24, 239–243. [Google Scholar] [CrossRef]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef]
- Ferris, F.L.; Fine, S.L.; Hyman, L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Coorey, N.J.; Zhang, M.; Zeng, S.; Madigan, M.C.; Zhang, X.; Gillies, M.C.; Zhu, L.; Zhang, T. Metabolism Dysregulation in Retinal Diseases and Related Therapies. Antioxidants 2022, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhang, F. Amino Acids Metabolism in Retinopathy: From Clinical and Basic Research Perspective. Metabolites 2022, 12, 1244. [Google Scholar] [CrossRef]
- Davis, T.A.; Fiorotto, M.L.; Reeds, P.J. Amino acid compositions of body and milk protein change during the suckling period in rats. J. Nutr. 1993, 123, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.D.; Jang, C.; Hui, S.; Murashige, D.S.; Chu, Q.; Morscher, R.J.; Li, X.; Zhan, L.; White, E.; Anthony, T.G.; et al. Quantitative Analysis of the Whole-Body Metabolic Fate of Branched-Chain Amino Acids. Cell Metab. 2019, 29, 417–429 e4. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jian, Q.; Hu, G.; Du, R.; Xu, X.; Zhang, F. Integrated Metabolomics and Transcriptomics Reveal Metabolic Patterns in Retina of STZ-Induced Diabetic Retinopathy Mouse Model. Metabolites 2022, 12, 1245. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, R.; Wei, F.; Fang, J.; Zhang, J.; Sun, J.; Sun, Q.; Wang, H. SGLT2 inhibitor-empagliflozin treatment ameliorates diabetic retinopathy manifestations and exerts protective effects associated with augmenting branched chain amino acids catabolism and transportation in db/db mice. Biomed. Pharmacother. 2022, 152, 113222. [Google Scholar] [CrossRef]
- Ola, M.S.; Alhomida, A.S.; LaNoue, K.F. Gabapentin Attenuates Oxidative Stress and Apoptosis in the Diabetic Rat Retina. Neurotox. Res. 2019, 36, 81–90. [Google Scholar] [CrossRef]
- Ichihara, A.; Koyama, E. Transaminase of branched chain amino acids. I. Branched chain amino acids-alpha-ketoglutarate transaminase. J. Biochem. 1966, 59, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A. Isozyme patterns of branched-chain amino acid transaminase during cellular differentiation and carcinogenesis. Ann. N. Y. Acad. Sci. 1975, 259, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Shinno, H.; Ichihara, A. Isozyme patterns of branched-chain amino acid transaminase in human tissues and tumors. Gan 1977, 68, 663–667. [Google Scholar] [PubMed]
- Johnson, W.A.; Connelly, J.L. Cellular localization and characterization of bovine liver branched-chain -keto acid dehydrogenases. Biochemistry 1972, 11, 1967–1973. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Rochfort, S. Metabolomics reviewed: A new “omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod. 2005, 68, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Jüppner, J.; Mubeen, U.; Leisse, A.; Caldana, C.; Brust, H.; Steup, M.; Herrmann, M.; Steinhauser, D.; Giavalisco, P. Dynamics of lipids and metabolites during the cell cycle of Chlamydomonas reinhardtii. Plant J. 2017, 92, 331–343. [Google Scholar] [CrossRef]
- Li, Q.; Wei, S.; Wu, D.; Wen, C.; Zhou, J. Urinary Metabolomics Study of Patients with Gout Using Gas Chromatography-Mass Spectrometry. Biomed. Res. Int. 2018, 2018, 3461572. [Google Scholar] [CrossRef]
