Determinants Related to Oxidative Stress Parameters in Pediatric Patients with Type 1 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Blood Sample Analysis of Selected Biomarkers
2.3. Nutritional Status and Nutrient Intake
2.4. Statistical Analysis
3. Results
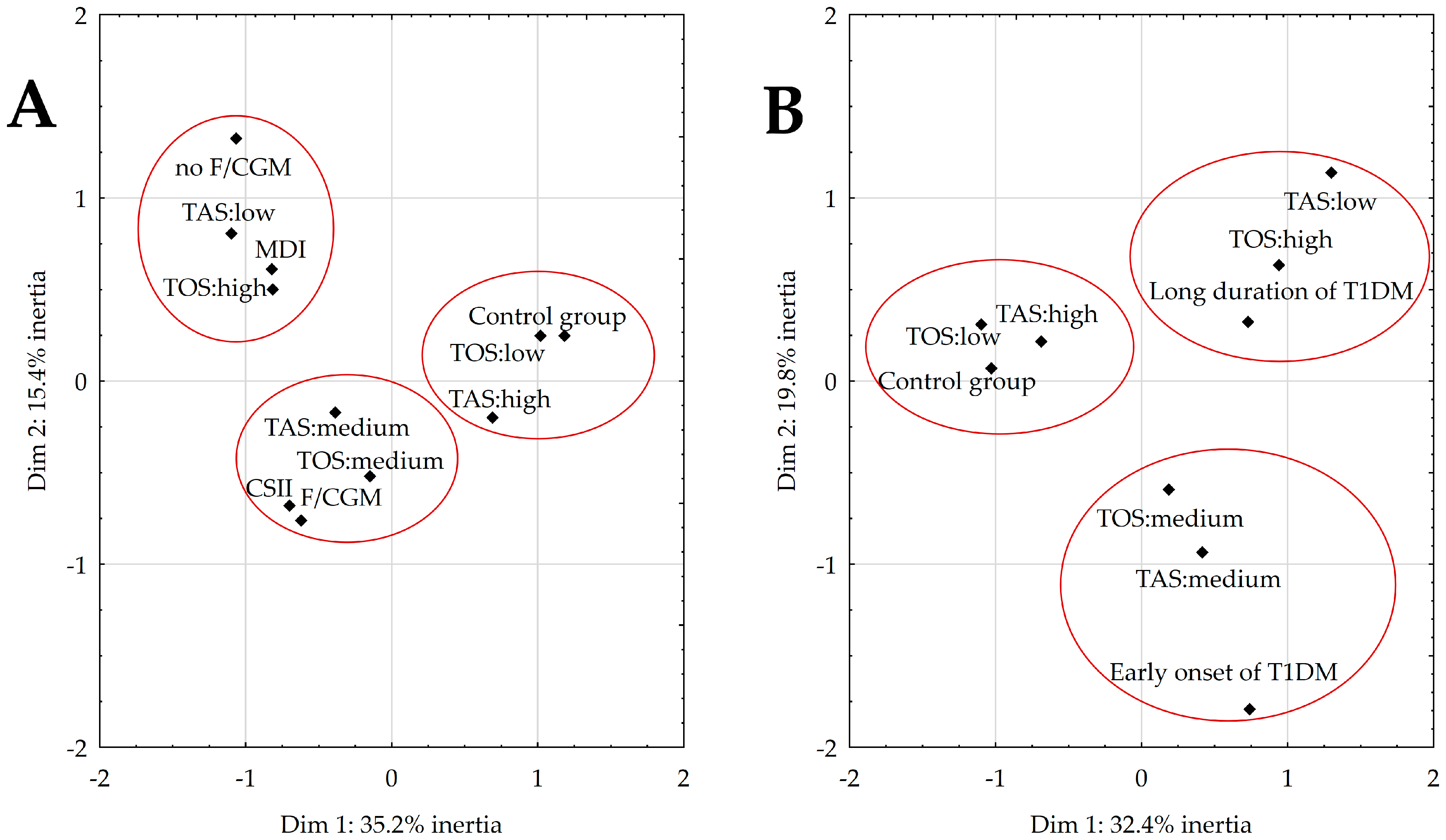
- (1)
- The first and fourth quadrants represent healthy peers with an above-average TAS range and low TOS.
- (2)
- The second quadrant includes diabetics with low TAS and high TOS who were not supported by FGM or CGM or who used MDI.
- (3)
- The final quadrant (III) consists of individuals with T1DM, who had medium TAS and TOS and who used FGM or CGM and CSII.
- (1)
- In the first quadrant are individuals who have had T1DM for more than 2 years, with low TAS and high TOS.
- (2)
- The second quadrant classifies healthy participants with an above-normal TAS and low TOS.
