Enokitake Mushroom and Its Active Component, Adenosine, Which Restores Testosterone Production in Impaired and Fatigued Mouse Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Mushroom and Other Extracts
2.3. Administration of Enokitake Extract and Adenosine to Animal Models
- (1)
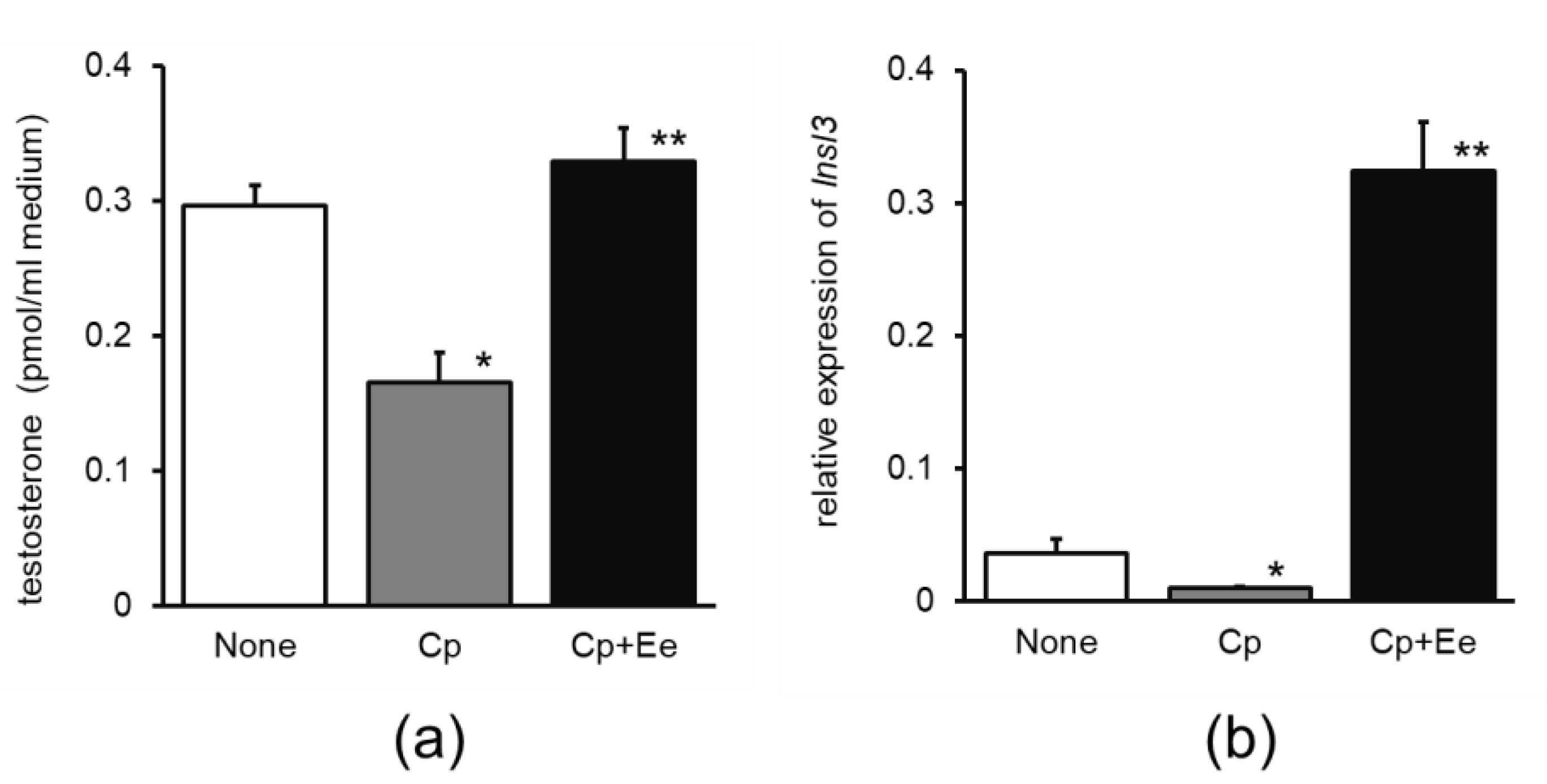
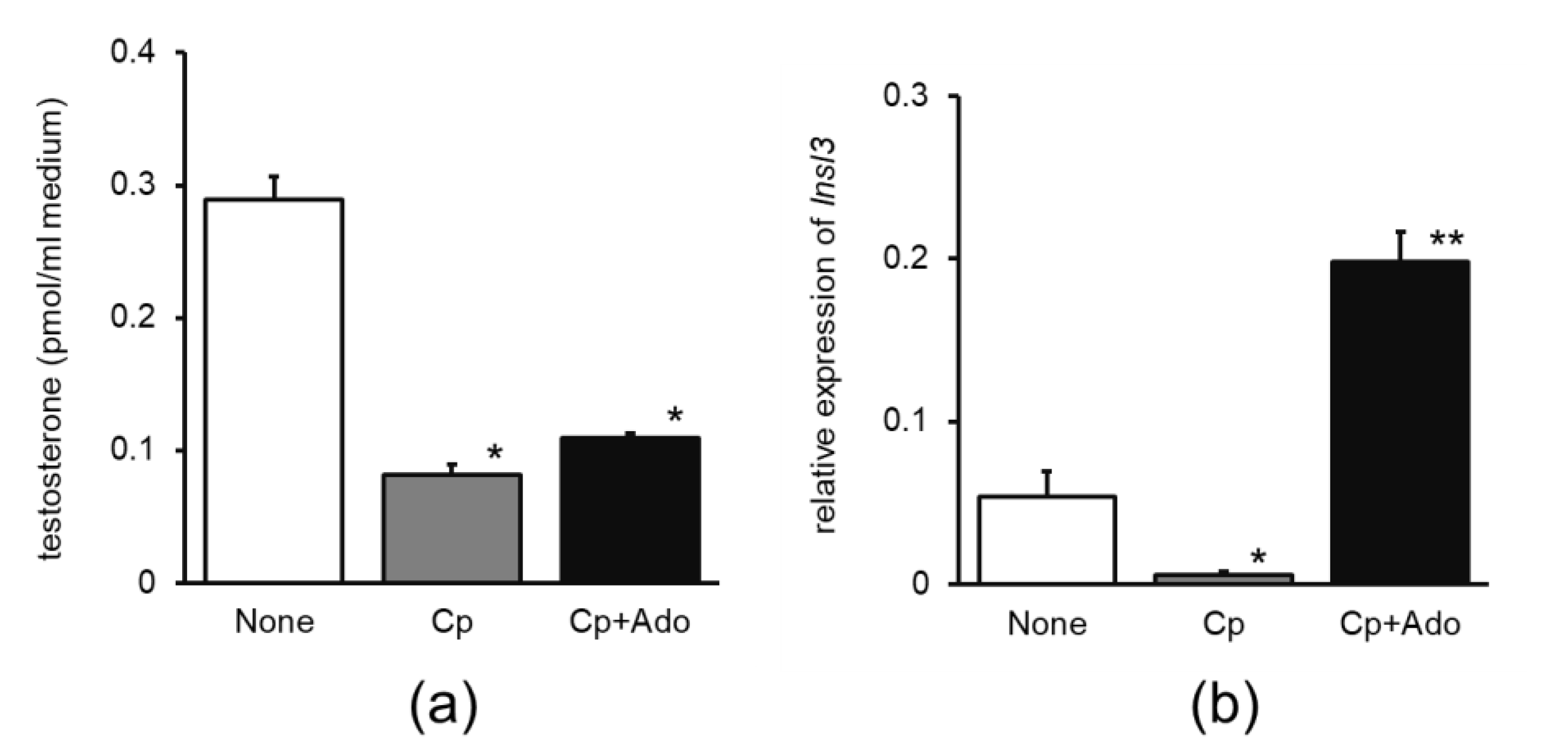
- Cp-impaired model. Mice (ddY, male, 7-week-old; Japan SLC Inc., Shizuoka, Japan) were assigned to three groups (four mice/group) as follows. In experiment 1, mice in the Cp and Cp + Ee groups were intraperitoneally administered Cp (0.2 mg/saline/mouse) on day 5. Mice in the Cp + Ee group were provided drinking water containing 6% of 30% ethanol extract of enokitake. Mice in the control group were provided water containing 1.8% ethanol without Cp. After 14 d, mice were euthanized at 09:00–11:00 h and the testes were dissected for culture and quantitative polymerase chain reaction (qPCR). In experiment 2, the Cp and Cp + Ado group mice were intraperitoneally administered Cp (0.2 mg/saline/mouse). Mice in the Cp + Ado group were provided drinking water containing 0.01% adenosine. Mice in the control group were provided water. After 12 d, the mice were euthanized at 09:00–11:00 h and the testes were dissected for culture and qPCR.