- Frayser, R.; Buse, M.G. Branched chain amino acid metabolism in the retina of diabetic rats. Diabetologia 1978, 14, 171–176. [Google Scholar] [CrossRef]
- Valle, D.; Walser, M.; Brusilow, S.W.; Kaiser-Kupfer, M. Gyrate atrophy of the choroid and retina: Amino acid metabolism and correction of hyperornithinemia with an arginine-deficient diet. J. Clin. Investig. 1980, 65, 371–378. [Google Scholar] [CrossRef]
- Masser, D.R.; Starkey, H.D.V.; Bixler, G.V.; Dunton, W.; Bronson, S.K.; Freeman, W.M. Insulin treatment normalizes retinal neuroinflammation but not markers of synapse loss in diabetic rats. Exp. Eye Res. 2014, 125, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Socha, O.; Dračínský, M. Dimerization of Acetic Acid in the Gas Phase-NMR Experiments and Quantum-Chemical Calculations. Molecules 2020, 25, 2150. [Google Scholar] [CrossRef] [PubMed]
- Chetwynd, A.J.; Dunn, W.B.; Rodriguez-Blanco, G. Collection and Preparation of Clinical Samples for Metabolomics. Adv. Exp. Med. Biol. 2017, 965, 19–44. [Google Scholar] [PubMed]
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Möller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef]
- Holekamp, N.M. The vitreous gel: More than meets the eye. Am. J. Ophthalmol. 2010, 149, 32–36. [Google Scholar] [CrossRef]
- Yalcinbayir, O.; Buyukuysal, R.L.; Gelisken, O.; Buyukuysal, C.; Can, B. Amino acid and vascular endothelial growth factor levels in subretinal fluid in rhegmatogenous retinal detachment. Mol. Vis. 2014, 20, 1357–1365. [Google Scholar]
- Bertram, K.M.; Bula, D.V.; Pulido, J.S.; Shippy, S.A.; Gautam, S.; Lu, M.J.; Hatfield, R.M.; Kim, J.H.; Quirk, M.T.; Arroyo, J.G. Amino-acid levels in subretinal and vitreous fluid of patients with retinal detachment. Eye 2008, 22, 582–589. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Chen, F.; Sun, Q.; Xu, X.; Lin, S.H.; Liu, K. Metabolomic profile of diabetic retinopathy: A GC-TOFMS-based approach using vitreous and aqueous humor. Acta Diabetol. 2020, 57, 41–51. [Google Scholar] [CrossRef]
- Xuan, Q.; Ouyang, Y.; Wang, Y.; Wu, L.; Li, H.; Luo, Y.; Zhao, X.; Feng, D.; Qin, W.; Hu, C.; et al. Multiplatform Metabolomics Reveals Novel Serum Metabolite Biomarkers in Diabetic Retinopathy Subjects. Adv. Sci. 2020, 7, 2001714. [Google Scholar] [CrossRef]
- Mendez, K.M.; Kim, J.; Laíns, I.; Nigalye, A.; Katz, R.; Pundik, S.; Kim, I.K.; Liang, L.; Vavvas, D.G.; Miller, J.B.; et al. Association of Human Plasma Metabolomics with Delayed Dark Adaptation in Age-Related Macular Degeneration. Metabolites 2021, 11, 183. [Google Scholar] [CrossRef]
- Lin, H.-T.; Cheng, M.-L.; Lo, C.-J.; Lin, G.; Lin, S.-F.; Yeh, J.-T.; Ho, H.-Y.; Lin, J.-R.; Liu, F.-C. 1H Nuclear Magnetic Resonance (NMR)-Based Cerebrospinal Fluid and Plasma Metabolomic Analysis in Type 2 Diabetic Patients and Risk Prediction for Diabetic Microangiopathy. J. Clin. Med. 2019, 8, 874. [Google Scholar] [CrossRef] [PubMed]
- Gowda, K.; Zinnanti, W.J.; LaNoue, K.F. The influence of diabetes on glutamate metabolism in retinas. J. Neurochem. 2011, 117, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tan, W.; Zou, J.; Cao, J.; Huang, Q.; Jiang, B.; Yoshida, S.; Li, Y. Metabolomics Analyses of Mouse Retinas in Oxygen-Induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2021, 62, 9. [Google Scholar] [CrossRef] [PubMed]
