A Scoping Review of the Relationship between Intermittent Fasting and the Human Gut Microbiota: Current Knowledge and Future Directions
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Data Sources and Search Terms
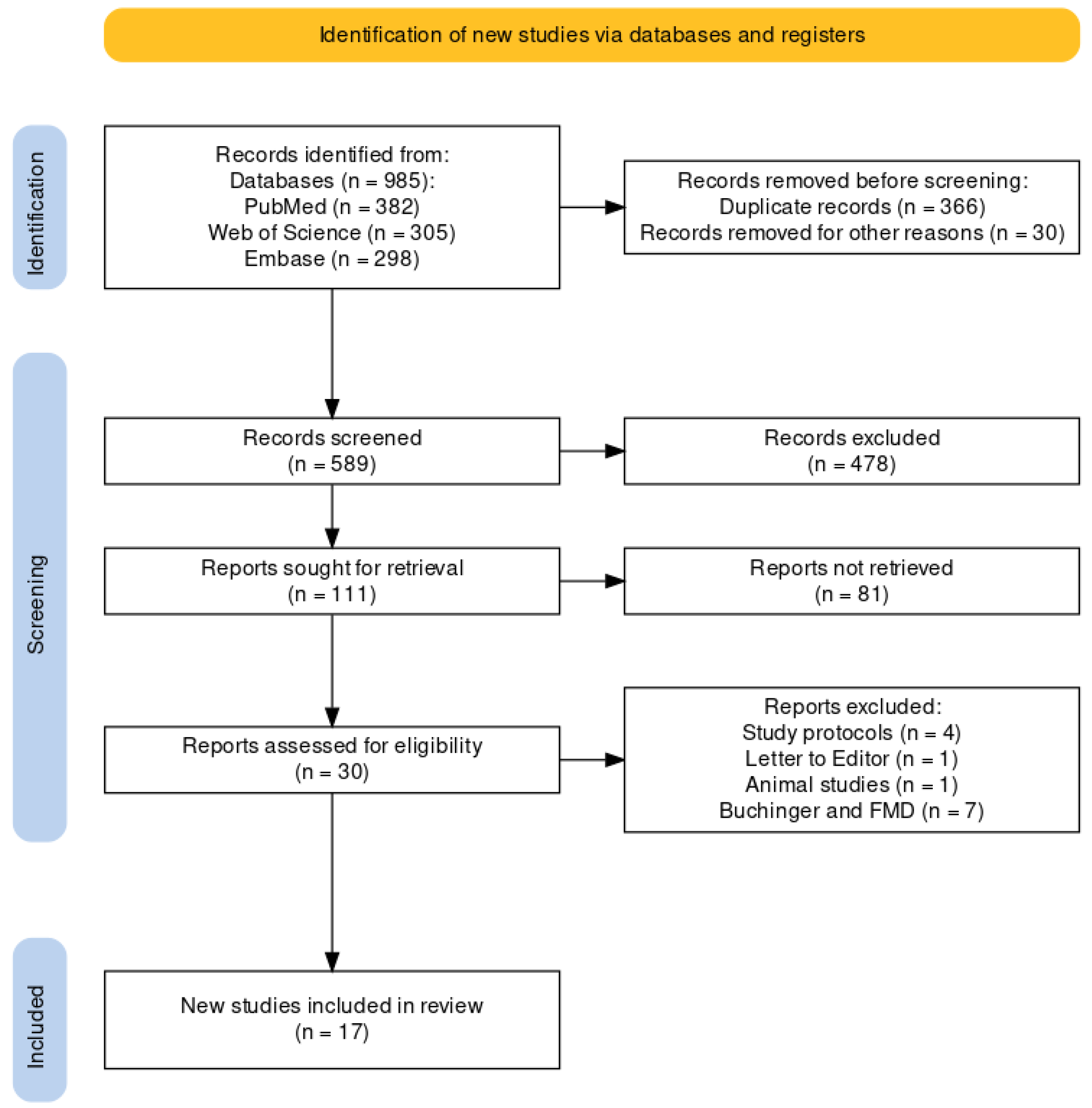
2.3. Study Selection Process
2.4. Quality Valuation
3. Results
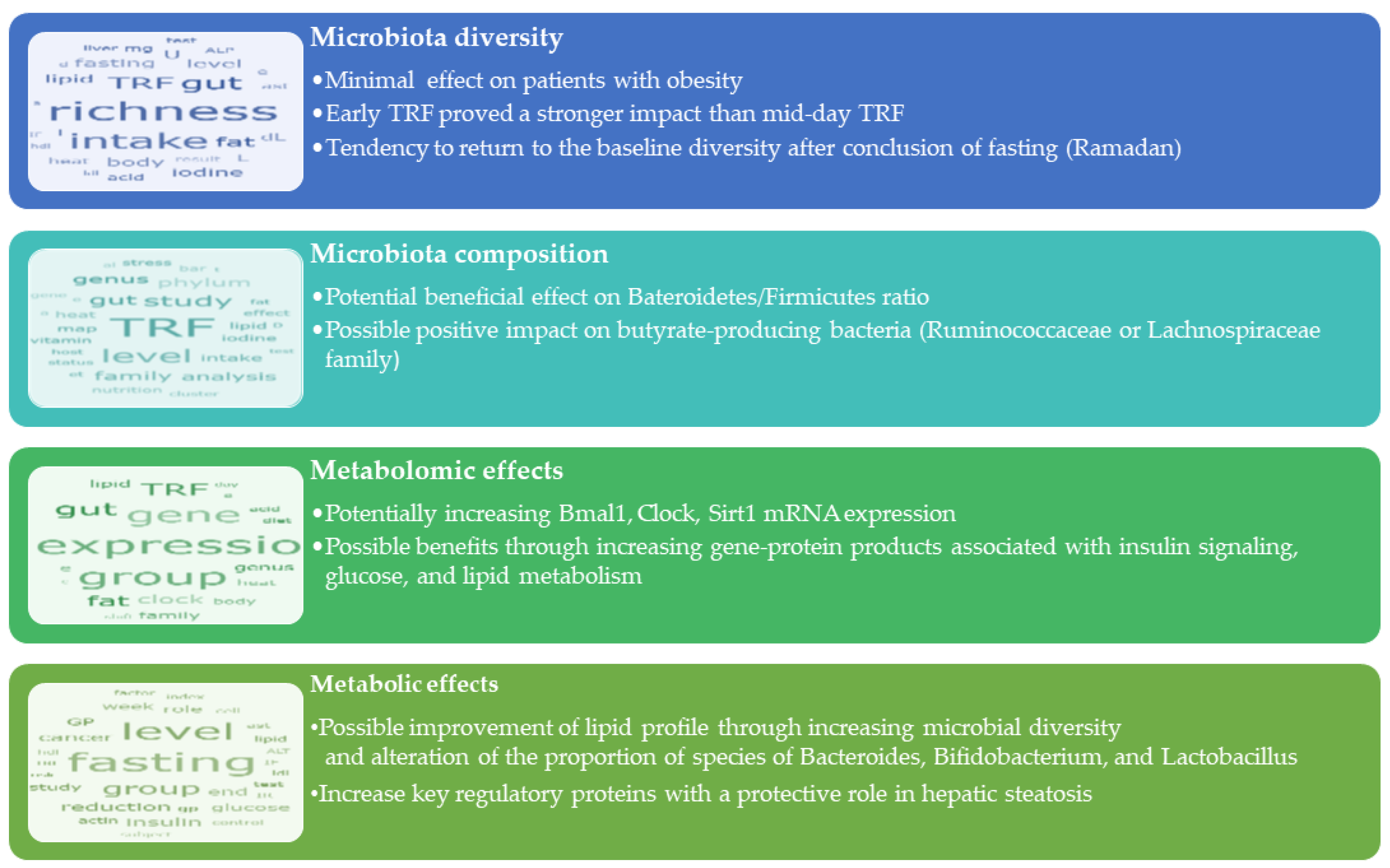
3.1. Time-Restricted Feeding (TRF)
3.1.1. Effects of TRF on Microbiota Diversity
3.1.2. Effects of TRF on Microbiota Community Composition
3.1.3. Metabolomic Effects
3.1.4. The Relation between TRF-Induced Weight Loss and Microbiota
3.1.5. Interactions with Metabolic Syndrome Components
3.2. Ramadan Fasting
3.2.1. Effects of Ramadan Fasting on Microbiota Diversity
3.2.2. Effects of Ramadan on Microbiota Community Composition
3.2.3. The Relation between Ramadan Fasting-Induced Weight Loss and Microbiota
3.2.4. Metabolomic Effects and Interactions with Metabolic Syndrome Components
3.3. Alternate-Day Fasting and 5:2 Diet
3.3.1. Effects on Microbiota Diversity
3.3.2. Effects on Microbiota Community Composition
3.3.3. Metabolomic Effects and Interactions with Metabolic Syndrome Components
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129 (Suppl. S2), S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Effects of Intermittent Fasting on Glucose and Lipid Metabolism. Proc. Nutr. Soc. 2017, 76, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ratiner, K.; Elinav, E. Circadian Influences of Diet on the Microbiome and Immunity. Trends Immunol. 2020, 41, 512–530. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Wang, Z.; Koonen, D.; Hofker, M.; Fu, J. Gut Microbiome and Lipid Metabolism: From Associations to Mechanisms. Curr. Opin. Lipidol. 2016, 27, 216–224. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Engen, P.A.; Keshavarzian, A. Circadian Rhythm and the Gut Microbiome. Int. Rev. Neurobiol. 2016, 131, 193–205. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Thompson, S.V.; Holscher, H.D. Complex interactions of circadian rhythms, eating behaviors, and the gastrointestinal microbiota and their potential impact on health. Nutr. Rev. 2017, 75, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T. Pathophysiological role of host microbiota in the development of obesity. Nutr. J. 2016, 15, 43. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut–Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Daas, M.C.; de Roos, N.M. Intermittent fasting contributes to aligned circadian rhythms through interactions with the gut microbiome. Benef. Microbes 2021, 12, 147–161. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Zang, B.-Y.; He, L.-X.; Xue, L. Intermittent Fasting: Potential Bridge of Obesity and Diabetes to Health? Nutrients 2022, 14, 981. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Ferrocino, I.; Pellegrini, M.; D’eusebio, C.; Goitre, I.; Ponzo, V.; Fadda, M.; Rosato, R.; Mengozzi, G.; Beccuti, G.; Merlo, F.D.; et al. The Effects of Time-Restricted Eating on Metabolism and Gut Microbiota: A Real-Life Study. Nutrients 2022, 14, 2569. [Google Scholar] [CrossRef]
