Risk Assessment of Micronutrients Deficiency in Vegetarian or Vegan Children: Not So Obvious
Abstract
1. Introduction
2. Search Strategy
3. Vitamins
3.1. Vitamin Supply from Foods from Plant Origin
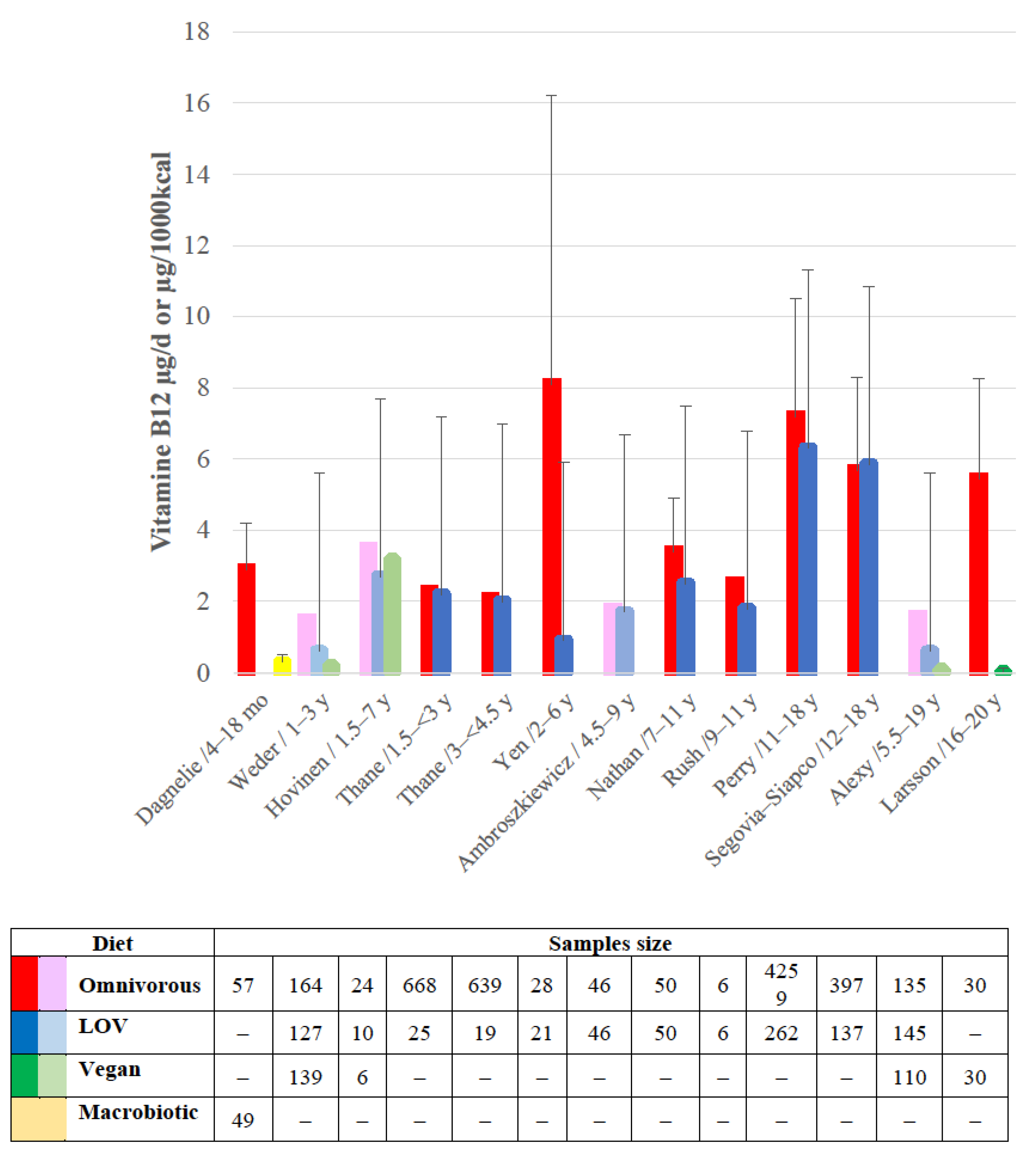
3.2. Vitamin B12 Concern
3.2.1. Newborns and Infants
3.2.2. Children and Adolescents
4. Minerals
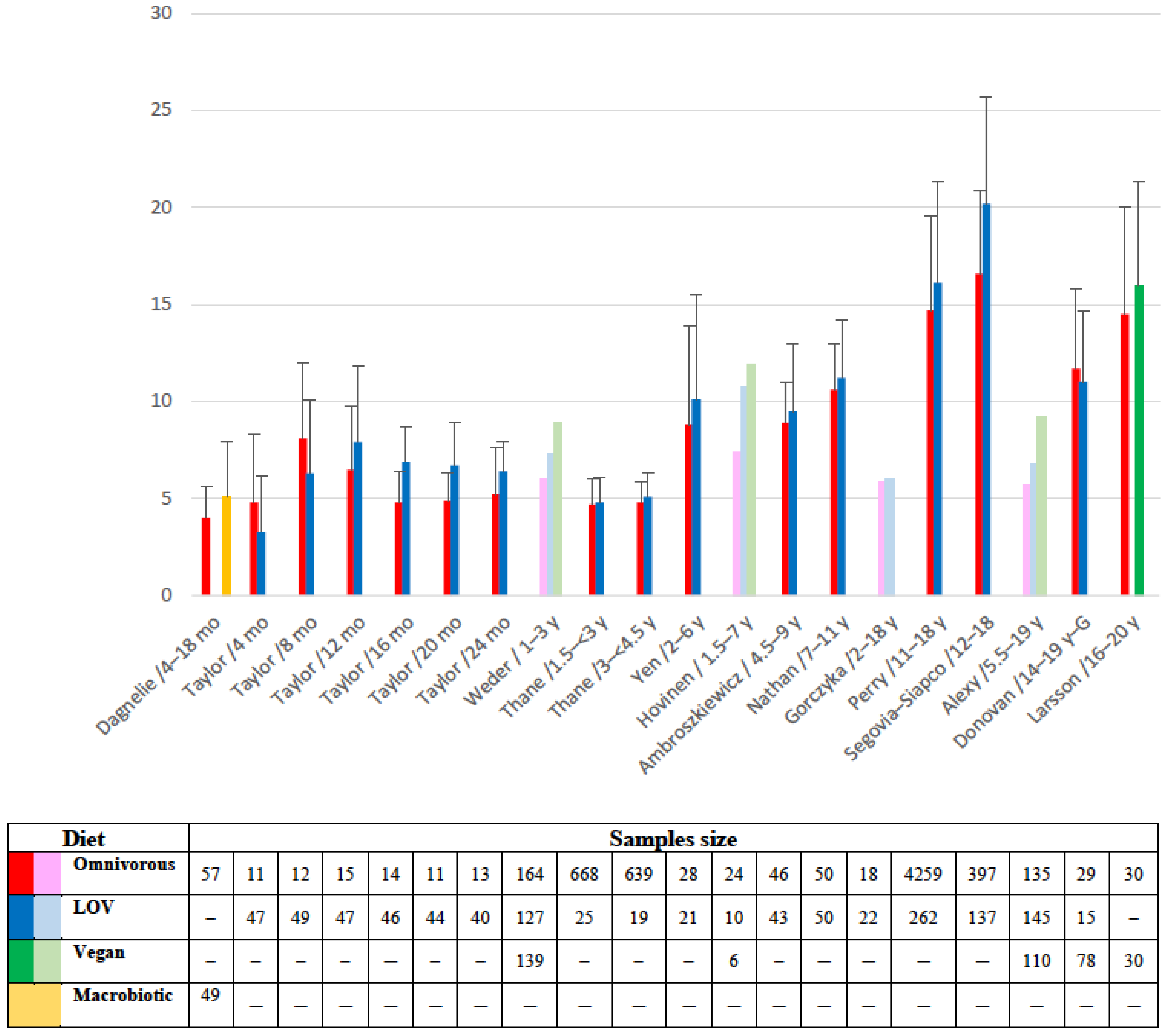
4.1. Iron
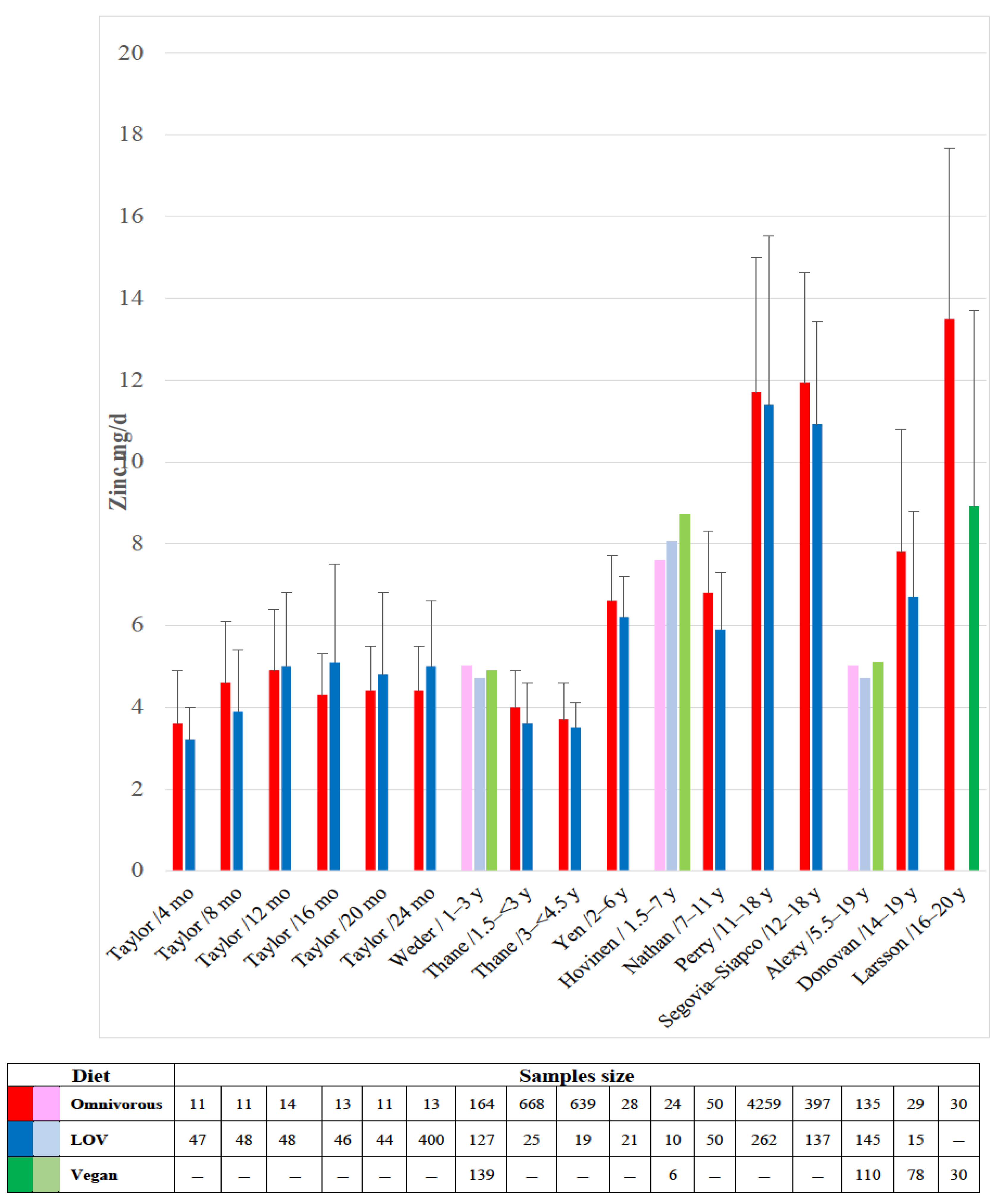
4.2. Zinc
4.3. Iodine
4.4. Other Microminerals
5. Discussion and Recommendations
5.1. Limitations
5.2. Recommendations
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASF | animal food sources |
| FPO | foods of plant origin |
| LOV | lacto-ovo-vegetarian |
References
- World Health Organization. Micronutrients. Geneva. 2022. Available online: https://www.who.int/health-topics/micronutrients#tab=tab_1 (accessed on 6 December 2022).
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451, Erratum in Lancet 2013, 382, 396. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Mullany, L.C.; Hurley, K.M.; Katz, J.; Black, R.E. Nutrition and maternal, neonatal, and child health. Semin. Perinatol. 2015, 39, 361–372, Erratum in Semin. Perinatol 2015, 39, 505. [Google Scholar] [CrossRef] [PubMed]
- Mattei, D.; Pietrobelli, A. Micronutrients and Brain Development. Curr. Nutr. Rep. 2019, 8, 99–107. [Google Scholar] [CrossRef]
- Inzaghi, E.; Pampanini, V.; Deodati, A.; Cianfarani, S. The Effects of Nutrition on Linear Growth. Nutrients 2022, 14, 1752. [Google Scholar] [CrossRef]