- (2)
- The wet floor fatigue model: Mice (ddY, male, 7-week-old; Japan SLC Inc.) were assigned to two groups (four mice/group) and housed in individual cages on wood chips. The test group was allowed to drink 1:20 diluted 30% ethanol extract of enokitake or 0.1% adenosine solution for 10 d. The mice were transferred to wet floor cages on day 6. A soft plastic plate was wrapped in six paper towels moistened with water and placed at the bottom of the cages to create a wet floor, and the mice were housed in these cages for 24 h. The amount of water was adjusted to ensure that the extremities of the mice were submerged to a depth of <5 mm, and chow was provided ad libitum. Mice were returned to the same individual cages. After 3 d, the mice were euthanized at 09:00–11:00 h, and the testes were dissected for culture and qPCR.
2.4. Effect of the Extracts and Compounds on the Primary Culture of Mouse Testicular Cells
2.5. EIAs
2.6. Determination and Quantification of Adenosine in Enokitake via LC–MS
2.7. qPCR
2.8. Statistical Analysis
3. Results
3.1. Enokitake Extract and Adenosine Treatment
3.1.1. Effect of Enokitake Extract or Adenosine on Testicular Testosterone Production
3.1.2. Direct Effect of Enokitake Extract on Testosterone Production in Primary Cultures of Testicular Cells
3.2. LC–MS Analysis of Enokitake Extract
3.3. Effect of Enokitake Extract or Adenosine on Testicular Testosterone Production in the Wet Floor Stress Model
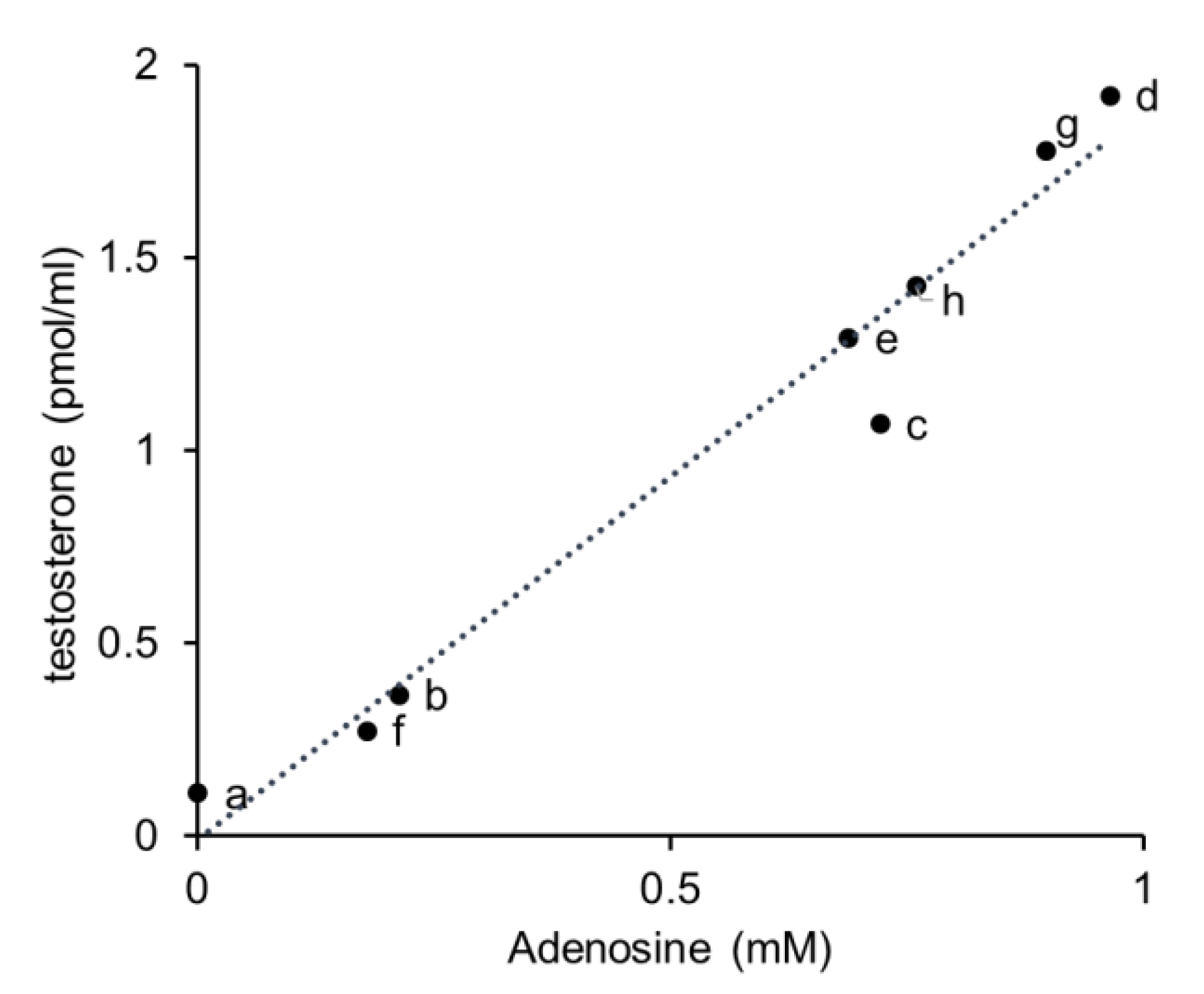
3.4. Correlation of Adenosine Concentration and the Effect of the Extracts of Mushrooms and Vegetables on Testosterone Production
3.5. Effect of Adenosine Structure-Related Compounds on Testosterone Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decaroli, M.C.; Rochira, V. Aging and sex hormones in males. Virulence 2017, 8, 545–570. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. Andropause: Current concepts. Indian J. Endocrinol. Metab. 2013, 17, 621. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, M. Testosterone, mood, behaviour and quality of life. Andrology 2020, 8, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Lopresti, A.L.; Teo, S.Y.M.; Fairchild, T.J. Examining the effects of herbs on testosterone concentrations in men: A systematic review. Adv. Nutr. 2021, 12, 744–765. [Google Scholar] [CrossRef]
- Wankhede, S.; Mohan, V.; Thakurdesai, P. Beneficial effects of fenugreek glycoside supplementation in male subjects during resistance training: A randomized controlled pilot study. J. Sport Health Sci. 2016, 5, 176–182. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R.; Wane, D. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: A randomized, double-blind, placebo-controlled study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef]
- Al-Qarawi, A.A. Stimulatory effect of the aqueous of Ruta chalepensis on the sex organs and hormones of male rats. J. Appl. Res. 2005, 5, 206–211. [Google Scholar]