- Gabel, K.; Marcell, J.; Cares, K.; Kalam, F.; Cienfuegos, S.; Ezpeleta, M.; Varady, K.A. Effect of time restricted feeding on the gut microbiome in adults with obesity: A pilot study. Nutr. Health 2020, 26, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Khan, S.I.; Rana, M.I.; Ayyaz, A.; Khan, M.Y.; Imran, M. Intermittent fasting positively modulates human gut microbial diversity and ameliorates blood lipid profile. Front. Microbiol. 2022, 13, 922727. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.-U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.; Chen, A.; Xu, C.; Jianglei, R.; Feng, Q.; Li, M. Time-restricted feeding is associated with changes in human gut microbiota related to nutrient intake. Nutrition 2020, 78, 110797. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Sanmiguel, C.; Gupta, A.; Mayer, E.A. Gut Microbiome and Obesity: A Plausible Explanation for Obesity. Curr. Obes. Rep. 2015, 4, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Schéle, E.; Grahnemo, L.; Anesten, F.; Hallén, A.; Bäckhed, F.; Jansson, J.-O. Regulation of body fat mass by the gut microbiota: Possible mediation by the brain. Peptides 2016, 77, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Mehr, N.S.; Mohamadi, M.H.; Shokri, F.; Heidary, M.; Sadeghifard, N.; Khoshnood, S. Obesity and gut–microbiota–brain axis: A narrative review. J. Clin. Lab. Anal. 2022, 36, e24420. [Google Scholar] [CrossRef] [PubMed]
- Omer, E.; Atassi, H. The Microbiome That Shapes Us: Can It Cause Obesity? Curr. Gastroenterol. Rep. 2017, 19, 59. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Yan, M.; Huang, R.; Wang, Y.; He, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zhang, F.; et al. CLOCK and BMAL1 Regulate Muscle Insulin Sensitivity via SIRT1 in Male Mice. Endocrinology 2016, 157, 2259–2269. [Google Scholar] [CrossRef]
- Rebolledo, C.; Cuevas, A.; Zambrano, T.; Acuña, J.J.; Jorquera, M.A.; Saavedra, K.; Martínez, C.; Lanas, F.; Seron, P.; Salazar, L.A.; et al. Bacterial Community Profile of the Gut Microbiota Differs between Hypercholesterolemic Subjects and Controls. BioMed Res. Int. 2017, 2017, 8127814. [Google Scholar] [CrossRef]