- Murphy, S.P.; Allen, L.H. Nutritional importance of animal source foods. J. Nutr. 2003, 133 (Suppl. S2), 3932S–3935S. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Salam, R.A.; Mahmood, S.B.; Moin, A.; Kumar, R.; Mukhtar, K.; Lassi, Z.S.; Bhutta, Z.A. Food fortification with multiple micronutrients: Impact on health outcomes in general population. Cochrane Database Syst. Rev 2019, 12, CD011400. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- World Health Organization. Ambition and Action in Nutrition 2016–2025. Geneva. 2017. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://apps.who.int/iris/bitstream/handle/10665/255485/9789241512435-eng.pdf?ua=1 (accessed on 6 December 2022).
- Allen, L.H. To what extent can food-based approaches improve micronutrient status? Asia. Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 103–105. [Google Scholar]
- Consalez, F.; Ahern, M.; Andersen, P.; Kjellevold, M. The Effect of the Meat Factor in Animal-Source Foods on Micronutrient Absorption: A Scoping Review. Adv. Nutr. 2022, 13, 2305–2315. [Google Scholar] [CrossRef]
- Rudloff, S.; Bührer, C.; Jochum, F.; Kauth, T.; Kersting, M.; Körner, A.; Koletzko, B.; Mihatsch, W.; Prell, C.; Reinehr, T.; et al. Vegetarian diets in childhood and adolescence: Position paper of the nutrition committee, German Society for Paediatric and Adolescent Medicine (DGKJ). Mol. Cell. Pediatr. 2019, 6, 4. [Google Scholar] [CrossRef]