- Luo, Q.; Li, Z.; Huang, X.; Yan, J.; Zhang, S.; Cai, Y.Z. Lycium barbarum polysaccharides: Protective effects against heat-induced damage of rat testes and H2O2-induced DNA damage in mouse testicular cells and beneficial effect on sexual behavior and reproductive function of hemicastrated rats. Life Sci. 2006, 79, 613–621. [Google Scholar] [CrossRef]
- Moundipa, F.P.; Kamtchouing, P.; Koueta, N.; Tantchou, J.; Foyang, N.P.R.; Mbiapo, F.T. Effects of aqueous extracts of Hibiscus macranthus and Basella alba in mature rat testis function. J. Ethnopharmacol. 1999, 65, 133–139. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Akanji, M.A.; Oladiji, A.T.; Adesokan, A.A. Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullock Ex Hoyl. stem in male wistar rats. J. Ethnopharmacol. 2008, 118, 508–513. [Google Scholar] [CrossRef]
- Mbongue, F.G.Y.; Kamtchouing, P.; Essame, O.; Yewah, P.M.; Dimo, T.; Lontsi, D. Effect of the aqueous extract of dry fruits of Piper guineense on the reproductive function of adult male rats. Indian J. Pharmacol. 2005, 37, 30–32. [Google Scholar] [CrossRef]
- Gauthaman, K.; Ganesan, A.P. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction—An evaluation using primates, rabbit and rat. Phytomedicine 2008, 15, 44–54. [Google Scholar] [CrossRef]
- Nantia, E.A.; Moundipa, P.F.; Monsees, T.K.; Carreau, S. Medicinal plants as potential male anti-infertility agents: A review. Andrologie 2009, 19, 148–158. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Sharma, V.; Dixit, V.K.; Thakur, M. A review on plants used for improvement of sexual performance and virility. BioMed Res. Int. 2014, 2014, 868062. [Google Scholar] [CrossRef]
- Hsu, C.C.; Huang, Y.L.; Tsai, S.J.; Sheu, C.C.; Huang, B.M. In vivo and in vitro stimulatory effects of Cordyceps Sinensis on testosterone production in mouse Leydig cells. Life Sci. 2003, 73, 2127–2136. [Google Scholar] [CrossRef]
- Kusama, K.; Miyagawa, M.; Ota, K.; Kuwabara, N.; Saeki, K.; Ohnishi, Y.; Kumaki, Y.; Aizawa, T.; Nakasone, T.; Okamatsu, S.; et al. Cordyceps militaris fruit body extract decreases testosterone catabolism and testosterone-stimulated prostate hypertrophy. Nutrients 2021, 13, 50. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, E.K.; Sung, S.H.; Fan, M.; Tang, Y.; Hwang, Y.J. Effect of Paecilomyces tenuipes extract on testosterone-induced benign prostatic hyperplasia in Sprague–Dawley rats. Int. J. Environ. Res. Public Health 2019, 16, 3764. [Google Scholar] [CrossRef]
- Okolo, K.O.; Siminialayi, I.M.; Orisakwe, O.E. Protective effects of Pleurotus tuber-regium on carbon-tetrachloride induced testicular injury in Sprague Dawley rats. Front. Pharmacol. 2016, 7, 480. [Google Scholar] [CrossRef]
- Okolo, K.O.; Orisakwe, O.E.; Siminialayi, I.M. Pleurotus tuber-regium mushrooms in the diet of rats ameliorates reproductive and testicular injury caused by carbon tetrachloride. Clin. Phytosci. 2017, 3, 14. [Google Scholar] [CrossRef]