- Van Herck, M.A.; Vonghia, L.; Francque, S.M. Animal Models of Nonalcoholic Fatty Liver Disease—A Starter’s Guide. Nutrients 2017, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Memel, Z.N.; Wang, J.; Corey, K.E. Intermittent Fasting as a Treatment for Nonalcoholic Fatty Liver Disease: What Is the Evidence? Clin. Liver Dis. 2022, 19, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Yalinay, M.; Karakan, T. Structural changes in gut microbiome after Ramadan fasting: A pilot study. Benef. Microbes 2020, 11, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Mindikoglu, A.L.; Abdulsada, M.M.; Jain, A.; Choi, J.M.; Jalal, P.K.; Devaraj, S.; Mezzari, M.P.; Petrosino, J.F.; Opekun, A.R.; Jung, S.Y. Intermittent fasting from dawn to sunset for 30 consecutive days is associated with anticancer proteomic signature and upregulates key regulatory proteins of glucose and lipid metabolism, circadian clock, DNA repair, cytoskeleton remodeling, immune system and cognitive function in healthy subjects. J. Proteom. 2020, 217, 103645. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Zhang, X.; Ma, M.; Xie, Z.; Pan, Q.; Ma, Z.; Peppelenbosch, M.P. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Am. J. Clin. Nutr. 2021, 113, 1332–1342. [Google Scholar] [CrossRef]
- Ali, I.; Liu, K.; Long, D.; Faisal, S.; Hilal, M.G.; Ali, I.; Huang, X.; Long, R. Ramadan Fasting Leads to Shifts in Human Gut Microbiota Structured by Dietary Composition. Front. Microbiol. 2021, 12, 642999. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Roshanravan, N.; Alamdari, N.M.; Safaiyan, A.; Mosharkesh, E.; Hadi, A.; Barati, M.; Ostadrahimi, A. The interplay between fasting, gut microbiota, and lipid profile. Int. J. Clin. Pract. 2021, 75, e14591. [Google Scholar] [CrossRef]
- Ozkul, C.; Yalinay, M.; Karakan, T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: A preliminary study on intermittent fasting. Turk. J. Gastroenterol. 2019, 30, 1030–1035. [Google Scholar] [CrossRef]
- Chen, S.; Ali, I.; Li, X.; Long, D.; Zhang, Y.; Long, R.; Huang, X. Shifts in Fecal Metabolite Profiles Associated With Ramadan Fasting Among Chinese and Pakistani Individuals. Front. Nutr. 2022, 9, 845086. [Google Scholar] [CrossRef]
- Kang, S.; Ma, W.; Li, F.Y.; Zhang, Q.; Niu, J.; Ding, Y.; Han, F.; Sun, X. Functional Redundancy Instead of Species Redundancy Determines Community Stability in a Typical Steppe of Inner Mongolia. PLoS ONE 2015, 10, e0145605. [Google Scholar] [CrossRef]
- Moya, A.; Ferrer, M. Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol. 2016, 24, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, F.; Wang, Y.; Su, Y.; Verhaar, A.; Ma, Z.; Peppelenbosch, M.P. Investigating Ramadan Like Fasting Effects on the Gut Microbiome in BALB/c Mice. Front. Nutr. 2022, 9, 832757. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Fernando, H.A.; Zibellini, J.; Harris, R.A.; Seimon, R.V.; Sainsbury, A. Effect of Ramadan Fasting on Weight and Body Composition in Healthy Non-Athlete Adults: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Wexler, A.G.; Goodman, A.L. An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2017, 2, 17026. