A Narrative Review of Factors Associated with Skin Carotenoid Levels
Abstract
1. Introduction
2. Methods
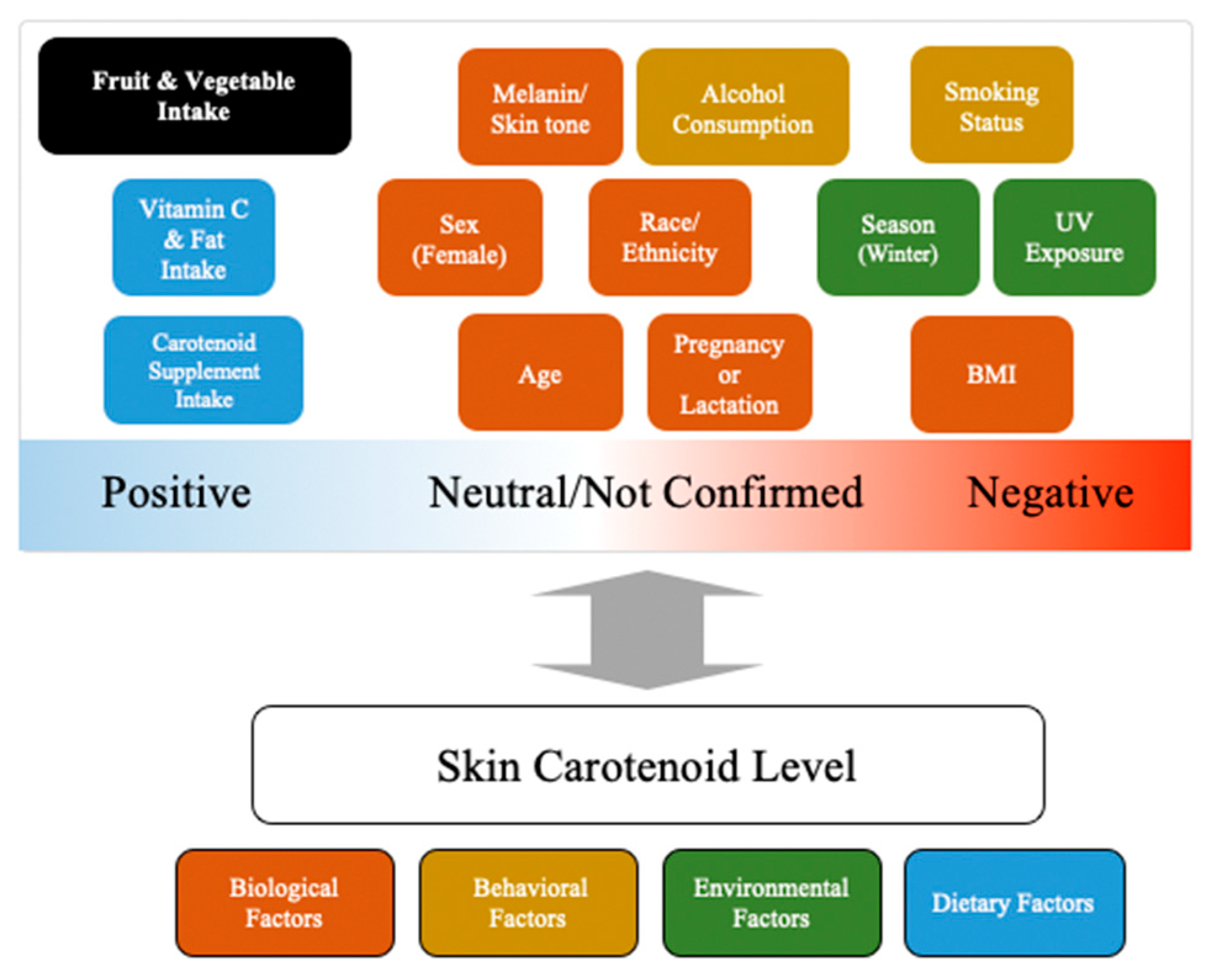
3. Factors/Covariates Affecting Skin Carotenoid Levels
4. Summary of Factors Affecting Skin Carotenoids and Comparison to Findings for Blood Carotenoids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnotti, K.; Bamber, M. Fruit and Vegetable Consumption in Overweight or Obese Individuals: A Meta-Analysis. West. J. Nurs. Res. 2020, 42, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Lunet, N.; Lacerda-Vieira, A.; Barros, H. Fruit and vegetables consumption and gastric cancer: A systematic review and meta-analysis of cohort studies. Nutr. Cancer 2005, 53, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Angelino, D.; Godos, J.; Ghelfi, F.; Tieri, M.; Titta, L.; Lafranconi, A.; Marventano, S.; Alonzo, E.; Gambera, A.; Sciacca, S.; et al. Fruit and vegetable consumption and health outcomes: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2019, 70, 652–667. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Barroso, S.; Trius-Soler, M.; Lamuela-Raventós, R.M.; Zamora-Ros, R. Vegetable and Fruit Consumption and Prognosis Among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Adv. Nutr. 2020, 11, 1569–1582. [Google Scholar] [CrossRef]
- Farvid, M.S.; Barnett, J.B.; Spence, N.D. Fruit and vegetable consumption and incident breast cancer: A systematic review and meta-analysis of prospective studies. Br. J. Cancer 2021, 125, 284–298. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Vingeliene, S.; Chan, D.S.M.; Aune, D.; Navarro-Rosenblatt, D.; Stevens, C.; Greenwood, D.; Norat, T. Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 81–96. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D.; He, Y. Fruit and vegetables consumption and incident hypertension: Dose–response meta-analysis of prospective cohort studies. J. Hum. Hypertens. 2016, 30, 573–580. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D.; Tan, Y. Intake of Fruit and Vegetables and the Incident Risk of Cognitive Disorders: A Systematic Review and Meta-Analysis of Cohort Studies. J. Nutr. Health Aging 2017, 21, 1284–1290. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality: Results from 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Pem, D.; Jeewon, R. Fruit and Vegetable Intake: Benefits and Progress of Nutrition Education Interventions- Narrative Review Article. Iran. J. Public Health 2015, 44, 1309–1321. [Google Scholar] [PubMed]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Shi, H.; Wang, K.; Wang, X.; Yu, N.; Guo, B. The Associations of Plasma/Serum Carotenoids with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2021, 82, 1055–1066. [Google Scholar] [CrossRef]
- Jiang, Y.-W.; Sun, Z.-H.; Tong, W.-W.; Yang, K.; Guo, K.-Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021, 12, 1723–1733. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Moore, L.V.; Thompson, F.E.; Demissie, Z. Percentage of Youth Meeting Federal Fruit and Vegetable Intake Recommendations, Youth Risk Behavior Surveillance System, United States and 33 States, 2013. J. Acad. Nutr. Diet. 2017, 117, 545–553.e3. [Google Scholar] [CrossRef]
- Lee, S.H.; Moore, L.V.; Park, S.; Harris, D.M.; Blanck, H.M. Adults Meeting Fruit and Vegetable Intake Recommendations—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef]
- Hebert, J.R.; Clemow, L.; Pbert, L.; Ockene, I.S.; Ockene, J.K. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int. J. Epidemiol. 1995, 24, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Naska, A.; Lagiou, A.; Lagiou, P. Dietary assessment methods in epidemiological research: Current state of the art and future prospects. F1000Research 2017, 6, 926. [Google Scholar] [CrossRef] [PubMed]
- Mayne, S.T.; Cartmel, B.; Scarmo, S.; Lin, H.; Leffell, D.J.; Welch, E.; Ermakov, I.; Bhosale, P.; Bernstein, P.S.; Gellermann, W. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am. J. Clin. Nutr. 2010, 92, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000.