- Danadapat, S.; Kumar, M.; Ranjan, R.; Sinha, M.P. Impact of mushroom Pleurotus tuber-regium (Rumph. Ex) Fr. extract on lipid profile and testosterone of rat. Turk. J. Food Agric. Sci. 2020, 8, 2268–2276. [Google Scholar] [CrossRef]
- Iqbal, T.; Jahan, S.; Ul Ain, Q.; Ullah, H.; Li, C.; Chen, L.; Zhou, X. Ameliorative effects of morel mushroom (Morchella esculenta) against cadmium-induced reproductive toxicity in adult male rats. Braz. J. Biol. 2022, 82, e250865. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, Y.-H.; Pan, B.-S.; Chang, M.-M.; Huang, B.-M. Functional study of Cordyceps sinensis and cordycepin in male reproduction: A review. J. Food Drug Anal. 2016, 25, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Assemie, A.; Abaya, G. The effect of edible mushroom on health and their biochemistry. Int. J. Microbiol. 2022, 2022, 8744788. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Hoo, P.C.X.; Tan, L.T.H.; Pusparajah, P.; Khan, T.M.; Lee, L.H.; Goh, B.H.; Chan, K.G. Golden needle mushroom: A culinary medicine with evidenced-based biological activities and health promoting properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Wang, Y.; Cui, S. Toxic effects of zearalenone and α-zearalenol on the regulation of steroidogenesis and testosterone production in mouse Leydig cells. Toxicol. Vitro 2007, 21, 558–565. [Google Scholar] [CrossRef]
- Hungerford, N.L.; Sortais, B.; Smart, C.G.; McKinney, A.R.; Ridley, D.D.; Stenhouse, A.M.; Suann, C.J.; Munn, K.J.; Sillence, M.N.; McLeod, M.D. Analysis of anabolic steroids in the horse: Development of a generic ELISA for the screening of 17α-alkyl anabolic steroid metabolites. J. Steroid Biochem. Mol. Biol. 2005, 96, 317–334. [Google Scholar] [CrossRef]
- Dressendörfer, R.A.; Kirschbaum, C.; Rohde, W.; Stahl, F.; Strasburger, C.J. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. Mol. Biol. 1992, 43, 683–692. [Google Scholar] [CrossRef]
- Sanada, S.; Suzuki, T.; Nagata, A.; Hashidume, T.; Yoshikawa, Y.; Miyoshi, N. Intestinal microbial metabolite stercobilin involvement in the chronic inflammation of Ob/Ob mice. Sci. Rep. 2020, 10, 6479. [Google Scholar] [CrossRef]
- Hashidume, T.; Sakano, T.; Mochizuki, A.; Ito, K.; Ito, S.; Kawarasaki, Y.; Miyoshi, N. Identification of soybean peptide leginsulin variants in different cultivars and their insulin-like activities. Sci. Rep. 2018, 8, 16847. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Sengupta, P.; Durairajanayagam, D.; Henkel, R.; Sadeghi, M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018, 16, 87. [Google Scholar] [CrossRef]
- Soni, K.K.; Kim, H.K.; Choi, B.R.; Karna, K.K.; You, J.H.; Cha, J.S.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Park, J.K. Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: Reactive oxygen species and endoplasmic reticulum stress. Drug. Des. Dev. Ther. 2016, 10, 3959–3968. [Google Scholar] [CrossRef]