- Bettinelli, M.E.; Bezze, E.; Morasca, L.; Plevani, L.; Sorrentino, G.; Morniroli, D.; Giannì, M.; Mosca, F. Knowledge of Health Professionals Regarding Vegetarian Diets from Pregnancy to Adolescence: An Observational Study. Nutrients 2019, 11, 1149. [Google Scholar] [CrossRef]
- Kiely, M.E. Risks and benefits of vegan and vegetarian diets in children. Proc. Nutr. Soc. 2021, 80, 159–164. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, C. Vegetarian nutrition: Past, present, future. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 496S–502S. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.N.; Key, T.J. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Mann, N.J. A brief history of meat in the human diet and current health implications. Meat. Sci. 2018, 144, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.P.; Turck, D.; Briend, A.; Darmaun, D.; Bocquet, A.; Feillet, F.; Frelut, M.L.; Girardet, J.P.; Guimber, D.; Hankard, R.; et al. Committee on Nutrition of the French Society of Pediatrics. Religious dietary rules and their potential nutritional and health consequences. Int. J. Epidemiol. 2021, 50, 12–26. [Google Scholar] [CrossRef]
- Rosenfeld, D.L.; Burrow, A.L. Vegetarian on purpose: Understanding the motivations of plant-based dieters. Appetite 2017, 116, 456–463. [Google Scholar] [CrossRef]
- IPSOS Mori. An Exploration into Diets around the World. 2018. Available online: https://www.ipsos.com/sites/default/files/ct/news/documents/2018-09/an_exploration_into_diets_around_the_world.pdf (accessed on 13 September 2022).