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Radtke, M.D.; Pitts, S.J.; Jahns, L.; Firnhaber, G.C.; Loofbourrow, B.M.; Zeng, A.; Scherr, R.E. Criterion-Related Validity of Spectroscopy-Based Skin Carotenoid Measurements as a Proxy for Fruit and Vegetable Intake: A Systematic Review. Adv. Nutr. 2020, 11, 1282–1299. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Gellermann, W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J. Biophotonics 2012, 5, 559–570. [Google Scholar] [CrossRef]
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W., Jr.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 1600685. [Google Scholar] [CrossRef]
- Allore, T.; Lemieux, S.; Vohl, M.-C.; Couture, P.; Lamarche, B.; Couillard, C. Correlates of the difference in plasma carotenoid concentrations between men and women. Br. J. Nutr. 2019, 121, 172–181. [Google Scholar] [CrossRef]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef]
- Djuric, Z.; Bassis, C.M.; Plegue, M.A.; Ren, J.; Chan, R.; Sidahmed, E.; Turgeon, D.K.; Ruffin, M.T.T.; Kato, I.; Sen, A. Colonic Mucosal Bacteria Are Associated with Inter-Individual Variability in Serum Carotenoid Concentrations. J. Acad. Nutr. Diet. 2018, 118, 606–616.e3. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L.; Hullar, M.A.J.; Maskarinec, G.; Monroe, K.R.; Shepherd, J.A.; Franke, A.A.; Randolph, T.W.; Wilkens, L.R.; Boushey, C.J.; Le Marchand, L.; et al. The Gut Microbiome Is Associated with Circulating Dietary Biomarkers of Fruit and Vegetable Intake in a Multiethnic Cohort. J. Acad. Nutr. Diet. 2022, 122, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda-Muñoz, M.; de Alvarenga, J.F.R.; Hernáez, Á.; Tresserra-Rimbau, A.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Malcampo, M.; Martínez, J.A.; Alonso-Gómez, Á.M.; et al. High Fruit and Vegetable Consumption and Moderate Fat Intake Are Associated with Higher Carotenoid Concentration in Human Plasma. Antioxidants 2021, 10, 473. [Google Scholar] [CrossRef]
- Ntanios, F.Y.; Duchateau, G.S.M.J.E. A healthy diet rich in carotenoids is effective in maintaining normal blood carotenoid levels during the daily use of plant sterol-enriched spreads. Int. J. Vitam. Nutr. Res. 2002, 72, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla-Alonso, B.; Beltrán-de-Miguel, B.; Estévez-Santiago, R.; Cuadrado-Vives, C. Markers of lutein and zeaxanthin status in two age groups of men and women: Dietary intake, serum concentrations, lipid profile and macular pigment optical density. Nutr. J. 2014, 13, 52. [Google Scholar] [CrossRef]
- Palli, D.; Decarli, A.; Russo, A.; Cipriani, F.; Giacosa, A.; Amadori, D.; Salkeld, R.; Salvini, S.; Buiatti, E. Plasma levels of antioxidant vitamins and cholesterol in a large population sample in central-northern Italy. Eur. J. Nutr. 1999, 38, 90–98. [Google Scholar] [CrossRef]
- Tsubono, Y.; Tsugane, S.; Gey, K.F. Differential effects of cigarette smoking and alcohol consumption on the plasma levels of carotenoids in middle-aged Japanese men. Jpn. J. Cancer Res. 1996, 87, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, I. Impact of Human Faecal Microbial Communities on Carotenoid Release from Taxo and Carrot Juice. Master’s Thesis, Ghent University, Ghent, Belgium, 2017. [Google Scholar]
- Van der Gaag, M.S.; van den Berg, R.; van den Berg, H.; Schaafsma, G.; Hendriks, H.F.J. Moderate consumption of beer, red wine and spirits has counteracting effects on plasma antioxidants in middle-aged men. Eur. J. Clin. Nutr. 2000, 54, 586–591. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef]
- Wu, K.; Schwartz, S.J.; Platz, E.A.; Clinton, S.K.; Erdman, J.W., Jr.; Ferruzzi, M.G.; Willett, W.C.; Giovannucci, E.L. Variations in plasma lycopene and specific isomers over time in a cohort of U.S. men. J. Nutr. 2003, 133, 1930–1936. [Google Scholar] [CrossRef]
- Nguyen, L.M.; Scherr, R.E.; Linnell, J.D.; Ermakov, I.V.; Gellermann, W.; Jahns, L.; Keen, C.L.; Miyamoto, S.; Steinberg, F.M.; Young, H.M.; et al. Evaluating the relationship between plasma and skin carotenoids and reported dietary intake in elementary school children to assess fruit and vegetable intake. Arch. Biochem. Biophys. 2015, 572, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, S.S.; Wengreen, H.J.; Lefevre, M.; Madden, G.J.; Gast, J. Skin carotenoids: A biomarker of fruit and vegetable intake in children. J. Acad. Nutr. Diet. 2014, 114, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Scarmo, S.; Henebery, K.; Peracchio, H.; Cartmel, B.; Lin, H.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S.; Duffy, V.B.; Mayne, S.T. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. Eur. J. Clin. Nutr. 2012, 66, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Sharifzadeh, M.; Liu, A.; Ermakov, I.; Nelson, K.; Sheng, X.; Panish, C.; Carlstrom, B.; Hoffman, R.O.; Gellermann, W. Blue-Light Reflectance Imaging of Macular Pigment in Infants and Children. Investig. Opthalmol. Vis. Sci. 2013, 54, 4034–4040. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, I.V.; Ermakova, M.R.; Bernstein, P.S.; Chan, G.M.; Gellermann, W. Resonance Raman based skin carotenoid measurements in newborns and infants. J. Biophotonics 2013, 6, 793–802. [Google Scholar] [CrossRef]
- Chan, G.M.; Chan, M.M.; Gellermann, W.; Ermakov, I.; Ermakova, M.; Bhosale, P.; Bernstein, P.; Rau, C. Resonance Raman spectroscopy and the preterm infant carotenoid status. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 556–559. [Google Scholar] [CrossRef]
- Seguin-Fowler, R.A.; Hanson, K.L.; Marshall, G.A.; Belarmino, E.H.; Jilcott Pitts, S.B.; Kolodinsky, J.; Sitaker, M.; Ammerman, A. Fruit and Vegetable Intake Assessed by Repeat 24 h Recalls, but Not by A Dietary Screener, Is Associated with Skin Carotenoid Measurements in Children. Nutrients 2021, 13, 980. [Google Scholar] [CrossRef]
- Smith, E.; Sutarso, T.; Kaye, G.L. Access With Education Improves Fruit and Vegetable Intake in Preschool Children. J. Nutr. Educ. Behav. 2020, 52, 145–151. [Google Scholar] [CrossRef]
- Nagao-Sato, S.; Baltaci, A.; Peralta Reyes, A.O.; Zhang, Y.; Hurtado Choque, G.A.; Reicks, M. Skin Carotenoid Scores Assessed with Reflection Spectroscopy Are Associated with Self-Reported Fruit and Vegetable Intake Among Latino Early Adolescents. J. Acad. Nutr. Diet. 2021, 121, 1507–1514. [Google Scholar] [CrossRef]
- Martinelli, S.; Acciai, F.; Tasevska, N.; Ohri-Vachaspati, P. Using the Veggie Meter in Elementary Schools to Objectively Measure Fruit and Vegetable Intake: A Pilot Study. Methods Protoc. 2021, 4, 33. [Google Scholar] [CrossRef]