- Natoli, R.; Fernando, N.; Dahlenburg, T.; Jiao, H.; Aggio-Bruce, R.; Barnett, N.L.; De La Barca, J.M.C.; Tcherkez, G.; Reynier, P.; Fang, J.; et al. Obesity-induced metabolic disturbance drives oxidative stress and complement activation in the retinal environment. Mol. Vis. 2018, 24, 201–217. [Google Scholar] [PubMed]
- Wei, P.; He, M.; Han, G. Metabolic Characterization of Ocular Tissues in Relation to Laser-Induced Choroidal Neovascularization in Rats. J. Proteome Res. 2022, 21, 2979–2986. [Google Scholar] [CrossRef]
- Xu, R.; Ritz, B.K.; Wang, Y.; Huang, J.; Zhao, C.; Gong, K.; Liu, X.; Du, J. The retina and retinal pigment epithelium differ in nitrogen metabolism and are metabolically connected. J. Biol. Chem. 2020, 295, 2324–2335. [Google Scholar] [CrossRef]
- Gibson, C.J. Diurnal alterations in retinal tyrosine level and dopamine turnover in diabetic rats. Brain. Res. 1988, 454, 60–66. [Google Scholar] [CrossRef]
- Elmi, A.; Ventrella, D.; Laghi, L.; Carnevali, G.; Zhu, C.; Pertile, G.; Barone, F.; Benfenati, F.; Bacci, M.L. 1H NMR Spectroscopy Characterization of Porcine Vitreous Humor in Physiological and Photoreceptor Degeneration Conditions. Investig. Ophthalmol. Vis. Sci. 2019, 60, 741–747. [Google Scholar] [CrossRef]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef]
- Lieth, E.; LaNoue, K.F.; Berkich, D.A.; Xu, B.; Ratz, M.; Taylor, C.; Hutson, S.M. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J. Neurochem. 2001, 76, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Contrusciere, V.; Paradisi, S.; Matteucci, A.; Malchiodi-Albedi, F. Branched-chain amino acids induce neurotoxicity in rat cortical cultures. Neurotox. Res. 2010, 17, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wei, X.Y.; Yang, A.A.; Liu, Z.; Wang, S.Q.; You, Y.Y.; Kuang, F.; You, S.W.; Wu, M.M. Branched-Chain Amino Acids Enhance Retinal Ganglion Cell Survival and Axon Regeneration after Optic Nerve Transection in Rats. Curr. Eye Res. 2018, 43, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Ikeda, H.O.; Iwai, S.; Muraoka, Y.; Tsuruyama, T.; Okamoto-Furuta, K.; Kohda, H.; Kakizuka, A.; Yoshimura, N. Branched chain amino acids attenuate major pathologies in mouse models of retinal degeneration and glaucoma. Heliyon 2018, 4, e00544. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T.; Mitala, C.M.; Kundu, S.; Verma, A.; Elkind, J.A.; Nissim, I.; Cohen, A.S. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc. Natl. Acad. Sci. USA 2010, 107, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Jeter, C.B.; Hergenroeder, G.W.; Ward, N.H., III; Moore, A.N.; Dash, P.K. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J. Neurotrauma 2013, 30, 671–679. [Google Scholar] [CrossRef]
- Mattson, M.P. Neuroprotective signal transduction, relevance to stroke. Neurosci. Biobehav. Rev. 1997, 21, 193–206. [Google Scholar] [CrossRef]
- Paschen, W.; Frandsen, A. Endoplasmic reticulum dysfunction--a common denominator for cell injury in acute and degenerative diseases of the brain? J. Neurochem. 2001, 79, 719–725. [Google Scholar] [CrossRef]
- Zhu, L.; Zang, J.; Liu, B.; Yu, G.; Hao, L.; Liu, L.; Zhong, J. Oxidative stress-induced RAC autophagy can improve the HUVEC functions by releasing exosomes. J. Cell. Physiol. 2020, 235, 7392–7409. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox. Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sun, G.F.; Pan, X.A.; Qu, X.H.; Yang, P.; Chen, Z.P.; Han, X.J.; Wang, T. BCATc inhibitor 2 ameliorated mitochondrial dysfunction and apoptosis in oleic acid-induced non-alcoholic fatty liver disease model. Front. Pharmacol. 2022, 13, 1025551. [Google Scholar] [CrossRef] [PubMed]