- Mori Sequeiros García, M.; Acquier, A.; Suarez, G.; Gomez, N.V.; Gorostizaga, A.; Mendez, C.F.; Paz, C. Cisplatin inhibits testosterone synthesis by a mechanism that includes the action of reactive oxygen species (ROS) at the level of P450scc. Chem. Biol. Interact. 2012, 199, 185–191. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, H.; Xu, J. Characterization, antioxidant and anti-inflammation capacities of fermented Flammulina velutipes polyphenols. Molecules 2021, 26, 6205. [Google Scholar] [CrossRef]
- Rahman, M.A.; Abdullah, N.; Aminudin, N. Antioxidative effects and inhibition of human low density lipoprotein oxidation in vitro of polyphenolic compounds in Flammulina velutipes (golden needle mushroom). Oxidative Med. Cell. Longev. 2015, 2015, 403023. [Google Scholar] [CrossRef]
- Chu, A.J. Quarter-century explorations of bioactive polyphenols: Diverse health benefits. Front. Biosci. 2022, 27, 134. [Google Scholar] [CrossRef]
- Wang, T.E.; Lai, Y.H.; Yang, K.C.; Lin, S.J.; Chen, C.L.; Tsai, P.S. Counteracting cisplatin-induced testicular damages by natural polyphenol constituent honokiol. Antioxidants 2020, 9, 723. [Google Scholar] [CrossRef]
- Tian, M.; Liu, F.; Liu, H.; Zhang, Q.; Li, L.; Hou, X.; Zhao, J.; Li, S.; Chang, X.; Sun, Y. Grape seed procyanidins extract attenuates cisplatin-induced oxidative stress and testosterone synthase inhibition in rat testes. Syst. Biol. Reprod. Med. 2018, 64, 246–259. [Google Scholar] [CrossRef]
- Ilbey, Y.O.; Ozbek, E.; Cekmen, M.; Simsek, A.; Otunctemur, A.; Somay, A. Protective effect of curcumin in cisplatin-induced oxidative injury in rat testis: Mitogen-activated protein kinase and nuclear factor-kappa B signaling pathways. Hum. Reprod. 2009, 24, 1717–1725. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.; Li, Y.; Pei, C.; Olatunji, O.J.; Tang, J.; Famurewa, A.C.; Wang, H.; Yan, B. Protective effects of nucleosides-rich extract from Cordyceps cicadae against cisplatin induced testicular damage. Chem. Biodivers. 2020, 17, e2000671. [Google Scholar] [CrossRef]
- Ivell, R.; Wade, J.D.; Anand-Ivell, R. INSL3 as a biomarker of Leydig cell functionality. Biol. Reprod. 2013, 88, 147. [Google Scholar] [CrossRef]
- Esteban-Lopez, M.; Agoulnik, A.I. Diverse functions of insulin-like 3 peptide. J. Endocrinol. 2020, 247, R1–R12. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Urtasun, N.; Pérez-Torras, S. Intestinal nucleoside transporters: Function, expression, and regulation. Compr. Physiol. 2018, 8, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.W.; Wilson, H.C. Studies in vitro of the digestion and absorption of purine ribonucleotides by the intestine. J. Biol. Chem. 1962, 237, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Salati, L.M.; Gross, C.J.; Henderson, L.M.; Savaiano, D.A. Absorption and metabolism of adenine, adenosine-5′-monophosphate, adenosine and hypoxanthine by the isolated vascularly perfused rat small intestine. J. Nutr. 1984, 114, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.; Cook, G.H. Activation of steroidogenesis and adenylate cyclase by adenosine in adrenal and Leydig tumor cells. J. Biol. Chem. 1977, 252, 687–693. [Google Scholar] [CrossRef]