- Burkholder, S.; Jilcott Pitts, S.; Wu, Q.; Bayles, J.; Baybutt, R.; Stage, V.C. Skin Carotenoid Status Over Time and Differences by Age and Sex Among Head Start Children (3–5 Years). J. Nutr. Educ. Behav. 2021, 53, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Ahmed, F.; Liu, A.; Allman, S.; Sheng, X.; Sharifzadeh, M.; Ermakov, I.; Gellermann, W. Macular pigment imaging in AREDS2 participants: An ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Investig. Opthalmol. Vis. Sci. 2012, 53, 6178–6186. [Google Scholar] [CrossRef] [PubMed]
- Toh, D.W.K.; Loh, W.W.; Sutanto, C.N.; Yao, Y.; Kim, J.E. Skin carotenoid status and plasma carotenoids: Biomarkers of dietary carotenoids, fruits and vegetables for middle-aged and older Singaporean adults. Br. J. Nutr. 2021, 126, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Meinke, M.C.; Lauer, A.; Taskoparan, B.; Gersonde, I.; Lademann, J.; Darvin, M.E. Influence on the Carotenoid Levels of Skin Arising from Age, Gender, Body Mass Index in Smoking/Non-Smoking Individuals. Free Radic. Antioxid. 2011, 1, 15–20. [Google Scholar] [CrossRef]
- Martina, C.M.; Sabine, S.; Silke, B.L.; Ihar, S.; Maxim, E.D.; Henning, V.; Björn, M.; Wolfang, K.; Jürgen, H.; Jürgen, L. Comparison of different cutaneous carotenoid sensors and influence of age, skin type, and kinetic changes subsequent to intake of a vegetable extract. J. Biomed. Opt. 2016, 21, 107002. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Ermakova, M.; Sharifzadeh, M.; Gorusupudi, A.; Farnsworth, K.; Bernstein, P.S.; Stookey, J.; Evans, J.; Arana, T.; Tao-Lew, L.; et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch. Biochem. Biophys. 2018, 646, 46–54. [Google Scholar] [CrossRef]
- Keller, J.E.; Taylor, M.K.; Smith, A.N.; Littrell, J.; Spaeth, K.; Boeckman, C.R.; Burns, J.M.; Sullivan, D.K. Correlation of Skin Carotenoid Content with 3-Day Dietary Intake in Community Dwelling Older Adults. J. Food Compost. Anal. 2022, 105, 104243. [Google Scholar] [CrossRef]
- Hill, C.M.; Paschall, M.J.; O’Brien, D.M.; Bersamin, A. Characterizing Vegetable and Fruit Intake in a Remote Alaska Native Community Using Reflection Spectroscopy and 24-Hour Recalls. J. Nutr. Educ. Behav. 2021, 53, 712–718. [Google Scholar] [CrossRef]
- Di Noia, J.; Gellermann, W. Use of the Spectroscopy-Based Veggie Meter® to Objectively Assess Fruit and Vegetable Intake in Low-Income Adults. Nutrients 2021, 13, 2270. [Google Scholar] [CrossRef]
- Obana, A.; Gohto, Y.; Gellermann, W.; Ermakov, I.V.; Sasano, H.; Seto, T.; Bernstein, P.S. Skin Carotenoid Index in a large Japanese population sample. Sci. Rep. 2019, 9, 9318. [Google Scholar] [CrossRef]
- McGuirt, J.T.; Jilcott Pitts, S.B.; Gustafson, A. Association between Spatial Access to Food Outlets, Frequency of Grocery Shopping, and Objectively-Assessed and Self-Reported Fruit and Vegetable Consumption. Nutrients 2018, 10, 1974. [Google Scholar] [CrossRef]
- Jilcott Pitts, S.B.; Moran, N.E.; Wu, Q.; Harnack, L.; Craft, N.E.; Hanchard, N.; Bell, R.; Moe, S.G.; Johnson, N.; Obasohan, J.; et al. Pressure-Mediated Reflection Spectroscopy Criterion Validity as a Biomarker of Fruit and Vegetable Intake: A 2-Site Cross-Sectional Study of 4 Racial or Ethnic Groups. J. Nutr. 2022, 152, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.; Amoah, I.; Diep, T.; Jalili-Moghaddam, S. Determinants and Suitability of Carotenoid Reflection Score as a Measure of Carotenoid Status. Nutrients 2020, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Obana, A.; Muto, S.; Asaoka, R.; Tanito, M.; Ermakov, I.V.; Bernstein, P.S.; Gellermann, W. Relationships between Skin Carotenoid Levels and Metabolic Syndrome. Antioxidants 2021, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Darvin, M.E.; Chung, H.S.; Jung, B.; Lee, S.H.; Lenz, K.; Chung, W.S.; Yu, R.X.; Patzelt, A.; Lee, B.N.; et al. Antioxidants in Asian-Korean and caucasian skin: The influence of nutrition and stress. Skin Pharmacol. Physiol. 2014, 27, 293–302. [Google Scholar] [CrossRef]
- Aguilar, S.S.; Wengreen, H.J.; Dew, J. Skin Carotenoid Response to a High-Carotenoid Juice in Children: A Randomized Clinical Trial. J. Acad. Nutr. Diet. 2015, 115, 1771–1778. [Google Scholar] [CrossRef]
- May, K.; Jilcott Pitts, S.; Stage, V.C.; Kelley, C.J.; Burkholder, S.; Fang, X.; Zeng, A.; Lazorick, S. Use of the Veggie Meter® as a tool to objectively approximate fruit and vegetable intake among youth for evaluation of preschool and school-based interventions. J. Hum. Nutr. Diet. 2020, 33, 869–875. [Google Scholar] [CrossRef]
- Takeuchi, J.; Kusunoki, T.; Morimoto, T. Association of Skin Carotenoid Score and Food Intake among School Children: A Multicenter Cross-Sectional Study. J. Nutr. Sci. Vitaminol. 2022, 68, 127–130. [Google Scholar] [CrossRef]
- Fultz, A.K.; Rex, S.M.; Mazelin, A.; McGarry, C.; Brewer, B.; Patterson, F.; Robson, S. Examining fruit and vegetable intake in low-income older adults using the Veggie Meter®. Nutr. Health 2022, 28, 13–17. [Google Scholar] [CrossRef]
- Henley, K.; Reeder, N.; Persell, A.; Tolar-Peterson, T. Fruit and vegetable liking and intake among college students: A cross-sectional study. J. Am. Coll. Health 2021, 1–7. [Google Scholar] [CrossRef]
- Jones, A.; Radtke, M.; Chodur, G.; Scherr, R. Assessing the Relationship Between Nutrition Knowledge and Skin Carotenoids in University Students. Curr. Dev. Nutr. 2020, 4, 1313. [Google Scholar] [CrossRef]
- Matsumoto, M.; Suganuma, H.; Shimizu, S.; Hayashi, H.; Sawada, K.; Tokuda, I.; Ihara, K.; Nakaji, S. Skin Carotenoid Level as an Alternative Marker of Serum Total Carotenoid Concentration and Vegetable Intake Correlates with Biomarkers of Circulatory Diseases and Metabolic Syndrome. Nutrients 2020, 12, 1825. [Google Scholar] [CrossRef] [PubMed]
- Beccarelli, L.M.; Scherr, R.E.; Dharmar, M.; Ermakov, I.V.; Gellermann, W.; Jahns, L.; Linnell, J.D.; Keen, C.L.; Steinberg, F.M.; Young, H.M.; et al. Using Skin Carotenoids to Assess Dietary Changes in Students After 1 Academic Year of Participating in the Shaping Healthy Choices Program. J. Nutr. Educ. Behav. 2017, 49, 73–78.e1. [Google Scholar] [CrossRef]
- Scarmo, S.; Cartmel, B.; Lin, H.; Leffell, D.J.; Ermakov, I.V.; Gellermann, W.; Bernstein, P.S.; Mayne, S.T. Single v. multiple measures of skin carotenoids by resonance Raman spectroscopy as a biomarker of usual carotenoid status. Br. J. Nutr. 2013, 110, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Rerksuppaphol, S.; Rerksuppaphol, L. Effect of fruit and vegetable intake on skin carotenoid detected by non-invasive Raman spectroscopy. J. Med. Assoc. Thail. 2006, 89, 1206–1212. [Google Scholar]