- Lian, K.; Guo, X.; Wang, Q.; Liu, Y.; Wang, R.-T.; Gao, C.; Li, G.-Y.; Li, C.-X.; Tao, L. PP2Cm overexpression alleviates MI/R injury mediated by a BCAA catabolism defect and oxidative stress in diabetic mice. Eur. J. Pharmacol. 2020, 866, 172796. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; Sun, S.-J.; Cao, W.-X.; Lan, X.-T.; Ni, M.; Fu, H.; Li, D.-J.; Wang, P.; Shen, F.-M. Excessive ROS production and enhanced autophagy contribute to myocardial injury induced by branched-chain amino acids: Roles for the AMPK-ULK1 signaling pathway and α7nAChR. Biochim. Biophys. Acta Mol. Basis. Dis. 2021, 1867, 165980. [Google Scholar] [CrossRef]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, R.; Mora, S.; Lawler, P.R.; Cook, N.R.; Ridker, P.M.; Buring, J.E.; Lee, I.-M.; Manson, J.E.; Tobias, D.K. Association of Plasma Branched-Chain Amino Acid With Biomarkers of Inflammation and Lipid Metabolism in Women. Circ. Genom. Precis. Med. 2021, 14, e003330. [Google Scholar] [CrossRef]
- Sorrentino, F.S.; Allkabes, M.; Salsini, G.; Bonifazzi, C.; Perri, P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. 2016, 162, 54–59. [Google Scholar] [CrossRef]
- Boss, J.D.; Singh, P.K.; Pandya, H.K.; Tosi, J.; Kim, C.; Tewari, A.; Juzych, M.S.; Abrams, G.W.; Kumar, A. Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients With Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5594–5603. [Google Scholar] [CrossRef]
- Nozaki, M.; Raisler, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Bogl, L.H.; Kaye, S.M.; Rämö, J.T.; Kangas, A.J.; Soininen, P.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Ortega-Alonso, A.; Rissanen, A.; et al. Abdominal obesity and circulating metabolites: A twin study approach. Metabolism 2016, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hu, C.; Yang, R.; Lv, Y.; Yuan, H.; Liang, Q.; He, B.; Pang, G.; Jiang, M.; Dong, J.; et al. Association of circulating branched-chain amino acids with cardiometabolic traits differs between adults and the oldest-old. Oncotarget 2017, 8, 88882–88893. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Sananbenesi, F.; Wang, X.; Dobbin, M.; Tsai, L.-H. Recovery of learning and memory is associated with chromatin remodelling. Nature 2007, 447, 178–182. [Google Scholar] [CrossRef]
- Heyward, F.D.; Gilliam, D.; Coleman, M.A.; Gavin, C.F.; Wang, J.; Kaas, G.; Trieu, R.; Lewis, J.; Moulden, J.; Sweatt, J.D.; et al. Obesity Weighs down Memory through a Mechanism Involving the Neuroepigenetic Dysregulation of Sirt1. J. Neurosci. 2016, 36, 1324–1335. [Google Scholar] [CrossRef]
- De Simone, R.; Vissicchio, F.; Mingarelli, C.; De Nuccio, C.; Visentin, S.; Ajmone-Cat, M.A.; Minghetti, L. Branched-chain amino acids influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals. Biochim. Biophys. Acta 2013, 1832, 650–659. [Google Scholar] [CrossRef]
- Rosa, L.; Scaini, G.; Furlanetto, C.B.; Galant, L.S.; Vuolo, F.; Dall’Igna, D.M.; Schuck, P.F.; Ferreira, G.C.; Dal-Pizzol, F.; Streck, E.L. Administration of branched-chain amino acids alters the balance between pro-inflammatory and anti-inflammatory cytokines. Int. J. Dev. Neurosci. 2016, 48, 24–30. [Google Scholar] [CrossRef]
