Effectiveness of a Smartphone Application for Dietary Sodium Intake Measurement
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
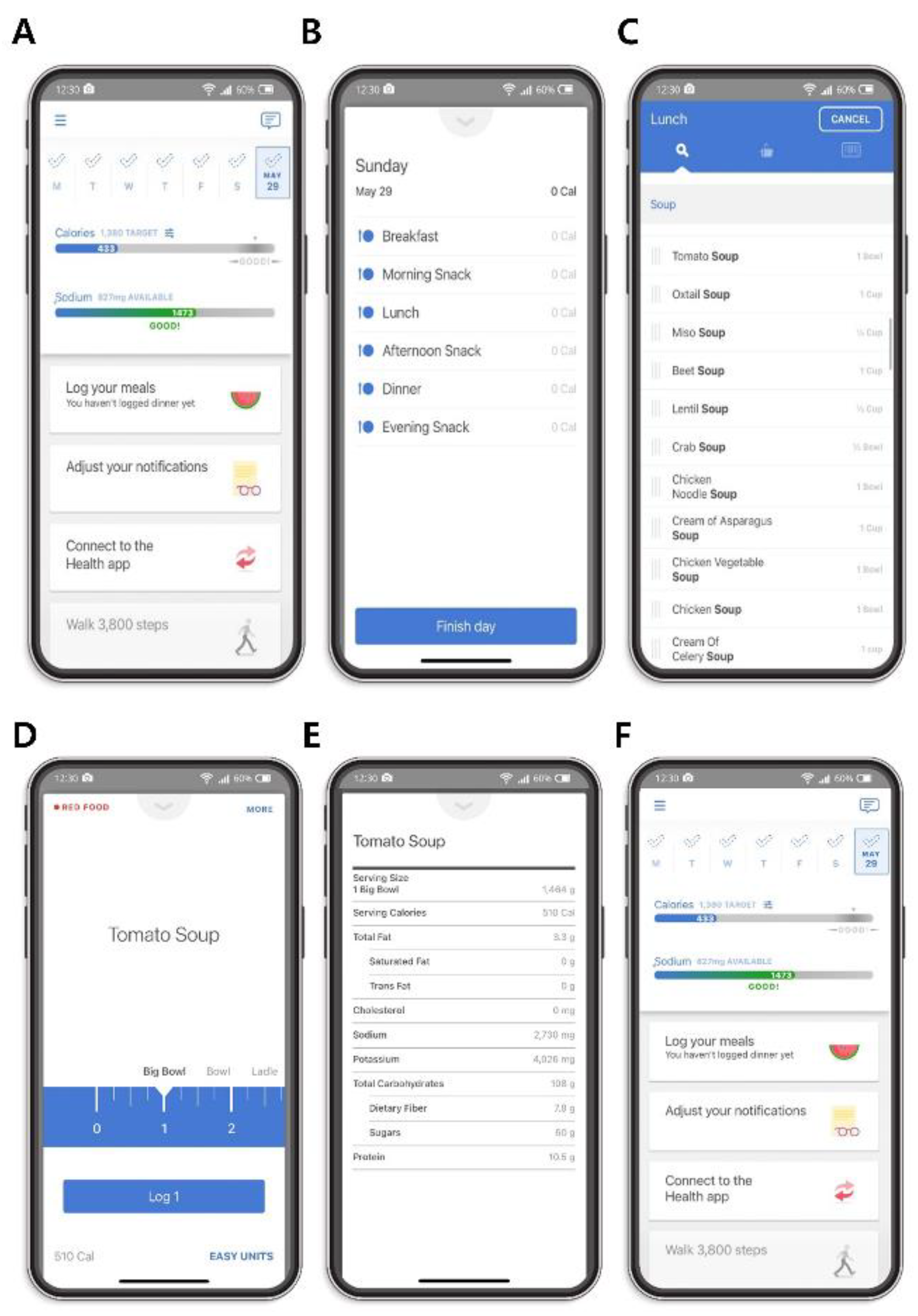
2.2. Smartphone Application-Based Dietary Assessment
2.3. Data Collection and Measurements
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Difference between Sodium Intake Measured by the Application and Calculated Using 24-h Urine Sodium Level
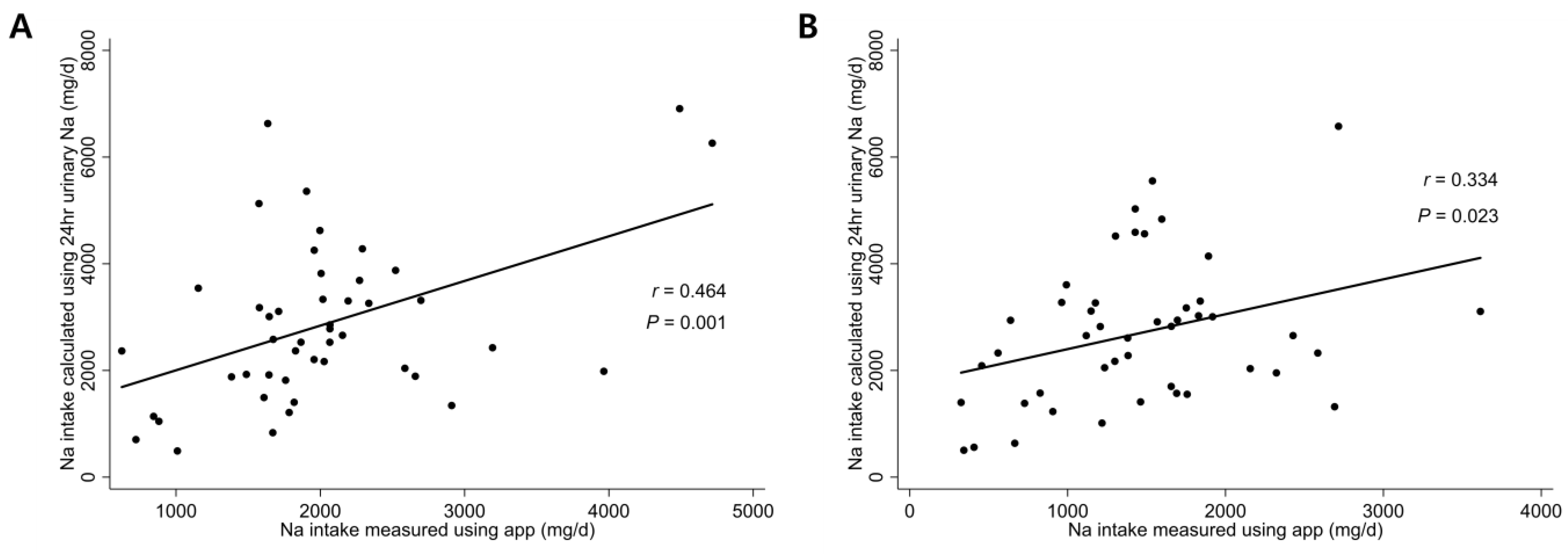
3.3. Correlation between Sodium Intake Measured by the Application and That Calculated Using 24-h Urine Sodium Level
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.; Trieu, K.; Yoshimura, S.; Neal, B.; Woodward, M.; Campbell, N.R.C.; Li, Q.; Lackland, D.T.; Leung, A.A.; Anderson, C.A.M.; et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomised trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Cook, N.R.; Cutler, J.A.; Obarzanek, E.; Buring, J.E.; Rexrode, K.M.; Kumanyika, S.K.; Appel, L.J.; Whelton, P.K. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007, 334, 885. [Google Scholar] [CrossRef]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D.; et al. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef]
- McCarron, D.A.; Kazaks, A.G.; Geerling, J.C.; Stern, J.S.; Graudal, N.A. Normal range of human dietary sodium intake: A perspective based on 24-hour urinary sodium excretion worldwide. Am. J. Hypertens. 2013, 26, 1218–1223. [Google Scholar] [CrossRef]
- World Health Organization. Effect of Reduced Sodium Intake on Blood Pressure, Renal Function, Blood Lipids and Other Potential Adverse Effects; World Health Organization: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/handle/10665/79325 (accessed on 13 September 2022).