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef]
- Milford, A.B.; Le Mouël, C.; Bodirsky, B.L.; Rolinski, S. Drivers of meat consumption. Appetite 2019, 141, 104313. [Google Scholar] [CrossRef]
- Dorard, G.; Mathieu, S. Vegetarian and omnivorous diets: A cross-sectional study of motivation, eating disorders, and body shape perception. Appetite 2021, 156, 104972. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.M. The effects of meat consumption on global health. Rev. Sci. Tech. 2018, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.E.; Panza, R.; Farella, I.; Posa, D.; Capozza, M.; Mauro, A.D.; Laforgia, N. Vegetarian and Vegan Weaning of the Infant: How Common and How Evidence-Based? A Population-Based Survey and Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 4835. [Google Scholar] [CrossRef]
- Bivi, D.; Di Chio, T.; Geri, F.; Morganti, R.; Goggi, S.; Baroni, L.; Mumolo, M.G.; de Bortoli, N.; Peroni, D.G.; Marchi, S.; et al. Raising Children on a Vegan Diet: Parents’ Opinion on Problems in Everyday Life. Nutrients 2021, 13, 1796. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Oh, J.; Cho, M. Differences between Vegetarians and Omnivores in Food Choice Motivation and Dietarian Identity. Foods 2022, 11, 539. [Google Scholar] [CrossRef]
- Larsen, J.K.; Hermans, R.C.; Sleddens, E.F.; Engels, R.C.; Fisher, J.O.; Kremers, S.P. How parental dietary behavior and food parenting practices affect children’s dietary behavior. Interacting sources of influence? Appetite 2015, 89, 246–257. [Google Scholar] [CrossRef]
- Eurispes. Quanti Sono i Vegani in Italia? 2022. Available online: https://www.veganok.com/vegani-in-italia/ (accessed on 13 September 2022).
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Rauma, A.L.; Mykkänen, H. Antioxidant status in vegetarians versus omnivores. Nutrition 2000, 16, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food. Res. 2019, 63, e1801045, Erratum in: Mol. Nutr. Food Res. 2020, 64, e2070024. [Google Scholar] [CrossRef]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Kujovská Krčmová, L.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef]
- Dagnelie, P.C.; van Staveren, W.A. Macrobiotic nutrition and child health: Results of a population-based, mixed-longitudinal cohort study in The Netherlands. Am. J. Clin. Nutr. 1994, 59 (Suppl. S5), 1187S–1196S. [Google Scholar] [CrossRef]
- Nathan, I.; Hackett, A.F.; Kirby, S. The dietary intake of a group of vegetarian children aged 7–11 years compared with matched omnivores. Br. J. Nutr. 1996, 75, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Donovan, U.M.; Gibson, R.S. Dietary intakes of adolescent females consuming vegetarian, semi–vegetarian, and omnivorous diets. J. Adolesc. Health 1996, 18, 292–300. [Google Scholar] [CrossRef]
- Krajcovicová-Kudlácková, M.; Simoncic, R.; Béderová, A.; Grancicová, E.; Magálová, T. Influence of vegetarian and mixed nutrition on selected haematological and biochemical parameters in children. Nahrung 1997, 41, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Thane, C.W.; Bates, C.J. Dietary intakes and nutrient status of vegetarian preschool children from a British national survey. J. Hum. Nutr. Diet. 2000, 13, 149–162. [Google Scholar] [CrossRef]
- Perry, C.L.; McGuire, M.T.; Neumark-Sztainer, D.; Story, M. Adolescent vegetarians: How well do their dietary patterns meet the healthy people 2010 objectives? Arch. Pediatr. Adolesc. Med. 2002, 156, 431–437. [Google Scholar] [CrossRef]
- Larsson, C.L.; Johansson, G.K. Dietary intake and nutritional status of young vegans and omnivores in Sweden. Am. J. Clin. Nutr. 2002, 76, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.E.; Yen, C.H.; Huang, M.C.; Cheng, C.H.; Huang, Y.C. Dietary intake and nutritional status of vegetarian and omnivorous preschool children and their parents in Taiwan. Nutr. Res. 2008, 28, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.E.; Yen, C.H.; Cheng, C.H.; Huang, Y.C. Vitamin B-12 status is not associated with plasma homocysteine in parents and their preschool children: Lacto-ovo, lacto, and ovo vegetarians and omnivores. J. Am. Coll. Nutr. 2010, 29, 7–13. [Google Scholar] [CrossRef]