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Marcos, A.; Wärnberg, J.; Martí, A.; Martin-Matillas, M.; Campoy, C.; Moreno, L.A.; Veiga, O.; Redondo-Figuero, C.; Garagorri, J.M.; et al. Interplay Between Weight Loss and Gut Microbiota Composition in Overweight Adolescents. Obesity 2009, 17, 1906–1915. [Google Scholar] [CrossRef]
- Lan, T.; Yu, Y.; Zhang, J.; Li, H.; Weng, Q.; Jiang, S.; Tian, S.; Xu, T.; Hu, S.; Yang, G.; et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology 2021, 74, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Templeman, I.; Gonzalez, J.T.; Thompson, D.; Betts, J.A. The role of intermittent fasting and meal timing in weight management and metabolic health. Proc. Nutr. Soc. 2020, 79, 76–87. [Google Scholar] [CrossRef]
- Pascual, P.E.; Rolands, M.R.; Eldridge, A.L.; Kassis, A.; Mainardi, F.; Lê, K.; Karagounis, L.G.; Gut, P.; Varady, K.A. A meta-analysis comparing the effectiveness of alternate day fasting, the 5:2 diet, and time-restricted eating for weight loss. Obesity 2023, 31, 9–21. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Jasbi, P.; Bowes, D.A.; Dirks, B.; Whisner, C.M.; Arciero, K.M.; Poe, M.; Gu, H.; Gumpricht, E.; Sweazea, K.L.; et al. Exploratory analysis of one versus two-day intermittent fasting protocols on the gut microbiome and plasma metabolome in adults with overweight/obesity. Front. Nutr. 2022, 9, 1036080. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Frank, D.N.; Borengasser, S.J.; Ostendorf, D.M.; Ir, D.; Jambal, P.; Bing, K.; Wayland, L.; Siebert, J.C.; Bessesen, D.H.; et al. The Gut Microbiota during a Behavioral Weight Loss Intervention. Nutrients 2021, 13, 3248. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.-M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the Gut Microbial Community of Obese Patients Following a Weight-Loss Intervention Using Whole Metagenome Shotgun Sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef] [PubMed]
| TRF | Study Type | Duration | Study Population | Control Group | Number of Participants | Results |
|---|---|---|---|---|---|---|
| Ferrocino et al. [21] | Real-life study | 12 weeks | BMI 30–45 kg/m2 | TRF (n = 25) non TRF (n = 24) | 49 | No differences in α and β diversity or gut microbiota composition. |
| Increase in Lachnospiraceae, Parasutterella, and Romboutsia in the TRF group. | ||||||
| Gabel et al. [22] | Pilot study | 12 weeks | BMI ≥ 30 kg/m2 | no | 14 | Regarding gut microbiota diversity, the abundance of Firmicutes, Bacteroidetes, or other phyla remained unchanged. |
| Khan et al. [23] | Clinical trial | 26 days | Healthy volunteers (normal weight or obesity) | no | 45 | Alteration of the gut metagenome after IF. |