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 2014, 129, 981–989. [Google Scholar] [CrossRef]
- Cook, N.R.; Appel, L.J.; Whelton, P.K. Sodium Intake and All-Cause Mortality Over 20 Years in the Trials of Hypertension Prevention. J. Am. Coll. Cardiol. 2016, 68, 1609–1617. [Google Scholar] [CrossRef]
- Hunter, R.W.; Dhaun, N.; Bailey, M.A. The impact of excessive salt intake on human health. Nat. Rev. Nephrol. 2022, 18, 321–335. [Google Scholar] [CrossRef]
- He, F.J.; Brinsden, H.C.; MacGregor, G.A. Salt reduction in the United Kingdom: A successful experiment in public health. J. Hum. Hypertens. 2014, 28, 345–352. [Google Scholar] [CrossRef]
- Humalda, J.K.; Klaassen, G.; de Vries, H.; Meuleman, Y.; Verschuur, L.C.; Straathof, E.J.; Laverman, G.D.; Bos, W.J.W.; van der Boog, P.J.; Vermeulen, K.M.; et al. A Self-management Approach for Dietary Sodium Restriction in Patients With CKD: A Randomized Controlled Trial. Am. J. Kidney Dis. 2020, 75, 847–856. [Google Scholar] [CrossRef]
- Heitmann, B.L.; Lissner, L. Dietary underreporting by obese individuals-is it specific or non-specific? BMJ 1995, 311, 986–989. [Google Scholar] [CrossRef]
- Garden, L.; Clark, H.; Whybrow, S.; Stubbs, R.J. Is misreporting of dietary intake by weighed food records or 24-hour recalls food specific? Eur. J. Clin. Nutr. 2018, 72, 1026–1034. [Google Scholar] [CrossRef]
- McLean, R.M.; Farmer, V.L.; Nettleton, A.; Cameron, C.M.; Cook, N.R.; Woodward, M.; Campbell, N.R.C.; TRUE Consortium (in Ternational Consortium for Quality Research on Dietary Sodium/Salt). Twenty-Four-Hour Diet recall and Diet records compared with 24-hour urinary excretion to predict an individual’s sodium consumption: A Systematic Review. J. Clin. Hypertens. 2018, 20, 1360–1376. [Google Scholar] [CrossRef]
- Khazen, W.; Jeanne, J.-F.; Demaretz, L.; Schäfer, F.; Fagherazzi, G. Rethinking the Use of Mobile Apps for Dietary Assessment in Medical Research. J. Med. Internet Res. 2020, 22, e15619. [Google Scholar] [CrossRef]
- GL, A.; Hurworth, M.; Giglia, R.; Trapp, G.; Strauss, P. Feasibility of a commercial smartphone application for dietary assessment in epidemiological research and comparison with 24-h dietary recalls. Nutr. J. 2018, 17, 5. [Google Scholar]
- Ahn, J.S.; Kim, D.W.; Kim, J.; Park, H.; Lee, J.E. Development of a Smartphone Application for Dietary Self-Monitoring. Front. Nutr. 2019, 6, 149. [Google Scholar] [CrossRef]
- Recio-Rodriguez, J.I.; Rodriguez-Martin, C.; Gonzalez-Sanchez, J.; Rodriguez-Sanchez, E.; Martin-Borras, C.; Martínez-Vizcaino, V.; Arietaleanizbeaskoa, M.S.; Magdalena-Gonzalez, O.; Fernandez-Alonso, C.; A Maderuelo-Fernandez, J.; et al. EVIDENT Smartphone App, a New Method for the Dietary Record: Comparison with a Food Frequency Questionnaire. JMIR mHealth uHealth 2019, 7, e11463. [Google Scholar] [CrossRef]
- Open Source Food Safety Database. Korean Ministry of Food and Drug Safety. 2022. Available online: https://www.mfds.go.kr/eng/index.do (accessed on 13 September 2022).