- Gorczyca, D.; Prescha, A.; Szeremeta, K.; Jankowski, A. Iron status and dietary iron intake of vegetarian children from Poland. Ann. Nutr. Metab. 2013, 62, 291–297. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Chełchowska, M.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. Bone status and adipokine levels in children on vegetarian and omnivorous diets. Clin. Nutr. 2019, 38, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Siapco, G.; Sabaté, J. Health and sustainability outcomes of vegetarian dietary patterns: A revisit of the EPIC-Oxford and the Adventist Health Study-2 cohorts. Eur. J. Clin. Nutr. 2019, 72 (Suppl. S1), 60–70, Erratum in Eur. J. Clin. Nutr 2019, 73, 968. [Google Scholar] [CrossRef] [PubMed]
- Hovinen, T.; Korkalo, L.; Freese, R.; Skaffari, E.; Isohanni, P.; Niemi, M.; Nevalainen, J.; Gylling, H.; Zamboni, N.; Erkkola, M.; et al. Vegan diet in young children remodels metabolism and challenges the statuses of essential nutrients. EMBO. Mol. Med. 2021, 13, e13492. [Google Scholar] [CrossRef]
- Alexy, U.; Fischer, M.; Weder, S.; Längler, A.; Michalsen, A.; Sputtek, A.; Keller, M. Nutrient Intake and Status of German Children and Adolescents Consuming Vegetarian, Vegan or Omnivore Diets: Results of the VeChi Youth Study. Nutrients 2021, 13, 1707. [Google Scholar] [CrossRef] [PubMed]
- Weder, S.; Keller, M.; Fischer, M.; Becker, K.; Alexy, U. Intake of micronutrients and fatty acids of vegetarian, vegan, and omnivorous children (1–3 years) in Germany (VeChi Diet Study). Eur. J. Nutr. 2022, 61, 1507–1520. [Google Scholar] [CrossRef]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. ESPGHAN Committee on Nutrition. Vitamin D in the healthy European paediatric population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, S.; Yuan, X.; Wang, Y.; Liu, Y.; Zhou, J. The role of vitamin D deficiency in the development of paediatric diseases. Ann. Med. 2023, 55, 127–135. [Google Scholar] [CrossRef]
- Jullien, S. Vitamin D prophylaxis in infancy. BMC Pediatr. 2021, 21 (Suppl. S1), 319. [Google Scholar] [CrossRef]
- Bacchetta, J.; Edouard, T.; Laverny, G.; Bernardor, J.; Bertholet-Thomas, A.; Castanet, M.; Garnier, C.; Gennero, I.; Harambat, J.; Lapillonne, A.; et al. Vitamin D and calcium intakes in general pediatric populations: A French expert consensus paper. Arch. Pediatr. 2022, 29, 312–325. [Google Scholar] [CrossRef]
- Chittaranjan, Y. Vitamin B12: An intergenerational story. Nestle. Nutr. Inst. Workshop Ser. 2020, 93, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Bjørke-Monsen, A.L.; Ueland, P.M. Cobalamin status in children. J. Inherit. Metab. Dis. 2011, 34, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F.; Yabuta, Y.; Tanioka, Y.; Bito, T. Biologically active vitamin B12 compounds in foods for preventing deficiency among vegetarians and elderly subjects. J. Agric. Food Chem. 2013, 61, 6769–6775. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.; La Ferrera, G.M.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among vegetarians: Status, assessment and supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef]
- Koebnick, C.; Hoffmann, I.; Dagnelie, P.C.; Heins, U.A.; Wickramasinghe, S.N.; Ratnayaka, I.D.; Gruendel, S.; Lindemans, J.; Leitzmann, C. Long-term ovo-lacto vegetarian diet impairs vitamin B-12 status in pregnant women. J. Nutr. 2004, 134, 3319–3326. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Layden, A.J.; Stover, P.J. Vitamin B-12 and Perinatal Health. Adv. Nutr. 2015, 6, 552–563. [Google Scholar] [CrossRef]
- Varsi, K.; Ueland, P.M.; Torsvik, I.K.; Bjørke-Monsen, A.L. Maternal Serum Cobalamin at 18 Weeks of Pregnancy Predicts Infant Cobalamin Status at 6 Months-A Prospective, Observational Study. J. Nutr. 2018, 148, 738–745. [Google Scholar] [CrossRef]
- Reischl-Hajiabadi, A.T.; Garbade, S.F.; Feyh, P.; Weiss, K.H.; Mütze, U.; Kölker, S.; Hoffmann, G.F.; Gramer, G. Maternal vitamin B12 deficiency detected by newborn screening—Evaluation of causes and characteristics. Nutrients 2022, 14, 3767. [Google Scholar] [CrossRef]
- Perrin, M.T.; Pawlak, R.; Dean, L.L.; Christis, A.; Friend, L. A cross-sectional study of fatty acids and brain-derived neurotrophic factor (BDNF) in human milk from lactating women following vegan, vegetarian, and omnivore diets. Eur. J. Nutr. 2019, 58, 2401–2410. [Google Scholar] [CrossRef]