- (3)
- The last quadrant (IV) contains recently diagnosed patients with medium TAS and TOS.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Renard, E. Automated insulin delivery systems: From early research to routine care of type 1 diabetes. Acta Diabetol. 2023, 60, 151–161. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Bandurska-Stankiewicz, E.; Borys, S.; Budzyński, A.; Cyganek, K.; Cypryk, K.; Czech, A.; Czupryniak, L.; Drzewoski, J.; Dzida, G.; et al. Zalecenia kliniczne dotyczące postępowania u osób z cukrzycą 2023-Stanowisko Polskiego Towarzystwa Diabetologicznego. Curr. Top. Diabet. 2023, 3, 1–140. [Google Scholar]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 7. Diabetes Technology: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S111–S127. [Google Scholar] [CrossRef] [PubMed]
- Seckin, D.; Ilhan, N.; Ilhan, N.; Ertugrul, S. Glycaemic control, markers of endothelial cell activation and oxidative stress in children with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2006, 73, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi. Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jaskulak, M.; Michalska, M.; Mrugacz, M.; Vandenbulcke, F. Air Pollution, Oxidative Stress, and the Risk of Development of Type 1 Diabetes. Antioxidants 2022, 11, 1908. [Google Scholar] [CrossRef]
- Zivić, S.; Vlaski, J.; Kocić, G.; Pesić, M.; Cirić, V.; Durić, Z. The importance of oxidative stress in pathogenesis of type 1 diabetes--determination of catalase activity in lymphocytes of diabetic patients. Med. Pregl. 2008, 61, 458–463. [Google Scholar] [CrossRef]
- Salmonowicz, B.; Krzystek-Korpacka, M.; Noczyńska, A. Trace elements, magnesium, and the efficacy of antioxidant systems in children with type 1 diabetes mellitus and in their siblings. Adv. Clin. Exp. Med. 2014, 23, 259–268. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; El-Afify, D.; Shabana, A. Relationship between bone turnover markers and oxidative stress in children with type 1 diabetes mellitus. Pediatr. Res. 2021, 89, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Grabia, M.; Markiewicz-Żukowska, R.; Socha, K.; Polkowska, A.; Zasim, A.; Boruch, K.; Bossowski, A. Prevalence of Metabolic Syndrome in Relation to Cardiovascular Biomarkers and Dietary Factors among Adolescents with Type 1 Diabetes Mellitus. Nutrients 2022, 14, 2435. [Google Scholar] [CrossRef]
- Rychert-Stos, M.; Walczak, M.; Horodnicka-Jozwa, A.; Romanowska, H.; Katuszonek, D.; Wyka, K.; Chojnacka, H.; Marciniak, W.; Lubiński, J.; Petriczko, E. Do trace elements influence the course of newly diagnosed type 1 diabetes mellitus? Neuro. Endocrinol. Lett. 2022, 43, 247–256. [Google Scholar]
- Gapys, B.; Raszeja-Specht, A.; Bielarczyk, H. Rola cynku w procesach fizjologicznych i patologicznych organizmu. Diagn. lab. 2014, 50, 45–52. [Google Scholar]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef]
- Darenskaya, M.; Chugunova, E.; Kolesnikov, S.; Semenova, N.; Michalevich, I.; Nikitina, O.; Lesnaya, A.; Kolesnikova, L. Receiver Operator Characteristic (ROC) Analysis of Lipids, Proteins, DNA Oxidative Damage, and Antioxidant Defense in Plasma and Erythrocytes of Young Reproductive-Age Men with Early Stages of Type 1 Diabetes Mellitus (T1DM) Nephropathy in the Irkutsk Region, Russia. Metabolites 2022, 12, 1282. [Google Scholar] [CrossRef]
- Alghobashy, A.A.; Alkholy, U.M.; Talat, M.A.; Abdalmonem, N.; Zaki, A.; Ahmed, I.A.; Mohamed, R.H. Trace elements and oxidative stress in children with type 1 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2018, 11, 85–92. [Google Scholar] [CrossRef]