- Korean Standard Food Composition Table. Korean Rural Development Administration National Institute of Agricultural Sciences. 2022. Available online: http://koreanfood.rda.go.kr/eng/fctFoodSrchEng/main (accessed on 13 September 2022).
- Computer Aided Nutritional Analysis Program. Korean Nutrition Society. 2022. Available online: http://canpro5.kns.or.kr/ (accessed on 13 September 2022).
- What Do the Green, Yellow, and Orange Food Color Categories Mean? Noom. 2022. Available online: https://www.noom.com/support/faqs/question-topics/food-logging/2015/12/what-do-the-green-yellow-and-orange-food-color-categories-mean/ (accessed on 13 September 2022).
- How Does Noom Determine my Daily Calorie Target? Noom. 2022. Available online: https://www.noom.com/support/faqs/question-topics/food-logging/2016/08/noom-determine-daily-calorie-target/ (accessed on 14 August 2023).
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Huang, L.; Crino, M.; Wu, J.H.; Woodward, M.; Barzi, F.; Land, M.-A.; McLean, R.; Webster, J.; Enkhtungalag, B.; Neal, B. Mean population salt intake estimated from 24-h urine samples and spot urine samples: A systematic review and meta-analysis. Leuk. Res. 2016, 45, 239–250. [Google Scholar] [CrossRef]
- Santos, J.A.; Rosewarne, E.; Hogendorf, M.; Trieu, K.; Pillay, A.; Ieremia, M.; Naseri, L.T.T.; Tukana, I.; Snowdon, W.; Petersen, K.; et al. Estimating mean population salt intake in Fiji and Samoa using spot urine samples. Nutr. J. 2019, 18, 55. [Google Scholar] [CrossRef]
- Hunter, D.; Van Dam, R. Nutritional Epidemiology, 3rd ed; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Land, M.-A.; Webster, J.; Christoforou, A.; Praveen, D.; Jeffery, P.; Chalmers, J.; Smith, W.; Woodward, M.; Barzi, F.; Nowson, C.; et al. Salt intake assessed by 24 h urinary sodium excretion in a random and opportunistic sample in Australia. BMJ Open 2014, 4, e003720. [Google Scholar] [CrossRef]
- Wielgosz, A.; Robinson, C.; Mao, Y.; Jiang, Y.; Campbell, N.R.; Muthuri, S.; Morrison, H. The Impact of Using Different Methods to Assess Completeness of 24-Hour Urine Collection on Estimating Dietary Sodium. J. Clin. Hypertens. 2016, 18, 581–584. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Ho, S.C.; Tang, N.; Chan, R.; Chen, Y.-M.; Woo, J. Urinary sodium excretion and dietary sources of sodium intake in Chinese postmenopausal women with prehypertension. PLoS ONE 2014, 9, e104018. [Google Scholar] [CrossRef]
- Birukov, A.; Rakova, N.; Lerchl, K.; Olde Engberink, R.H.; Johannes, B.; Wabel, P.; Moissl, U.; Rauh, M.; Luft, F.C.; Titze, J. Ultra-long-term human salt balance studies reveal interrelations between sodium, potassium, and chloride intake and excretion. Am. J. Clin. Nutr. 2016, 104, 49–57. [Google Scholar] [CrossRef]
- Manunta, P.; Lavery, G.; Lanzani, C.; Braund, P.S.; Simonini, M.; Bodycote, C.; Zagato, L.; Delli Carpini, S.; Tantardini, C.; Brioni, E.; et al. Physiological interaction between α-adducin and WNK1-NEDD4L pathways on sodium related blood pressure regulation. Hypertension 2008, 52, 366–372. [Google Scholar] [CrossRef]
- Dahlberg, J.; Nilsson, L.-O.; von Wowern, F.; Melander, O. Physiological interaction between α-adducin and WNK1-NEDD4L pathways on sodium-related blood pressure regulation. PLoS ONE 2007, 2, e432. [Google Scholar] [CrossRef]