- Li, D.G.; LeCompte, G.; Golod, L.; Cecchi, G.; Irwin, D.; Harken, A.; Matecki, A. Dermal carotenoid measurement is inversely related to anxiety in patients with breast cancer. J. Investig. Med. 2018, 66, 329–333. [Google Scholar] [CrossRef]
- Faraji, B.; Bukowski, M.; Thompson-Johnson, T.; Krusinski, L.; Goldberg, J.; Brooks, C.; Snyder, S. Skin Carotenoid Status of Black/African American College Students Correlates with Plasma Carotenoids and Fruit and Vegetable Intake Independent of Skin Tone. Int. J. Clin. Nutr. Diet. 2022, 8, 161. [Google Scholar] [CrossRef]
- Ermakov, I.V.; Sharifzadeh, M.; Ermakova, M.; Gellermann, W. Resonance Raman detection of carotenoid antioxidants in living human tissue. J. Biomed. Opt. 2005, 10, 064028. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Patzelt, A. Alcohol Consumption Decreases the Protection Efficiency of the Antioxidant Network and Increases the Risk of Sunburn in Human Skin. Skin Pharmacol. Physiol. 2013, 26, 45–51. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Schanzer, S.; Richter, H.; Patzelt, A.; Meinke, M.C.; Zastrow, L.; Golz, K.; Doucet, O.; Sterry, W.; et al. Dermal carotenoid level and kinetics after topical and systemic administration of antioxidants: Enrichment strategies in a controlled in vivo study. J. Dermatol. Sci. 2011, 64, 53–58. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Jungmann, H.; von Laar, J.; Schietzel, M.; Sies, H.; Tronnier, H. Increased Dermal Carotenoid Levels Assessed by Noninvasive Reflection Spectrophotometry Correlate with Serum Levels in Women Ingesting Betatene. J. Nutr. 1998, 128, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Brady, W.E.; Mares-Perlman, J.A.; Bowen, P.; Stacewicz-Sapuntzakis, M. Human Serum Carotenoid Concentrations Are Related to Physiologic and Lifestyle Factors. J. Nutr. 1996, 126, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.K.; Adetona, O.; Aguilar-Villalobos, M.; Cassidy, B.E.; Pfeiffer, C.M.; Schleicher, R.L.; Caldwell, K.L.; Needham, L.L.; Rathbun, S.L.; Vena, J.E.; et al. Changes in the concentrations of biochemical indicators of diet and nutritional status of pregnant women across pregnancy trimesters in Trujillo, Peru, 2004–2005. Nutr. J. 2013, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega, H.; Alvino, G.; Giovannini, N.; Amusquivar, E.; Cetin, I. Relationship between plasma fatty acid profile and antioxidant vitamins during normal pregnancy. Eur. J. Clin. Nutr. 2004, 58, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Schweigert, F.J.; Bathe, K.; Chen, F.; Büscher, U.; Dudenhausen, J.W. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur. J. Nutr. 2004, 43, 39–44. [Google Scholar] [CrossRef]
- Lindqvist, A.; Sharvill, J.; Sharvill, D.E.; Andersson, S. Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J. Nutr. 2007, 137, 2346–2350. [Google Scholar] [CrossRef]
- Ferrucci, L.; Perry, J.R.; Matteini, A.; Perola, M.; Tanaka, T.; Silander, K.; Rice, N.; Melzer, D.; Murray, A.; Cluett, C.; et al. Common variation in the beta-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2009, 84, 123–133. [Google Scholar] [CrossRef]
- Hendrickson, S.J.; Hazra, A.; Chen, C.; Eliassen, A.H.; Kraft, P.; Rosner, B.A.; Willett, W.C. β-Carotene 15,15′-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. Am. J. Clin. Nutr. 2012, 96, 1379–1389. [Google Scholar] [CrossRef]
- Borel, P. Genetic variations involved in interindividual variability in carotenoid status. Mol. Nutr. Food Res. 2012, 56, 228–240. [Google Scholar] [CrossRef]
- Lobo, G.P.; Amengual, J.; Baus, D.; Shivdasani, R.A.; Taylor, D.; von Lintig, J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J. Biol. Chem. 2013, 288, 9017–9027. [Google Scholar] [CrossRef]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J. Nutr. 2007, 137, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; de Edelenyi, F.S.; Vincent-Baudry, S.; Malezet-Desmoulin, C.; Margotat, A.; Lyan, B.; Gorrand, J.M.; Meunier, N.; Drouault-Holowacz, S.; Bieuvelet, S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann. Med. 2011, 43, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.J.; Mares, J.A.; Igo, R.P., Jr.; Truitt, B.; Liu, Z.; Millen, A.E.; Klein, M.; Johnson, E.J.; Engelman, C.D.; Karki, C.K.; et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Investig. Opthalmol. Vis. Sci. 2014, 55, 587–599. [Google Scholar] [CrossRef]
- Zubair, N.; Kooperberg, C.; Liu, J.; Di, C.; Peters, U.; Neuhouser, M.L. Genetic variation predicts serum lycopene concentrations in a multiethnic population of postmenopausal women. J. Nutr. 2015, 145, 187–192. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.J.; Loane, E.; Nolan, J.M.; Patterson, C.C.; Meyers, K.J.; Mares, J.A.; Yonova-Doing, E.; Hammond, C.J.; Beatty, S.; Silvestri, G. Investigation of genetic variation in scavenger receptor class B, member 1 (SCARB1) and association with serum carotenoids. Ophthalmology 2013, 120, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Maubaret, C.; Korobelnik, J.-F.; Delyfer, M.-N.; Rougier, M.-B.; Lambert, J.-C.; Amouyel, P.; Malet, F.; Le Goff, M.; Dartigues, J.-F.; et al. Association of HDL-Related Loci with Age-Related Macular Degeneration and Plasma Lutein and Zeaxanthin: The Alienor Study. PLoS ONE 2013, 8, e79848. [Google Scholar] [CrossRef]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. Lycopene bioavailability is associated with a combination of genetic variants. Free Radic. Biol. Med. 2015, 83, 238–244. [Google Scholar] [CrossRef]
- Borel, P.; Moussa, M.; Reboul, E.; Lyan, B.; Defoort, C.; Vincent-Baudry, S.; Maillot, M.; Gastaldi, M.; Darmon, M.; Portugal, H.; et al. Human fasting plasma concentrations of vitamin E and carotenoids, and their association with genetic variants in apo C-III, cholesteryl ester transfer protein, hepatic lipase, intestinal fatty acid binding protein and microsomal triacylglycerol transfer protein. Br. J. Nutr. 2009, 101, 680–687. [Google Scholar] [CrossRef]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R.; Morange, S.; Lesavre, N. Interindividual variability of lutein bioavailability in healthy men: Characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am. J. Clin. Nutr. 2014, 100, 168–175. [Google Scholar] [CrossRef]
- Herron, K.L.; McGrane, M.M.; Waters, D.; Lofgren, I.E.; Clark, R.M.; Ordovas, J.M.; Fernandez, M.L. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J. Nutr. 2006, 136, 1161–1165. [Google Scholar] [CrossRef]