| Increased bacterial α diversity. | ||||||
| Lactobacillus and Bifidobacterium increased. | ||||||
| Xie et al. [24] | RCT | 5 weeks | Healthy individuals without obesity | eTRF (n = 28) | 82 | No significant alteration in the gut microbiota diversity or overall composition. |
| mTRF (n = 26) | eTRF > mTRF in improving insulin sensitivity. | |||||
| control group (n = 28) | Only eTRF improved fasting glycemia, decreased adiposity, ameliorated inflammation, and increased gut microbiota diversity. | |||||
| Zeb et al. [25] | RCT | 25 days | Healthy men | TRF (n = 56) non TRF (n = 24) | 80 | Enrichment of Prevotellaceae and Bacteroideaceae in the IF group. |
| Activation of sirtuin-1 was positively related with gut microbiota richness (TRF). | ||||||
| Zeb et al. [26] | RCT | 12 weeks | Healthy men | TRF (n = 15) | 30 | Increased Prevotella_9, Faecalibacterium, and Dialister in TRF. |
| non-TRF (n = 15) | Bacteroidetes were the most abundant in the TRF group, while in the non-TRF group, Firmicutes was the prevailing phylum |
| Study | Study Type | Control Group | Number of Participants | Results |
|---|---|---|---|---|
| Ozkul et al. [43] | Pilot study | No | 9 | Microbial richness increased. No significant difference in terms of α-diversity. Significant differences between baseline and after Ramadan in microbial community structure:
|
| Mindikoglu et al. [44] | Clinical trial | No | 14 | Bacterial richness and diversity did not change significantly. |
| Increase in gene-protein products associated with glucose and lipid metabolism and insulin signaling (perilipin 4, pyruvate kinase M1/2). | ||||
| Su et al. [45] | Cohort | 30 young men and 37 middle-aged men (10 control, 27 Ramadan) | 67 | Increased microbiome diversity associated with upregulation of the Clostridiales-order-derived Lachnospiraceae and Ruminococcaceae. |
| Decreased abundance of the Prevotellaceae family. | ||||
| Microbiome composition returned to baseline when IF finished. | ||||
| Ali et al. [46] | Cohort | No | 34 (16 Chinese and 18 Pakistani adults) | α-diversity significantly altered among Chinese subjects. |
| IF could affect β-diversity in both populations. | ||||
| Increased relative abundance of Proteobacteria (combined groups analyses). | ||||
| Decreased relative abundance of Firmicutes (Pakistani group). | ||||
| Increased relative abundance of Bacteroidetes phylum (Chinese group). | ||||
| Decreased relative abundance of Bacteroidetes phylum (Pakistani group). | ||||
| Mohammadzadeh et al. [47] | Cross-sectional | No | 30 | Bacteroides and Firmicutes increased. |
| Bacteroides increased in both sexes. | ||||
| Firmicutes increased only in women. | ||||
| Increased butyrate levels after IF. | ||||