- Sanada, H.; Jones, J.E.; Jose, P.A. Genetics of Salt-Sensitive Hypertension. Curr. Hypertens. Rep. 2011, 13, 55–66. [Google Scholar] [CrossRef]
- Nishimoto, M.; Fujita, T. Renal mechanisms of salt-sensitive hypertension: Contribution of two steroid receptor-associated pathways. Am. J. Physiol. Physiol. 2015, 308, F377–F387. [Google Scholar] [CrossRef]
- Kye, S.; Kwon, S.-O.; Lee, S.-Y.; Lee, J.; Kim, B.H.; Suh, H.-J.; Moon, H.-K. Under-reporting of Energy Intake from 24-hour Dietary Recalls in the Korean National Health and Nutrition Examination Survey. Osong Public Health Res. Perspect. 2014, 5, 85–91. [Google Scholar] [CrossRef]
- Lerchl, K.; Rakova, N.; Dahlmann, A.; Rauh, M.; Goller, U.; Basner, M.; Dinges, D.F.; Beck, L.; Agureev, A.; Larina, I.; et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled ensvironment. Hypertension 2015, 66, 850–857. [Google Scholar] [CrossRef]
- Campino, C.; Hill, C.; Baudrand, R.; Martínez-Aguayo, A.; Aglony, M.; Carrasco, C.A.; Ferrada, C.; Loureiro, C.; Vecchiola, A.; Bancalari, R.; et al. Usefulness and Pitfalls in Sodium Intake Estimation: Comparison of Dietary Assessment and Urinary Excretion in Chilean Children and Adults. Am. J. Hypertens. 2016, 29, 1212–1217. [Google Scholar] [CrossRef]
- Ali, S.H.; Luo, R.; Li, Y.; Liu, X.; Tang, C.; Zhang, P. Application of Mobile Health Technologies Aimed at Salt Reduction: Systematic Review. JMIR mHealth uHealth 2019, 7, e13250. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, H.Y.; Cheng, L.; Sit, J.W. The effectiveness of electronic health interventions on blood pressure control, self-care behavioural outcomes and psychosocial well-being in patients with hypertension: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2019, 92, 27–46. [Google Scholar] [CrossRef]
- Eyles, H.; Grey, J.; Jiang, Y.; Umali, E.; McLean, R.; Te Morenga, L.; Neal, B.; Rodgers, A.; Doughty, R.N.; Mhurchu, C.N. Effectiveness of a Sodium-Reduction Smartphone App and Reduced-Sodium Salt to Lower Sodium Intake in Adults With Hypertension: Findings From the Salt Alternatives Randomized Controlled Trial. JMIR Mhealth Uhealth 2023, 11, e43675. [Google Scholar] [CrossRef]
- John, K.A.; Cogswell, M.E.; Campbell, N.R.; Nowson, C.A.; Legetic, B.; Hennis, A.J.M.; Patel, S.M. Accuracy and Usefulness of Select Methods for Assessing Complete Collection of 24-Hour Urine: A Systematic Review. J. Clin. Hypertens. 2016, 18, 456–467. [Google Scholar] [CrossRef]
- Kawasaki, T.; Itoh, K.; Uezono, K.; Sasaki, H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin. Exp. Pharmacol. Physiol. 1993, 20, 7–14. [Google Scholar] [CrossRef]
- Lee, H.-S.; Duffey, K.J.; Popkin, B.M. Sodium and potassium intake patterns and trends in South Korea. J. Hum. Hypertens. 2013, 27, 298–303. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.-J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data Resource Profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/handle/10665/42665 (accessed on 13 September 2022).
- Food Sanitation Act. Korea Law Translation Center. 2023. Available online: https://elaw.klri.re.kr/eng_service/lawView.do?lang=ENG&hseq=32542#:~:text=The%20purpose%20of%20this%20Act,giving%20accurate%20information%20on%20foods (accessed on 14 August 2023).