- Rommerts, F.F.G.; Molenaar, R.; Hoogerbrugge, J.W.; van der Molen, H.J. Development of adenosine responsiveness after isolation of Leydig cells. Biol. Reprod. 1984, 30, 842–847. [Google Scholar] [CrossRef]
- Leu, S.F.; Poon, S.L.; Pao, H.Y.; Huang, B.M. The in vivo and in vitro stimulatory effects of cordycepin on mouse Leydig cell steroidogenesis. Biosci. Biotechnol. Biochem. 2011, 75, 723–731. [Google Scholar] [CrossRef]
- Huang, B.M.; Pan, B.S.; Lin, C.Y. The effect of cordycepin on steroidogenesis and apoptosis in MA-10 mouse Leydig tumor cells. Evid. Based Complement. Altern. Med. 2011, 2011, 750468. [Google Scholar] [CrossRef]
- Kopalli, S.R.; Cha, K.M.; Lee, S.H.; Hwang, S.Y.; Lee, Y.J.; Koppula, S.; Kim, S.K. Cordycepin, an active constituent of nutrient powerhouse and potential medicinal mushroom Cordyceps militaris Linn., ameliorates age-related testicular dysfunction in rats. Nutrients 2019, 11, 906. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.Z.; Chen, Y.Y.; Wang, X.F.; Zhou, X.W. Determination and analysis of cordycepin and adenosine in the products of Cordyceps Spp. Afr. J. Microbiol. Res. 2009, 3, 957–961. [Google Scholar]
- Monaco, L.; Conti, M. Localization of adenosine receptors in rat testicular cells. Biol. Reprod. 1986, 35, 258–266. [Google Scholar] [CrossRef]
- Conti, M.; Boitani, C.; Demanno, D.; Migliaccio, S.; Monaco, L.; Szymeczek, C. Characterization and function of adenosine receptors in the testis. Ann. N. Y. Acad. Sci. 1989, 564, 39–47. [Google Scholar] [CrossRef]
- Rivkees, S.A. Localization and characterization of adenosine receptor expression in rat testis. Endocrinology 1994, 135, 2307–2313. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakamura, F.; Mizokawa, S.; Matsumura, A.; Nozaki, S.; Watanabe, Y. Establishment and assessment of a rat model of fatigue. Neurosci. Lett. 2003, 352, 159–162. [Google Scholar] [CrossRef]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to cordycepin. 3 Biotech 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Sohn, S.H.; Lee, S.C.; Hwang, S.Y.; Kim, S.W.; Kim, I.W.; Ye, M.B.; Kim, S.K. Effect of long-term administration of cordycepin from Cordyceps militaris on testicular function in middle-aged rats. Planta Med. 2012, 78, 1620–1625. [Google Scholar] [CrossRef]
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from mushroom: Health attributes and food industry applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef]
- Gunes, S.; Hekim, G.N.T.; Arslan, M.A.; Asci, R. Effects of aging on the male reproductive system. J. Assist. Reprod. Genet. 2016, 33, 441–454. [Google Scholar] [CrossRef]