- Herbeth, B.; Gueguen, S.; Leroy, P.; Siest, G.; Visvikis-Siest, S. The lipoprotein lipase serine 447 stop polymorphism is associated with altered serum carotenoid concentrations in the Stanislas Family Study. J. Am. Coll. Nutr. 2007, 26, 655–662. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, C.R.; D’Urso, A.; Ryan, K.A.; Yerges-Armstrong, L.M.; Semba, R.D.; Steinle, N.I.; Mitchell, B.D.; Shuldiner, A.R.; McArdle, P.F. A Common Variant in the SETD7 Gene Predicts Serum Lycopene Concentrations. Nutrients 2016, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R. A Combination of Single-Nucleotide Polymorphisms Is Associated with Interindividual Variability in Dietary β-Carotene Bioavailability in Healthy Men. J. Nutr. 2015, 145, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Yonova-Doing, E.; Hysi, P.G.; Venturini, C.; Williams, K.M.; Nag, A.; Beatty, S.; Liew, S.H.; Gilbert, C.E.; Hammond, C.J. Candidate gene study of macular response to supplemental lutein and zeaxanthin. Exp. Eye Res. 2013, 115, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Mancina, R.M.; Burza, M.A.; Maglio, C.; Pirazzi, C.; Sentinelli, F.; Incani, M.; Montalcini, T.; Pujia, A.; Congiu, T.; Loche, S.; et al. The COBLL1 C allele is associated with lower serum insulin levels and lower insulin resistance in overweight and obese children. Diabetes Metab. Res. Rev. 2013, 29, 413–416. [Google Scholar] [CrossRef]
- Meyers, K.J.; Johnson, E.J.; Bernstein, P.S.; Iyengar, S.K.; Engelman, C.D.; Karki, C.K.; Liu, Z.; Igo, R.P., Jr.; Truitt, B.; Klein, M.L.; et al. Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2333–2345. [Google Scholar] [CrossRef]
- Forman, M.R.; Beecher, G.R.; Muesing, R.; Lanza, E.; Olson, B.; Campbell, W.S.; McAdam, P.; Raymond, E.; Schulman, J.D.; Graubard, B.I. The fluctuation of plasma carotenoid concentrations by phase of the menstrual cycle: A controlled diet study. Am. J. Clin. Nutr. 1996, 64, 559–565. [Google Scholar] [CrossRef]
- Forman, M.R.; Johnson, E.J.; Lanza, E.; Graubard, B.I.; Beecher, G.R.; Muesing, R. Effect of menstrual cycle phase on the concentration of individual carotenoids in lipoproteins of premenopausal women: A controlled dietary study. Am. J. Clin. Nutr. 1998, 67, 81–87. [Google Scholar] [CrossRef]
- Mumford, S.L.; Browne, R.W.; Schliep, K.C.; Schmelzer, J.; Plowden, T.C.; Michels, K.A.; Sjaarda, L.A.; Zarek, S.M.; Perkins, N.J.; Messer, L.C.; et al. Serum Antioxidants Are Associated with Serum Reproductive Hormones and Ovulation among Healthy Women123. J. Nutr. 2016, 146, 98–106. [Google Scholar] [CrossRef]
- Gabriel, H.E.; Liu, Z.; Crott, J.W.; Choi, S.-W.; Song, B.C.; Mason, J.B.; Johnson, E.J. A Comparison of Carotenoids, Retinoids, and Tocopherols in the Serum and Buccal Mucosa of Chronic Cigarette Smokers versus Nonsmokers. Cancer Epidemiol. Biomark. Prev. 2006, 15, 993–999. [Google Scholar] [CrossRef]
- Rydén, M.; Leanderson, P.; Kastbom, K.O.; Jonasson, L. Effects of simvastatin on carotenoid status in plasma. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Vasankari, T.; Ahotupa, M.; Viikari, J.; Nuotio, I.; Strandberg, T.; Vanhanen, H.; Gylling, H.; Miettinen, T.; Tikkanen, M.J. Effect of 12-month statin therapy on antioxidant potential of LDL and serum antioxidant vitamin concentrations. Ann. Med. 2004, 36, 618–622. [Google Scholar] [CrossRef]
- Sundl, I.; Pail, E.; Mellitzer, K.; Toplak, H.; Winklhofer-Roob, B.M. Effects of orlistat therapy on plasma concentrations of oxygenated and hydrocarbon carotenoids. Lipids 2006, 41, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Lidebjer, C.; Leanderson, P.; Ernerudh, J.; Jonasson, L. Low plasma levels of oxygenated carotenoids in patients with coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 448–456. [Google Scholar] [CrossRef]
- Coyne, T.; Ibiebele, T.I.; Baade, P.D.; Dobson, A.; McClintock, C.; Dunn, S.; Leonard, D.; Shaw, J. Diabetes mellitus and serum carotenoids: Findings of a population-based study in Queensland, Australia. Am. J. Clin. Nutr. 2005, 82, 685–693. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High-serum carotenoids associated with lower risk for developing type 2 diabetes among Japanese subjects: Mikkabi cohort study. BMJ Open Diabetes Res. Care 2015, 3, e000147. [Google Scholar] [CrossRef]
- Scanlon, G.; Connell, P.; Ratzlaff, M.; Foerg, B.; McCartney, D.; Murphy, A.; O’Connor, K.; Loughman, J. Macular pigment optical density is lower in type 2 diabetes, compared with type 1 diabetes and normal controls. Retina 2015, 35, 1808–1816. [Google Scholar] [CrossRef]
- Floreani, A.; Baragiotta, A.; Martines, D.; Naccarato, R.; D’Odorico, A. Plasma antioxidant levels in chronic cholestatic liver diseases. Aliment. Pharmacol. Ther. 2000, 14, 353–358. [Google Scholar] [CrossRef]
- Cohn, W.; Thürmann, P.; Tenter, U.; Aebischer, C.; Schierle, J.; Schalch, W. Comparative multiple dose plasma kinetics of lycopene administered in tomato juice, tomato soup or lycopene tablets. Eur. J. Nutr. 2004, 43, 304–312. [Google Scholar] [CrossRef]
- Chung, H.Y.; Rasmussen, H.M.; Johnson, E.J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. 2004, 134, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, J.J.; West, C.E.; Linssen, J.P.; van het Hof, K.H.; Voragen, A.G. The food matrix of spinach is a limiting factor in determining the bioavailability of beta-carotene and to a lesser extent of lutein in humans. J. Nutr. 1999, 129, 349–355. [Google Scholar] [CrossRef] [PubMed]
- van het Hof, K.H.; Brouwer, I.A.; West, C.E.; Haddeman, E.; Steegers-Theunissen, R.P.; van Dusseldorp, M.; Weststrate, J.A.; Eskes, T.K.; Hautvast, J.G. Bioavailability of lutein from vegetables is 5 times higher than that of beta-carotene. Am. J. Clin. Nutr. 1999, 70, 261–268. [Google Scholar] [CrossRef]
- Handelman, G.J.; Nightingale, Z.D.; Lichtenstein, A.H.; Schaefer, E.J.; Blumberg, J.B. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am. J. Clin. Nutr. 1999, 70, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.L.; Chitchumronchokchai, C.; Ferruzzi, M.G.; Goltz, S.R.; Campbell, W.W. Unsaturated fatty acids promote bioaccessibility and basolateral secretion of carotenoids and α-tocopherol by Caco-2 cells. Food Funct. 2014, 5, 1101–1112. [Google Scholar] [CrossRef]
- Goltz, S.R.; Campbell, W.W.; Chitchumroonchokchai, C.; Failla, M.L.; Ferruzzi, M.G. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol. Nutr. Food Res. 2012, 56, 866–877. [Google Scholar] [CrossRef]