- Allison, D.B.; Heshka, S.; Sepulveda, D.; Heymsfield, S.B. Counting calories caveat emptor. JAMA 1993, 270, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.E.; Dallal, G.E.; Robinson, L.M.; Ausman, L.M.; Saltzman, E.; Roberts, S.B. The accuracy of stated energy contents of reduced energy, commercially prepared foods. J. Am. Diet. Assoc. 2010, 110, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Venti, C.A.; Le, D.S.; Michaels, J.; Parrington, S.; Krakoff, J.; Votruba, S. Food label accuracy of common snack foods. Obesity 2012, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]


| Study Participants (n = 46) | |
|---|---|
| Age, years | 40.2 (12.3) |
| Male, % | 22 (48) |
| Body mass index, kg/m2 | 23.8 (3.9) |
| Comorbidities, % | |
| Hypertension | 46 (100) |
| Medication, % | |
| Anti-hypertensive | 46 (100) |
| Anti-dyslipidemic | 11 (24) |
| Laboratory parameters | |
| BUN, mg/dL | 17.0 (6.6) |
| Creatinine, mg/dL | 1.0 (0.3) |
| eGFR, mL/min/1.73 m2 | 86.5 (26.5) |
| Glucose, mg/dL | 93.7 (8.1) |
| Cholesterol, mg/dL | 200.7 (34.0) |
| Albumin, g/dL | 4.3 (0.3) |
| Uric acid, mg/dL | 6.1 (1.6) |
| Na+, mmol/L | 141.1 (1.5) |
| K+, mmol/L | 4.5 (0.4) |
| Random urine Na+, mmol/L | 88.1 (40.1) |
| 24-h urine Na+, mmol/24 h | 124.3 (65.6) |
| Albumin/creatinine ratio (mg/gCr) | 287.1 (155.8–499.5) |
| 24-h urine albumin, mg/24 h | 229.6 (103.0–613.7) |
| App usage | |
| Meals logged, times/week | 16.2 (10.3) |
| Messages sent, times/week | 3.8 (3.4) |
| Visit | 24-h Urine Sodium (mmol/24 h) | Sodium Intake Measured by App (mg/24 h) | Sodium Intake Calculated Using 24-h Urine Sodium (mg/24 h) | p a |
|---|---|---|---|---|
| 1st visit (Baseline) | 124.3 (65.6) | 2020.9 (835.2) | 2857.6 (1508.8) | <0.001 |
| 2nd visit (six months after the initial visit) | 117.4 (58.5) | 1456.0 (687.3) | 2698.3 (1345.2) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, C.-Y.; Kim, Y.; Kim, H.W.; Han, S.H.; Yoo, T.-H.; Kang, S.-W.; Park, J.T. Effectiveness of a Smartphone Application for Dietary Sodium Intake Measurement. Nutrients 2023, 15, 3590. https://doi.org/10.3390/nu15163590
Jung C-Y, Kim Y, Kim HW, Han SH, Yoo T-H, Kang S-W, Park JT. Effectiveness of a Smartphone Application for Dietary Sodium Intake Measurement. Nutrients. 2023; 15(16):3590. https://doi.org/10.3390/nu15163590
Chicago/Turabian StyleJung, Chan-Young, Youngin Kim, Hyung Woo Kim, Seung Hyeok Han, Tae-Hyun Yoo, Shin-Wook Kang, and Jung Tak Park. 2023. "Effectiveness of a Smartphone Application for Dietary Sodium Intake Measurement" Nutrients 15, no. 16: 3590. https://doi.org/10.3390/nu15163590
APA StyleJung, C.-Y., Kim, Y., Kim, H. W., Han, S. H., Yoo, T.-H., Kang, S.-W., & Park, J. T. (2023). Effectiveness of a Smartphone Application for Dietary Sodium Intake Measurement. Nutrients, 15(16), 3590. https://doi.org/10.3390/nu15163590