- Haskó, G.; Antonioli, L.; Cronstein, B.N. Adenosine metabolism, immunity and joint health. Biochem. Pharmacol. 2018, 151, 307–313. [Google Scholar] [CrossRef]
- Fredholm, B.B. Adenosine—A physiological or pathophysiological agent? J. Mol. Med. 2014, 92, 201–206. [Google Scholar] [CrossRef]
- Missel, A.; Walenta, L.; Eubler, K.; Mundt, N.; Heikelä, H.; Pickl, U.; Trottmann, M.; Popper, B.; Poutanen, M.; Strauss, L.; et al. Testicular adenosine acts as a pro-inflammatory molecule: Role of testicular peritubular cells. Mol. Hum. Reprod. 2021, 27, gaab037. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef] [PubMed]
- Belardin, L.B.; Brochu, K.; Légaré, C.; Battistone, M.A.; Breton, S. Purinergic signaling in the male reproductive tract. Front. Endocrinol. 2022, 13, 1049511. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M. Paracrine control of the testis. Clin. Endocrinol. Metab. 1986, 15, 185–207. [Google Scholar] [CrossRef]
- Huleihel, M.; Lunenfeld, E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J. Androl. 2004, 6, 259–268. [Google Scholar]
- Foresta, C.; Rossato, M.; Nogara, A.; Gottardello, F.; Bordon, P.; Di Virgilio, F. Role of P2-purinergic receptors in rat Leydig cell steroidogenesis. Biochem. J. 1996, 320, 499–504. [Google Scholar] [CrossRef]
- Artur Poletto Chaves, L.; Piva Pontelli, E.; Antonio Varanda, W.; Chaves, P.; Artur, L.; Antonio Varanda, W. P2X receptors in mouse Leydig cells. Am. J. Physiol. Cell Physiol. 2006, 290, 1009–1017. [Google Scholar] [CrossRef]
- Bjelobaba, I.; Janjic, M.M.; Stojilkovic, S.S. Purinergic signaling pathways in endocrine system. Auton. Neurosci. 2015, 191, 102–116. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iguchi, K.; Nagashima, K.; Mochizuki, J.; Yamamoto, H.; Unno, K.; Miyoshi, N. Enokitake Mushroom and Its Active Component, Adenosine, Which Restores Testosterone Production in Impaired and Fatigued Mouse Models. Nutrients 2023, 15, 2142. https://doi.org/10.3390/nu15092142
Iguchi K, Nagashima K, Mochizuki J, Yamamoto H, Unno K, Miyoshi N. Enokitake Mushroom and Its Active Component, Adenosine, Which Restores Testosterone Production in Impaired and Fatigued Mouse Models. Nutrients. 2023; 15(9):2142. https://doi.org/10.3390/nu15092142
Chicago/Turabian StyleIguchi, Kazuaki, Koji Nagashima, Jun Mochizuki, Hiroyuki Yamamoto, Keiko Unno, and Noriyuki Miyoshi. 2023. "Enokitake Mushroom and Its Active Component, Adenosine, Which Restores Testosterone Production in Impaired and Fatigued Mouse Models" Nutrients 15, no. 9: 2142. https://doi.org/10.3390/nu15092142
APA StyleIguchi, K., Nagashima, K., Mochizuki, J., Yamamoto, H., Unno, K., & Miyoshi, N. (2023). Enokitake Mushroom and Its Active Component, Adenosine, Which Restores Testosterone Production in Impaired and Fatigued Mouse Models. Nutrients, 15(9), 2142. https://doi.org/10.3390/nu15092142