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176. [Google Scholar] [CrossRef]
- Tang, G.; Ferreira, A.L.; Grusak, M.A.; Qin, J.; Dolnikowski, G.G.; Russell, R.M.; Krinsky, N.I. Bioavailability of synthetic and biosynthetic deuterated lycopene in humans. J. Nutr. Biochem. 2005, 16, 229–235. [Google Scholar] [CrossRef]
- Gärtner, C.; Stahl, W.; Sies, H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am. J. Clin. Nutr. 1997, 66, 116–122. [Google Scholar] [CrossRef]
- Grainger, E.M.; Hadley, C.W.; Moran, N.E.; Riedl, K.M.; Gong, M.C.; Pohar, K.; Schwartz, S.J.; Clinton, S.K. A comparison of plasma and prostate lycopene in response to typical servings of tomato soup, sauce or juice in men before prostatectomy. Br. J. Nutr. 2015, 114, 596–607. [Google Scholar] [CrossRef]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef]
- Baumgartner, S.; Ras, R.T.; Trautwein, E.A.; Mensink, R.P.; Plat, J. Plasma fat-soluble vitamin and carotenoid concentrations after plant sterol and plant stanol consumption: A meta-analysis of randomized controlled trials. Eur. J. Nutr. 2017, 56, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.S. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996, 10, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Suganuma, H.; Ozato, N.; Shimizu, S.; Katashima, M.; Katsuragi, Y.; Mikami, T.; Itoh, K.; Nakaji, S. Association between Serum Concentration of Carotenoid and Visceral Fat. Nutrients 2021, 13, 912. [Google Scholar] [CrossRef]
- Canas, J.A.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of Mixed Carotenoids on Adipokines and Abdominal Adiposity in Children: A Pilot Study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Pezdirc, K.; Hutchesson, M.J.; Williams, R.L.; Rollo, M.E.; Burrows, T.L.; Wood, L.G.; Oldmeadow, C.; Collins, C.E. Consuming High-Carotenoid Fruit and Vegetables Influences Skin Yellowness and Plasma Carotenoids in Young Women: A Single-Blind Randomized Crossover Trial. J. Acad. Nutr. Diet. 2016, 116, 1257–1265. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids-A Review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Carotenoids and UV protection. Photochem. Photobiol. Sci. 2004, 3, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Vinet, C.; David, I.P. Effect of beta-carotene supplementation on African skin. J. Biomed. Opt. 2014, 19, 025004. [Google Scholar] [CrossRef]
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Jilcott Pitts, S.B.; Johnson, N.S.; Wu, Q.; Firnhaber, G.C.; Preet Kaur, A.; Obasohan, J. A meta-analysis of studies examining associations between resonance Raman spectroscopy-assessed skin carotenoids and plasma carotenoids among adults and children. Nutr. Rev. 2022, 80, 230–241. [Google Scholar] [CrossRef] [PubMed]
| Findings | |
|---|---|
| Age | Children RRS [43] Age NS in 9–12 year US children (n = 128) [44] Age NS in US children/adolescents 5–17 year (n = 45) [45] Age + in US children 3–5 year in low-income homes (n = 381) * [46] Age + US infants/children in <1–7 year (n = 51) [47] Gestational Age + in US preterm infants (n = 16) [48] Age − in US formula-fed and NS in breast-fed male infants, birth–9 w (n = 40) [49] Age − in US children 2–12 year in low-income homes (n = 177) * [50] Age NS in healthy US children 3–5 year (n = 209) RS [51] Age NS in US Latino children 10–14 year (n = 195) [52] Age NS in US children 9–12 year (n = 143) [53] Age + in US children 3–5 year in low-income homes (n = 112) Adults RRS [23] Age NS in US adults 21–65 year (n = 74) [54] Age NS in US adults 50–85 year with AMD (n = 44) [55] Age NS in Singaporean adults 50–75 year (n = 103) * [56] Age NS in German adults 18+ year (n = 151) [57] Age NS in German adults 19–79 year (RRS and RS, n = 33) RS [58] Age NS in Japanese eye clinic patients 6–98 year (n = 569) [59] Age NS in older US adults 65–86 year (n = 95) [60] Age NS in US adults 20–84 year (n = 80) [61] Age NS in low-income US adults (n = 287) [62] Age NS in Japanese adults 16–97 year (n = 985) [63] Age NS in US adults 18+ year (n = 136) [64] Age NS in healthy US adults 18–65 year (n = 213) * [65] Age + in a diverse sample of NZ adults 16+ year (n = 571) * [66] Age + in Japanese adults 22–90 year (n = 1812) [67] Age + in healthy Koreans and Germans 7–75 year (n = 714) |
| Sex | Children RRS [44] Sex NS in US children/adolescents 5–17 year (n = 45) [45] Sex NS in US children 3–5 year in low-income homes (n = 381) * [49] Sex NS in US children 2–12 year in low-income homes (n = 177) [68] Sex NS in US children 5–17 year (n = 58) [50] Sex NS in healthy US children 3–5 year (n = 209) [43] Males + in US children 9–12 year (n = 128) RS [52] Sex NS in US children 9–12 year (n = 143) [51] Sex NS in US Latino children 10–14 year (n = 195) [69] Males + in US pre-school (n = 112) and middle-school (n = 94) children; Sex NS in US high school children (n = 58) [53] Males + in US children 3–5 year in low-income homes (n = 112) [70] Sex NS in Japanese children 10 year (n = 315) Adults RRS [23] Sex NS in US adults 21–65 year = (n = 74) [55] Females + in Singaporean adults 50–75 year (n = 103) * [56] Females + in German adults 18+ year (n = 151) RS [71] Sex NS in low-income older US adults > 60 year (n = 154) [72] Sex NS in US college students 18–25 year (n = 66) [73] Sex NS in US college students (n = 40) [64] Sex NS in healthy US adults 18–65 year (n = 213) * [66] Females + in Japanese adults 22–90 year (n = 1812) [62] Females + in Japanese adults 16–97 year (n = 985) [58] Females + in Japanese eye clinic patients 6–98 year (n = 569) [60] Males + US adults 20–84 year (n = 80) [63] Males + in US adults 18+ year (n = 136) [74] Females + in healthy Japanese adults 20+ year (n = 811) |
| BMI | Children RRS [44] BMI% NS in US children/adolescents 5–17 year (n = 45) [45] BMI − in US children 3–5 year in low-income homes (n = 381) * [49] BMI NS in US children 2–12 year in low-income homes (n = 177) * [68] BMI NS in US children 5–17 year (n = 58) [43] BMI − (Normal < Overweight < Obese) in US children 9–12 year (n = 128) [50] BMI NS in healthy US children 3–5 year (n = 209) [75] BMI NS in US 4th grade students (n = 30) * RS [51] BMI NS in US Latino children 10–14 year (n = 195) [69] BMI NS in US preschool (n = 112), middle school (n = 94), and high school children (n = 58) Adults RRS [23] BMI NS in US adults 21–65 year (n = 74) [76] BMI NS in US adults 21–65 year (n = 74) * [56] BMI NS (p < 0.1) in German adults 18+ year (n = 151) [77] BMI NS in Thai adult health professionals (n = 29) [55] BMI − in Singaporean adults 50–75 year (n = 103) * [78] BMI − in US women with breast cancer 18–90 year (n = 102) * RS [72] BMI NS in US college students 18–25 year (n = 66) [64] BMI NS in healthy US adults 18–65 year (n = 213) * [59] BMI − in older US adults 65–86 year (n = 95) [61] BMI − in low-income US adults (n = 287) * [73] BMI − in US college students (n = 40) [65] BMI − in a diverse sample of NZ adults 16+ year (n = 571) * [62] BMI − in Japanese adults 16–97 year (n = 985) [79] BMI NS in US African American college students 18–30 year (n = 98) * [74] BMI − in healthy Japanese adults 20+ year (n = 811) |