- Feijo GD, S.; Jantsch, J.; Correia, L.L.; Eller, S.; Furtado-Filho, O.V.; Giovenardi, M.; Porawski, M.; Braganhol, E.; Guedes, R.P. Neuroinflammatory responses following zinc or branched-chain amino acids supplementation in obese rats. Metab. Brain Dis. 2022, 37, 1875–1886. [Google Scholar] [CrossRef]
- Murgas Torrazza, R.; Suryawan, A.; Gazzaneo, M.C.; Orellana, R.A.; Frank, J.W.; Nguyen, H.V.; Fiorotto, M.L.; El-Kadi, S.; Davis, T.A. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J. Nutr. 2010, 140, 2145–2152. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Wang, W.; Guo, Q.; Wen, C.; Yin, Y. Alteration of muscle fiber characteristics and the AMPK-SIRT1-PGC-1α axis in skeletal muscle of growing pigs fed low-protein diets with varying branched-chain amino acid ratios. Oncotarget 2017, 8, 107011–107021. [Google Scholar] [CrossRef]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Macotela, Y.; Emanuelli, B.; Bång, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary leucine—An environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 2011, 6, e21187. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Lapworth, A.L.; An, J.; Wang, L.; McGarrah, R.W.; Stevens, R.D.; Ilkayeva, O.; George, T.; Muehlbauer, M.J.; Bain, J.R.; et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016, 5, 538–551. [Google Scholar] [CrossRef]
- Chuang, J.L.; Wynn, R.M.; Chuang, D.T. The C-terminal hinge region of lipoic acid-bearing domain of E2b is essential for domain interaction with branched-chain alpha-keto acid dehydrogenase kinase. J. Biol. Chem. 2002, 277, 36905–36908. [Google Scholar] [CrossRef]
- Tso, S.-C.; Qi, X.; Gui, W.-J.; Chuang, J.L.; Morlock, L.K.; Wallace, A.L.; Ahmed, K.; Laxman, S.; Campeau, P.M.; Lee, B.H.; et al. Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain α-ketoacid dehydrogenase kinase. Proc. Natl. Acad. Sci. USA 2013, 110, 9728–9733. [Google Scholar] [CrossRef]
- Burrage, L.C.; Nagamani, S.C.; Campeau, P.M.; Lee, B.H. Branched-chain amino acid metabolism: From rare Mendelian diseases to more common disorders. Hum. Mol. Genet. 2014, 23, R1-8. [Google Scholar] [CrossRef]
- Maguolo, A.; Rodella, G.; Giorgetti, A.; Nicolodi, M.; Ribeiro, R.; Dianin, A.; Cantalupo, G.; Monge, I.; Carcereri, S.; De Bernardi, M.L.; et al. A Gain-of-Function Mutation on BCKDK Gene and Its Possible Pathogenic Role in Branched-Chain Amino Acid Metabolism. Genes 2022, 13, 233. [Google Scholar] [CrossRef]
- Oyarzabal, A.; Martínez-Pardo, M.; Merinero, B.; Navarrete, R.; Desviat, L.R.; Ugarte, M.; Rodríguez-Pombo, P. A novel regulatory defect in the branched-chain α-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum. Mutat. 2013, 34, 355–362. [Google Scholar] [CrossRef]
- Lotta, L.A.; Scott, R.A.; Sharp, S.J.; Burgess, S.; Luan, J.; Tillin, T.; Schmidt, A.F.; Imamura, F.; Stewart, I.D.; Perry, J.R.B. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS Med. 2016, 13, e1002179. [Google Scholar] [CrossRef]