- Karcz, K.; Królak-Olejnik, B. Vegan or vegetarian diet and breast milk composition—A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1081–1098. [Google Scholar] [CrossRef]
- Mathey, C.; Di Marco, J.N.; Poujol, A.; Cournelle, M.A.; Brevaut, V.; Livet, M.O.; Chabrol, B.; Michel, G. Stagnation pondérale et régression psychomotrice révélant une carence en vitamine B12 chez 3 nourrissons [Failure to thrive and psychomotor regression revealing vitamin B12 deficiency in 3 infants]. Arch. Pediatr. 2007, 14, 467–471. [Google Scholar] [CrossRef]
- Sahgal, N.; Evans, J.; Salazar, A.M.; Starr, K.J.; Corichi, M. Religion in India: Tolerance and Segregation. 10: Religion and Food. Pew Research Center 2021. Available online: https://www.pewresearch.org/religion/2021/06/29/religion-and-food/ (accessed on 13 February 2023).
- Kadiyala, A.; Palani, A.; Rajendraprasath, S.; Venkatramanan, P. Prevalence of vitamin B12 deficiency among exclusively breast fed term infants in South India. J. Trop. Pediatr. 2021, 67, fmaa114. [Google Scholar] [CrossRef]
- Dubaj, C.; Czyż, K.; Furmaga-Jabłońska, W. Vitamin B12 deficiency as a cause of severe neurological symptoms in breast fed infant—A case report. Ital. J. Pediatr. 2020, 46, 40. [Google Scholar] [CrossRef]
- Honzik, T.; Adamovicova, M.; Smolka, V.; Magner, M.; Hruba, E.; Zeman, J. Clinical presentation and metabolic consequences in 40 breastfed infants with nutritional vitamin B12 deficiency--what have we learned? Eur. J. Paediatr. Neurol. 2010, 14, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kocaoglu, C.; Akin, F.; Caksen, H.; Böke, S.B.; Arslan, S.; Aygün, S. Cerebral atrophy in a vitamin B12-deficient infant of a vegetarian mother. J. Health Popul. Nutr. 2014, 32, 367–371. [Google Scholar] [PubMed]
- Reghu, A.; Hosdurga, S.; Sandhu, B.; Spray, C. Vitamin B12 deficiency presenting as oedema in infants of vegetarian mothers. Eur. J. Pediatr. 2005, 164, 257–258. [Google Scholar] [CrossRef]
- von Schenck, U.; Bender-Götze, C.; Koletzko, B. Persistence of neurological damage induced by dietary vitamin B-12 deficiency in infancy. Arch. Dis. Child. 1997, 77, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Ambroszkiewicz, J.; Klemarczyk, W.; Mazur, J.; Gajewska, J.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Serum Hepcidin and Soluble Transferrin Receptor in the Assessment of Iron Metabolism in Children on a Vegetarian Diet. Biol. Trace. Elem. Res. 2017, 180, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C.; Chhichhia, P.; Hinckson, E.; Nabiryo, C. Dietary patterns and vitamin B(12) status of migrant Indian preadolescent girls. Eur. J. Clin. Nutr 2009, 63, 585–587. [Google Scholar] [CrossRef]
- Agnoli, C.; Baroni, L.; Bertini, I.; Ciappellano, S.; Fabbri, A.; Papa, M.; Pellegrini, N.; Sbarbati, R.; Scarino, M.L.; Siani, V.; et al. Position paper on vegetarian diets from the working group of the Italian Society of Human Nutrition. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Schneede, J.; Dagnelie, P.C.; van Staveren, W.A.; Vollset, S.E.; Refsum, H.; Ueland, P.M. Methylmalonic acid and homocysteine in plasma as indicators of functional cobalamin deficiency in infants on macrobiotic diets. Pediatr. Res. 1994, 36, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Světnička, M.; Sigal, A.; Selinger, E.; Heniková, M.; El-Lababidi, E.; Gojda, J. Cross-Sectional Study of the Prevalence of Cobalamin Deficiency and Vitamin B12 Supplementation Habits among Vegetarian and Vegan Children in the Czech Republic. Nutrients 2022, 14, 535. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.S.; Lee, R.H.; Sung, R.Y.; Luo, H.Y.; Kam, C.W.; Yuen, M.P.; Hjelm, M.; Lee, S.H. Growth and nutrition of Chinese vegetarian children in Hong Kong. J. Paediatr. Child. Health 2001, 37, 247–253. [Google Scholar] [CrossRef]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How prevalent is vitamin B(12) deficiency among vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef]
- Kalyan, G.B.; Mittal, M.; Jain, R. Compromised vitamin B12 status of Indian infants and toddlers. Food. Nutr. Bull. 2020, 41, 430–437. [Google Scholar] [CrossRef]