| Race/Ethnicity | Children RRS [44] Ethnicity NS in US children/adults 5–17 year (n = 45) [45] White non-Hispanic > Hispanic in US children 3–5 year in low-income homes (n = 381) * [49] Ethnicity NS in US children 2–12 year in low-income homes (n = 177) * [68] Ethnicity NS in US children 5–17 year (n = 58) [50] Ethnicity NS in healthy US children 3–5 year (n = 209) RS [69] Ethnicity NS in US preschool (n = 112), middle school (n = 94), and high school children (n = 58) [52] Ethnicity NS in US children 9–12 year (n = 143) Adults RRS [23] Ethnicity NS in US adults 21–65 year (n = 74) [55] Ethnicity NS in Singaporean adults 50–75 year (n = 103) * [76] Asian > White in US adults 21–65 year (n = 74) * RS [60] Ethnicity NS in US adults 20–84 year (n = 80) [61] Ethnicity NS in low-income US adults (n = 287) [72] Ethnicity NS in US college students 18–25 year (n = 66) [63] Ethnicity NS in US adults 18+ year (n = 136) [64] Ethnicity NS in healthy US adults 18–65 year (n = 213) * [65] Asian > other ethnicities in a diverse sample of NZ adults 16+ year (n = 571) * [71] Non-white > white in low-income older US adults > 60 year (n = 154) |
| Pregnancy/Lactation | RS [61] Pregnancy NS and breastfeeding NS in low-income US adults (n = 287) * |
| Melanin/Skin Tone | RRS [58] Skin melanin NS in an ethnically diverse sample of US adults (RRS and RS, n = 160) [23] Darker skin tone NS in US adults 21–65 year (n = 74) [76] Skin tone NS in US adults 21–65 year (n = 74) * [57] Skin tone NS in German adults 19–79 year (RRS & RS, n = 33) RS [79] Skin tone NS in US African American college students 18–30 year (n = 98) * [64] Skin melanin NS in healthy US adults 18–65 year (n = 213) |
| Smoking Status | RRS [23] Smoking NS in US adults 21–65 year (n = 74) [76] Smoking NS in US adults 21–65 year (n = 74) * [55] Smoking − in Singaporean adults 50–75 year (n = 103) * [56] Smoking − in German adults 18+ year (n = 108) [80] Smoking − in healthy British subjects (n = 1375) * [78] Smoking − in US women with breast cancer 18–90 year (n = 102) * RS [60] Smoking NS US adults 20–84 year (n = 76) [58] Smoking − in Japanese eye clinic patients 6–98 year (n = 569) [61] Smoking NS in low-income US adults (n = 287) * [65] Smoking − in a diverse sample NZ adults 16+ year, NS in multiple regression (n = 571) * [66] Smoking − in Japanese adults 22–90 year (n = 1812) [62] Current < Past < Never smokers in Japanese adults 16–97 year (n = 985) |
| Alcohol Consumption | RRS [23] Alcohol drinker NS in US adults 21–65 year (n = 74) [81] Alcohol consumption − in German adult males 21–54 year (n = 6) * |
| Medications | RRS [55] Cholesterol, hypertension, and diabetes medications − in Singaporean adults 50–75 year (n = 103) * RS [66] Hypolipidemic agents + in Japanese adults 22–90 year (n = 1812) |
| Season | RRS [76] Spring/Autumn and Summer > Winter in US adults 21–65 year (n = 74) * RS [51] Fall > Winter in US Latino children 10–14 year (n = 195) * |
| UV Exposure | RRS [76] Recent sun exposure – in US adults 21–65 year (n = 74) * [80] High sunlight exposure – in healthy British subjects (n = 1375) * |
| Carotenoid Supplement Intake | RRS [82] Beta-carotene + lycopene supplement + in healthy German female adults 21–72 year (n = 129) * RS [58] Lutein supplements + in Japanese eye clinic patients 6–98 year (n = 569) [62] Lutein supplements + in Japanese adults 16–97 year (n = 985) [83] Beta-carotene supplement + in healthy German female adults 20–45 year (n = 12) * |
| Nutrient Intake | RRS [55] Vitamin C intake + in Singaporean adults 50–75 year (n = 103) * [49] Fat intake (% kcal) + in US children 2–12 year in low-income homes (n = 177) * |
| Factor/Covariate | Blood Carotenoids | Skin Carotenoids | Summary |
|---|---|---|---|
| Biological Factors | |||
| Age | NS: [30] | Children +: [45] NS: [49] Adults NS: [55,64] +: [65] | In most of the available studies on adults, age does not appear to significantly impact blood or skin carotenoid levels. One study on a group of children aged 3–5 found that age was significantly positively associated with skin carotenoids, but another on a group aged 2–12 did not. |
| Sex | Females +: [30] | Children NS: [45] Adults Females +: [55] NS: [64] | Studies have consistently observed that females have significantly greater blood carotenoid concentrations independently of dietary carotenoid intake, but conflicting results exist with respect to the potential differences in skin carotenoid levels between males and females. |
| BMI | −: [30,84] | Children −: [45] NS: [49,75] Adults −: [55,61,65,78] NS: [64,76,79] | Most studies have found that a greater BMI is associated with significantly lower blood carotenoids, but the relationship between BMI and skin carotenoids is not quite as consistent, especially among children. This may be at least in part due to the heterogeneity of the size and composition of the studied populations. |
| Race/Ethnicity | NS: [55] | Children NH White > Hispanic: [45] NS: [49] Adults Asian +: [65,76] NS: [55,64] | Most of the existing research has not identified significant differences in skin carotenoids across racial/ethnic groups after accounting for dietary carotenoid and FV intake, though a few studies have observed that among adults, Asian individuals have significantly greater skin carotenoids than other racial/ethnic groups. |
| Pregnancy or Lactation | Pregnancy (third trimester) +: [85,86] Lactation –: [87] | Breastfeeding NS: [61] Pregnancy NS: [61] | Blood carotenoids have been found to increase significantly in the third trimester of pregnancy and decrease during lactation, though the potential of these observations being a result of dietary changes was not ruled out. The limited data on skin carotenoids suggest breastfeeding may not affect skin carotenoids independently of FV/carotenoid intake, and pregnancy is not associated with a significant difference in skin carotenoid levels (without dietary adjustment). |