- Xuan, L.; Hou, Y.; Wang, T.; Li, M.; Zhao, Z.; Lu, J.; Xu, Y.; Chen, Y.; Qi, L.; Wang, W.; et al. Association of branched chain amino acids related variant rs1440581 with risk of incident diabetes and longitudinal changes in insulin resistance in Chinese. Acta Diabetol. 2018, 55, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Bao, S.; Zhang, C.; Zhang, J.; Lv, J.; Li, X.; Chudhary, M.; Ren, X.; Kong, L. Stimulation of AMPK Prevents Diabetes-Induced Photoreceptor Cell Degeneration. Oxid. Med. Cell Longev. 2021, 2021, 5587340. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr. 2004, 134 (Suppl. S6), 1583s–1587s. [Google Scholar] [CrossRef] [PubMed]
- Lian, K.; Du, C.; Liu, Y.; Zhu, D.; Yan, W.; Zhang, H.; Hong, Z.; Liu, P.; Zhang, L.; Pei, H.; et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice. Diabetes 2015, 64, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Uchitomi, R.; Hatazawa, Y.; Miura, S.; Kamei, Y. Metabolomic analysis on blood of transgenic mice overexpressing PGC-1α in skeletal muscle. Biosci. Biotechnol. Biochem. 2021, 85, 579–586. [Google Scholar] [CrossRef]
- Hatazawa, Y.; Tadaishi, M.; Nagaike, Y.; Morita, A.; Ogawa, Y.; Ezaki, O.; Takai-Igarashi, T.; Kitaura, Y.; Shimomura, Y.; Kamei, Y.; et al. PGC-1α-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS ONE 2014, 9, e91006. [Google Scholar] [CrossRef]
- Tomi, M.; Mori, M.; Tachikawa, M.; Katayama, K.; Terasaki, T.; Hosoya, K.I. L-type amino acid transporter 1-mediated L-leucine transport at the inner blood-retinal barrier. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2522–2530. [Google Scholar] [CrossRef]
- Akanuma, S.-I.; Yamakoshi, A.; Sugouchi, T.; Kubo, Y.; Hartz, A.M.S.; Bauer, B.; Hosoya, K.-I. Role of l-Type Amino Acid Transporter 1 at the Inner Blood-Retinal Barrier in the Blood-to-Retina Transport of Gabapentin. Mol. Pharm. 2018, 15, 2327–2337. [Google Scholar] [CrossRef]
- Huang, P.; Frohman, M.A. The role of phospholipase D in Glut-4 translocation. Diabetes Metab. Res. Rev. 2003, 19, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.Y.; Crary, J.F.; Rao, C.; Sacktor, T.C.; Mirra, S.S. A typical protein kinase C in neurodegenerative disease II: PKCiota/lambda in tauopathies and alpha-synucleinopathies. J. Neuropathol. Exp. Neurol. 2006, 65, 327–335. [Google Scholar] [CrossRef]
- Thong, F.S.; Bilan, P.J.; Klip, A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 2007, 56, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.T.; Yang, C.M.; Yang, C.H. Astaxanthin Protects Retinal Photoreceptor Cells against High Glucose-Induced Oxidative Stress by Induction of Antioxidant Enzymes via the PI3K/Akt/Nrf2 Pathway. Antioxidants 2020, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Janani, R.; Anitha, R.E.; Divya, P.; Chonche, M.; Baskaran, V. Astaxanthin ameliorates hyperglycemia induced inflammation via PI3K/Akt-NF-κB signaling in ARPE-19 cells and diabetic rat retina. Eur. J. Pharmacol. 2022, 926, 174979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ren, M.; Zeng, X.; He, P.; Ma, X.; Qiao, S. Leucine stimulates ASCT2 amino acid transporter expression in porcine jejunal epithelial cell line (IPEC-J2) through PI3K/Akt/mTOR and ERK signaling pathways. Amino Acids 2014, 46, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, W.; Shao, J.; Wang, Y.; Zhou, M.; Sun, H. Branched-Chain Amino Acid Negatively Regulates KLF15 Expression via PI3K-AKT Pathway. Front. Physiol. 2017, 8, 853. [Google Scholar] [CrossRef]
- Kimball, S.R. Regulation of translation initiation by amino acids in eukaryotic cells. Prog. Mol. Subcell. Biol. 2001, 26, 155–184. [Google Scholar]