- Ferrara, P.; Corsello, G.; Quattrocchi, E.; Dell’Aquila, L.; Ehrich, J.; Giardino, I.; Pettoello-Mantovani, M. Caring for Infants and Children Following Alternative Dietary Patterns. J. Pediatr. 2017, 187, 339–340.e1. [Google Scholar] [CrossRef]
- Miller, D.R.; Specker, B.L.; Ho, M.L.; Norman, E.J. Vitamin B-12 status in a macrobiotic community. Am. J. Clin. Nutr. 1991, 53, 524–529. [Google Scholar] [CrossRef]
- Goraya, J.S.; Kaur, S.; Mehra, B. Neurology of Nutritional Vitamin B12 Deficiency in Infants: Case Series from India and Literature Review. J. Child. Neurol. 2015, 30, 1387–1831. [Google Scholar] [CrossRef]
- Johnson, M.A. If high folic acid aggravates vitamin B12 deficiency what should be done about it? Nutr. Rev. 2007, 65, 451–458. [Google Scholar] [CrossRef]
- Chouraqui, J.P. Dietary Approaches to Iron Deficiency Prevention in Childhood-A Critical Public Health Issue. Nutrients 2022, 14, 1604. [Google Scholar] [CrossRef]
- Willoughby, J.L.; Bowen, C.N. Zinc deficiency and toxicity in pediatric practice. Curr. Opin. Pediatr. 2014, 26, 579–584. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Bioavailability of Micronutrients from Plant Foods: An Update. Crit. Rev. Food Sci. Nutr. 2016, 56, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Krebs, N.F.; Hambidge, K.M. Trace elements. In Nutrition in Pediatrics, 5th ed.; Duggan, C., Watkins, J.B., Koletzko, B., Walker, W.A., Eds.; Peoples Medical Publishing House: Shelton, CT, USA, 2016; Volume 1, pp. 95–116. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Mensink, G.B.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and Mineral Status in a Vegan Diet. Dtsch. Arztebl. Int. 2020, 117, 575–582. [Google Scholar] [CrossRef]
- Morris, A.L.; Mohiuddin, S.S. Biochemistry, Nutrients; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554545/ (accessed on 8 March 2023).
- Taylor, A.; Redworth, E.W.; Morgan, J.B. Influence of diet on iron, copper, and zinc status in children under 24 months of age. Biol. Trace. Elem. Res. 2004, 97, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Le Louer, B.; Lemale, J.; Garcette, K.; Orzechowski, C.; Chalvon, A.; Girardet, J.P.; Tounian, P. Conséquences nutritionnelles de l’utilisation de boissons végétales inadaptées chez les nourrissons de moins d’un an [Severe nutritional deficiencies in young infants with inappropriate plant milk consumption]. Arch. Pediatr. 2014, 21, 483–488. [Google Scholar] [CrossRef]
- Maywald, M.; Rink, L. Zinc in Human Health and Infectious Diseases. Biomolecules 2022, 12, 1748. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Panel on Dietetic Products Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef]
- Eveleigh, E.R.; Coneyworth, L.J.; Avery, A.; Welham, S.J.M. Vegans, Vegetarians, and Omnivores: How Does Dietary Choice Influence Iodine Intake? A Systematic Review. Nutrients 2020, 12, 1606. [Google Scholar] [CrossRef]
- Groufh-Jacobsen, S.; Hess, S.Y.; Aakre, I.; Folven Gjengedal, E.L.; Blandhoel Pettersen, K.; Henjum, S. Vegans, Vegetarians and Pescatarians Are at Risk of Iodine Deficiency in Norway. Nutrients 2020, 12, 3555. [Google Scholar] [CrossRef] [PubMed]
- Comerford, K.B.; Miller, G.D.; Reinhardt Kapsak, W.; Brown, K.A. The Complementary Roles for Plant-Source and Animal-Source Foods in Sustainable Healthy Diets. Nutrients 2021, 13, 3469. [Google Scholar] [CrossRef] [PubMed]
- Messina, V.; Mangels, A.R. Considerations in planning vegan diets: Children. J. Am. Diet. Assoc. 2001, 101, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Boeing, H.; Grünewald-Funk, D.; Heseker, H.; Kroke, A.; Leschik-Bonnet, E.; Oberritter, H.; Strohm, D.; Watzl, B. Vegan diet. Position of the German Nutrition Society (DGE). Ernähr. Umsch. 2016, 63, 92–102, Erratum in Ernähr. Umsch. 2016, 63, M262. [Google Scholar] [CrossRef]
- Menal-Puey, S.; Marques-Lopes, I. Development of a food guide for the vegetarians of Spain. J. Acad. Nutr. Diet. 2017, 117, 1509–1516. [Google Scholar] [CrossRef]
- Federal Commission for Nutrition (FCN). Vegan Diets: Review of Nutritional Benefits and Risks. Expert Report of the FCN. Bern: Federal Food Safety and Veterinary Office. 2018. Available online: https://www.blv.admin.ch/blv/en/home/das-blv/organisation/kommissionen/eek/vor-und-nachteile-vegane-ernaehrung.html (accessed on 13 February 2023).