| Özkul et al. [48] | Pilot study | No | 9 | Increased abundance of A. muciniphila and B. fragilis groups. |
| Chen et al. [49] | Cohort | No | 34 (16 Chinese and 18 Pakistani adults) | L-histidine, lycofawcine, and cordycepin levels were higher after IF in the Chinese group. |
| Brucine increased in the Pakistani group. | ||||
| Bacterial taxa were correlated with specific metabolites unique to each ethnic group. |
| Study | Study Type | Duration | Study Population | Control Group | Number of Participants | Results |
|---|---|---|---|---|---|---|
| Cignarella et al. [61] | Pilot study/ADF | 15 days | Metabolic syndrome patients | 8 ad libitum | 16 | IF confers protection through gut microbiota changes in metabolic syndrome patients. |
| 8 IF | ||||||
| Mohr et al. [62] | RCT/5:2 | 5 weeks (4 weeks intervention) | Sedentary volunteers with overweight/obesity | 1-day fasting (n = 10) | 20 | β-diversity was altered. Increased abundance of Ruminococcaceae Incertae Sedis in both groups. Increased abundance of Eubacterium fissicatena in the 1-day fasting group. |
| 2-day fasting (n = 10) | ||||||
| Guo et al. [63] | RCT/5:2 | 8 weeks | Metabolic syndrome patients | 21 IF | 39 | Increased production of SCFAs. |
| 18 controls | Decreased levels of lipopolysaccharides. | |||||
| Stanislawski et al. [64] | RCT/ADF | 12 months | Overweight/obese participants | 25 DCR | 71 | Increased number of bacterial taxa were associated with weight loss and reduction in waist circumference. |
| 34 IF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, A.D.; Niță, O.; Gherasim, A.; Enache, A.I.; Caba, L.; Mihalache, L.; Arhire, L.I. A Scoping Review of the Relationship between Intermittent Fasting and the Human Gut Microbiota: Current Knowledge and Future Directions. Nutrients 2023, 15, 2095. https://doi.org/10.3390/nu15092095
Popa AD, Niță O, Gherasim A, Enache AI, Caba L, Mihalache L, Arhire LI. A Scoping Review of the Relationship between Intermittent Fasting and the Human Gut Microbiota: Current Knowledge and Future Directions. Nutrients. 2023; 15(9):2095. https://doi.org/10.3390/nu15092095
Chicago/Turabian StylePopa, Alina Delia, Otilia Niță, Andreea Gherasim, Armand Iustinian Enache, Lavinia Caba, Laura Mihalache, and Lidia Iuliana Arhire. 2023. "A Scoping Review of the Relationship between Intermittent Fasting and the Human Gut Microbiota: Current Knowledge and Future Directions" Nutrients 15, no. 9: 2095. https://doi.org/10.3390/nu15092095
APA StylePopa, A. D., Niță, O., Gherasim, A., Enache, A. I., Caba, L., Mihalache, L., & Arhire, L. I. (2023). A Scoping Review of the Relationship between Intermittent Fasting and the Human Gut Microbiota: Current Knowledge and Future Directions. Nutrients, 15(9), 2095. https://doi.org/10.3390/nu15092095