- Towle, H.C. The metabolic sensor GCN2 branches out. Cell Metab. 2007, 5, 85–87. [Google Scholar] [CrossRef]
- Bonvini, A.; Coqueiro, A.Y.; Tirapegui, J.; Calder, P.C.; Rogero, M.M. Immunomodulatory role of branched-chain amino acids. Nutr. Rev. 2018, 76, 840–856. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Sabatini, D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005, 17, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Avet-Rochex, A.; Carvajal, N.; Christoforou, C.P.; Yeung, K.; Maierbrugger, K.T.; Hobbs, C.; Lalli, G.; Cagin, U.; Plachot, C.; McNeill, H.; et al. Unkempt is negatively regulated by mTOR and uncouples neuronal differentiation from growth control. PLoS Genet. 2014, 10, e1004624. [Google Scholar] [CrossRef]
- Teotia, P.; Van Hook, M.J.; Fischer, D.; Ahmad, I. Human retinal ganglion cell axon regeneration by recapitulating developmental mechanisms: Effects of recruitment of the mTOR pathway. Development 2019, 146, dev178012. [Google Scholar] [CrossRef] [PubMed]
- Yagasaki, R.; Nakahara, T.; Mori, A.; Sakamoto, K.; Ishii, K. Effects of mTOR inhibition on normal retinal vascular development in the mouse. Exp. Eye Res. 2014, 129, 127–134. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Martina, J.A.; Puertollano, R. Rag GTPases mediate amino acid-dependent recruitment of TFEB and MITF to lysosomes. J. Cell Biol. 2013, 200, 475–491. [Google Scholar] [CrossRef]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef]
- Balka, K.R.; Louis, C.; Saunders, T.L.; Smith, A.M.; Calleja, D.J.; D’Silva, D.B.; Moghaddas, F.; Tailler, M.; Lawlor, K.E.; Zhan, Y.; et al. TBK1 and IKKε Act Redundantly to Mediate STING-Induced NF-κB Responses in Myeloid Cells. Cell Rep. 2020, 31, 107492. [Google Scholar] [CrossRef]
- Mao, X.; Sun, R.; Wang, Q.; Chen, D.; Yu, B.; He, J.; Yu, J.; Luo, J.; Luo, Y.; Yan, H.; et al. l-Isoleucine Administration Alleviates DSS-Induced Colitis by Regulating TLR4/MyD88/NF-κB Pathway in Rats. Front. Immunol. 2021, 12, 817583. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, S.; Liu, X.; Li, S.; Mao, X.; Zeng, X.; Qiao, S. Different Lipopolysaccharide Branched-Chain Amino Acids Modulate Porcine Intestinal Endogenous β-Defensin Expression through the Sirt1/ERK/90RSK Pathway. J. Agric. Food Chem. 2016, 64, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, Y. A new branch connecting thermogenesis and diabetes. Nat. Metab. 2019, 1, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xia, M.; Wu, Y.; Zhang, F. Branched-Chain Amino Acids Metabolism and Their Roles in Retinopathy: From Relevance to Mechanism. Nutrients 2023, 15, 2161. https://doi.org/10.3390/nu15092161
Zhang X, Xia M, Wu Y, Zhang F. Branched-Chain Amino Acids Metabolism and Their Roles in Retinopathy: From Relevance to Mechanism. Nutrients. 2023; 15(9):2161. https://doi.org/10.3390/nu15092161
Chicago/Turabian StyleZhang, Xiaonan, Mengxue Xia, Yingjie Wu, and Fang Zhang. 2023. "Branched-Chain Amino Acids Metabolism and Their Roles in Retinopathy: From Relevance to Mechanism" Nutrients 15, no. 9: 2161. https://doi.org/10.3390/nu15092161
APA StyleZhang, X., Xia, M., Wu, Y., & Zhang, F. (2023). Branched-Chain Amino Acids Metabolism and Their Roles in Retinopathy: From Relevance to Mechanism. Nutrients, 15(9), 2161. https://doi.org/10.3390/nu15092161