- Dietary Guidelines Advisory Committee. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf (accessed on 13 February 2023).
- Van Winckel, M.; Vande Velde, S.; De Bruyne, R.; Van Biervliet, S. Clinical practice: Vegetarian infant and child nutrition. Eur. J. Pediatr. 2011, 170, 1489–1494. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan Nutrition for Mothers and Children: Practical Tools for Healthcare Providers. Nutrients 2018, 11, 5. [Google Scholar] [CrossRef]
- Müller, P. Vegan diet in young children. Nestle. Nutr. Inst. Workshop Ser. 2020, 93, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.P.; Turck, D.; Tavoularis, G.; Ferry, C.; Dupont, C. The Role of Young Child Formula in Ensuring a Balanced Diet in Young Children (1–3 Years Old). Nutrients 2019, 11, 2213. [Google Scholar] [CrossRef]
- Bocquet, A.; Dupont, C.; Chouraqui, J.P.; Darmaun, D.; Feillet, F.; Frelut, M.L.; Girardet, J.P.; Hankard, R.; Lapillonne, A.; Rozé, J.C.; et al. Committee on Nutrition of the French Society of Pediatrics (CNSFP). Efficacy and safety of hydrolyzed rice-protein formulas for the treatment of cow’s milk protein allergy. Arch. Pediatr. 2019, 26, 238–246. [Google Scholar] [CrossRef]
- Amit, M. Vegetarian diets in children and adolescents. Paediatr. Child Health 2010, 15, 303–314. [Google Scholar]
- National Health and Medical Research Council. Eat for health—Australian dietary guidelines. 2013; 212p. Available online: https://www.eatforhealth.gov.au/sites/default/files/content/n55_australian_dietary_guidelines.pdf (accessed on 13 February 2023).
- Gomes Silva, S.C.; Pinho, J.P.; Borges, C.; Teixeira Santos, C.; Santos, A.; Graça, A. Guidelines for a healthy vegetarian diet. In National Programme for the Promotion of a Healthy Diet; Direção-Geral da Saúde: Lisbon, Portugal, 2015; 45p, Available online: https://nutrimento.pt/activeapp/wp–content/uploads/2015/12/Guidelines-for-a-healthy-vegetarian-diet.pdf (accessed on 13 February 2023).
- Patel, P.; Kassam, S. Evaluating nutrition education interventions for medical students: A rapid review. J. Hum. Nutr. Diet. 2022, 35, 861–871. [Google Scholar] [CrossRef] [PubMed]
- STAGE (Strategic Technical Advisory Group of Experts); Duke, T.; AlBuhairan, F.S.; Agarwal, K.; Arora, N.K.; Arulkumaran, S.; Bhutta, Z.A.; Binka, F.; Castro, A.; Claeson, M.; et al. World Health Organization and knowledge translation in maternal, newborn, child and adolescent health and nutrition. Arch. Dis. Child. 2022, 107, 644–649. [Google Scholar] [CrossRef]
- Kushner, R.F.; Van Horn, L.; Rock, C.L.; Edwards, M.S.; Bales, C.W.; Kohlmeier, M.; Akabas, S.R. Nutrition education in medical school: A time of opportunity. Am. J. Clin. Nutr. 2014, 99 (Suppl. S5), 1167S–1173S. [Google Scholar] [CrossRef]
- Bassin, S.R.; Al-Nimr, R.I.; Allen, K.; Ogrinc, G. The state of nutrition in medical education in the United States. Nutr. Rev. 2020, 78, 764–780. [Google Scholar] [CrossRef]
- Villette, C.; Vasseur, P.; Lapidus, N.; Debin, M.; Hanslik, T.; Blanchon, T.; Steichen, O.; Rossignol, L. Vegetarian and Vegan Diets: Beliefs and Attitudes of General Practitioners and Pediatricians in France. Nutrients 2022, 14, 3101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouraqui, J.-P. Risk Assessment of Micronutrients Deficiency in Vegetarian or Vegan Children: Not So Obvious. Nutrients 2023, 15, 2129. https://doi.org/10.3390/nu15092129
Chouraqui J-P. Risk Assessment of Micronutrients Deficiency in Vegetarian or Vegan Children: Not So Obvious. Nutrients. 2023; 15(9):2129. https://doi.org/10.3390/nu15092129
Chicago/Turabian StyleChouraqui, Jean-Pierre. 2023. "Risk Assessment of Micronutrients Deficiency in Vegetarian or Vegan Children: Not So Obvious" Nutrients 15, no. 9: 2129. https://doi.org/10.3390/nu15092129
APA StyleChouraqui, J.-P. (2023). Risk Assessment of Micronutrients Deficiency in Vegetarian or Vegan Children: Not So Obvious. Nutrients, 15(9), 2129. https://doi.org/10.3390/nu15092129