| Genetics | Many gene variants influence blood carotenoids or modify blood responses to carotenoid ingestion: [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108] | Not yet studied | Quite a few gene variants have the ability to affect blood carotenoid concentrations and responses to dietary carotenoid intake, but the implications for skin carotenoids have not been studied directly. |
| Menstrual Cycle | Lowest during menses, highest in the midluteal phase: [109,110,111] | Not yet studied | Studies have revealed that blood carotenoids decrease during menses and are greatest in the midluteal phase. No research has been conducted to assess how skin carotenoids may vary across stages of the menstrual cycle. |
| Melanin/Skin Tone | Not yet studied | NS: [64,76,79] | A few studies have found that melanin or skin tone does not significantly affect skin carotenoids when adjusting for dietary carotenoid or FV intake, but more research on larger and more diverse samples is warranted. |
| Behavioral Factors | |||
| Smoking Status | −: [38,112] | −: [55,65,78,80] NS: [61,76] | Current and previous smokers tend to have significantly lower blood and skin carotenoid levels. |
| Alcohol Consumption | −: [40] | −: [81] | One interventional study found that increasing alcohol intake elicits a significant decrease in blood carotenoids, and a very small pilot study found that alcohol consumption significantly decreased skin carotenoid levels. Future research should be conducted to confirm these findings. |
| Medication Use | Statins -: [113,114] Lipase Inhibitor -: [115] | Cholesterol, hypertension, and diabetes medications −: [55] | Statins and lipase inhibitors significantly decrease blood carotenoids, and one study on skin carotenoids found that medication use (including statins) is associated with lower levels after adjustment for dietary carotenoid intake. Additional research is needed to better quantify the influence of medications on skin carotenoids. |
| Health Status | CVD −: [116,117] Diabetes/IR −: [118,119,120] Chronic Cholestasis −: [121] | Not yet studied | Many studies have found CVD, diabetes/IR, and chronic cholestasis are associated with lower blood carotenoid levels; however, dietary carotenoid intake was not accounted for. Currently, there is no research on the effect of these or other diseases on skin carotenoid levels. |
| Environmental Factors | |||
| Season | Not yet studied | Winter −: [76] | In one study, skin carotenoid levels were significantly higher in spring/summer and autumn than in winter after accounting for dietary carotenoid intake. The potential seasonal influence on skin carotenoids should continue to be investigated to further confirm these findings. |
| UV/Sun Exposure | Not yet studied | Recent sun exposure −: [76,80] | Two studies found that participants with recent or greater usual sun exposure had significantly lower skin carotenoid levels. These findings should be confirmed in future research. |
| Dietary Factors | |||
| Carotenoid Supplement Intake | Supplement carotenoid bioavailability is frequently greater than food bioavailability [122,123,124,125] | Carotenoid supplement intake +: [82,83] | Interventional studies have shown carotenoid supplement bioavailability is higher than that of carotenoids from certain foods. While a few studies have suggested carotenoid supplement ingestion significantly increases skin carotenoid levels, it remains unclear if these increases are greater than those elicited by ingestion of similar amounts of carotenoids from food. |
| Dietary Fat Intake | Acute feeding studies suggest co-consumption of fat increases carotenoid absorption (UFAs > SFAs), but associations between self-reported intakes of fat and blood carotenoids have been inconsistent [34,55,126,127,128] | Intake of total, saturated, and unsaturated fats (g/d) NS: [55] | Acute feeding studies found that co-consumption of fat can increase carotenoid bioavailability. However, one study found that greater habitual fat intake does not significantly influence blood or skin carotenoids independently of dietary carotenoid intake, and another found that a very high fat intake is associated with reduced blood carotenoid concentrations compared to a low/moderate fat intake. Additional research should seek to clarify whether or not fat intake meaningfully affects skin carotenoids. |
| Dietary Fiber Intake | One study found that fiber enrichment of meals may decrease carotenoid bioavailability, but another found a greater habitual dietary fiber intake did not significantly affect serum carotenoid concentrations [55,129] | Fiber NS: [55] | One interventional study found that the addition of fiber to meals may decrease carotenoid bioavailability, though in other research a greater dietary fiber intake did not significantly affect blood or skin carotenoids. Upcoming research should seek to confirm these findings. |
| Food Matrix | Carotenes from “softer” processed foods are more bioavailable than those from minimally processed foods [122,130,131,132,133] | Not yet studied | Processing of carotenoid-rich foods into “softer” forms seems to increase their bioavailability and thus their ability to increase blood carotenoid levels, but whether this also translates to skin carotenoid levels has yet to be studied. |
| Plant Stanols/Sterols | Intake of plant stanols/sterols appears to decrease absorption and plasma concentrations of blood carotenoids [35,134] | Not yet studied | Multiple interventional trials have found that plant stanols/sterols can decrease blood carotenoid concentrations, but no studies have investigated their influence on skin carotenoid levels. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madore, M.P.; Hwang, J.-E.; Park, J.-Y.; Ahn, S.; Joung, H.; Chun, O.K. A Narrative Review of Factors Associated with Skin Carotenoid Levels. Nutrients 2023, 15, 2156. https://doi.org/10.3390/nu15092156
Madore MP, Hwang J-E, Park J-Y, Ahn S, Joung H, Chun OK. A Narrative Review of Factors Associated with Skin Carotenoid Levels. Nutrients. 2023; 15(9):2156. https://doi.org/10.3390/nu15092156
Chicago/Turabian StyleMadore, Matthew P., Jeong-Eun Hwang, Jin-Young Park, Seoeun Ahn, Hyojee Joung, and Ock K. Chun. 2023. "A Narrative Review of Factors Associated with Skin Carotenoid Levels" Nutrients 15, no. 9: 2156. https://doi.org/10.3390/nu15092156
APA StyleMadore, M. P., Hwang, J.-E., Park, J.-Y., Ahn, S., Joung, H., & Chun, O. K. (2023). A Narrative Review of Factors Associated with Skin Carotenoid Levels. Nutrients, 15(9), 2156. https://doi.org/10.3390/nu15092156