- Forte, G.; Bocca, B.; Peruzzu, A.; Tolu, F.; Asara, Y.; Farace, C.; Oggiano, R.; Madeddu, R. Blood metals concentration in type 1 and type 2 diabetics. Biol. Trace. Elem. Res. 2013, 156, 79–90. [Google Scholar] [CrossRef]
- Lin, C.-C.; Tsweng, G.-J.; Lee, C.-F.; Chen, B.-H.; Huang, Y.-L. Magnesium, zinc, and chromium levels in children, adolescents, and young adults with type 1 diabetes. Clin. Nutr. 2016, 35, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The double face of metals: The intriguing case of chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Ludvigsson, J.; Andersson-White, P.; Guerrero-Bosagna, C. Toxic metals in cord blood and later development of Type 1 diabetes. Pediatr. Dimens. 2019, 4, 2. [Google Scholar]
- De Leon, J.A.D.; Borges, C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 2020, 22, e61122. [Google Scholar] [CrossRef]
| Parameter | Unit | Wavelength | Material |
|---|---|---|---|
| Cu | mg/L | 324.8 nm | Serum |
| Cr | µg/L | 359.3 nm | Serum |
| Se | µg/L | 196 nm | Serum |
| Zn | mg/L | 213.9 nm | Serum |
| TAS | mmol/L | 600 nm | Serum |
| SOD | U/mL | 450 nm | Serum |
| CAT | n/mol/min | 540 nm | Serum |
| GPx | U/L whole blood | 340 nm | Whole blood |
| TOS | μmol H2O2 equiv./L | 560/800 nm | Serum |
| MDA | µmol/L | 535 nm | Serum |
| As | µg/L | As75 74.92 U * | Whole blood |
| Cd | µg/L | 228.8 nm | Whole blood |
| Hg | µg/L | 254 nm | Whole blood |
| Pb | µg/L | 283.3 nm | Whole blood |
| Parameter | T1DM Group (n = 103) | Control Group (n = 65) | |
|---|---|---|---|
| Age (years) | Me (Q1–Q3) | 13 (11–15) | 15 (14–15) |
| Height (cm) | 164 (155–173) | 167 (158–178) | |
| Body weight (kg) | 57 (46–67) | 56 (48–67) | |
| HbA1c (%) | 8 (6–10) | – | |
| Age at diagnosis (years) | 9 (6–11) | – | |
| T1DM duration (years) | 4 (1–7) | – | |
| Gender (girls/boys) | % | 51/49 | 38/62 |
| Newly diagnosed (<2 years) | 27 | – | |
| Type of insulin therapy (MDI/CSII) | 41/59 | – | |
| Type of glucose monitoring system (Glucometer only/FGM/CGM) | 29/41/30 | – |
| Parameter | T1DM (n = 103) | Control (n = 65) | p-Value (T1DM vs. Control) |
|---|---|---|---|
| Cu (mg/L) | 0.874 (0.724–1.154) | 0.903 (0.706–1.130) | NS |
| Cu/Zn ratio | 1.057 (0.842–1.458) | 0.981 (0.669–1.179) | <0.05 |
| Cr (µg/L) | 0.648 (0.568–0.960) | 1.530 (1.088–1.809) | <0.001 |
| Se (µg/L) | 60.9 (50.2–69.8) | 61.4 (51.1–69.0) | NS |
| Zn (mg/L) | 0.891 (0.796–1.020) | 0.979 (0.898–1.119) | <0.001 |
| TAS (mmol/L) | 1.304 (1.173–1.525) | 1.580 (1.476–1.761) | <0.001 |
| SOD (U/mL) | 1.470 (1.068–2.049) | 2.114 (1.676–2.356) | <0.001 |
| CAT (n/mol/min) | 43.2 (27.7–72.4) | 58.3 (48.2–75.2) | <0.01 |
| GPx (U/L) | 1329 (756–2168) | 1749 (967–3049) | NS |
| TOS (μmol H2O2 Equiv./L) | 7.568 (6.0–9.295) | 4.847 (3.851–5.839) | <0.001 |
| OSI | 0.575 (0.439–0.771) | 0.284 (0.241–0.382) | <0.001 |
| MDA (µmol/L) | 3.912 (2.512–5.312) | 2.520 (1.690–4.950) | <0.01 |
| As (µg/L) | 0.593 (0.385–0.766) | 0.581 (0.480–0.658) | NS |
| Cd (µg/L) | 0.696 (0.577–1.330) | 0.862 (0.569–1.650) | NS |
| Hg (µg/L) | 0.456 (0.246–0.767) | 0.344 (0.243–0.562) | NS |
| Pb (µg/L) | 22.9 (15.2–32.6) | 27.1 (20.1–31.5) | NS |
| Parameter | Low | Medium | High | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| % | Me (Q1–Q3) | % | Me (Q1–Q3) | % | Me (Q1–Q3) | Low vs. Medium | Low vs. High | |
| TAS | 31 | 9.0 (7.4–10.7) | 41 | 7.8 (6.6–9.8) | 28 | 6.9 (6.3–8.7) | NS | <0.01 |
| TOS | 11 | 6.6 (6.1–7.9) | 47 | 7.7 (6.8–9.8) | 43 | 8.1 (7.0–10.6) | <0.05 | <0.05 |
| OSI | 27 | 7.5 (6.6–9.8) | 41 | 7.8 (6.6–10.8) | 32 | 8.2 (7.3–9.8) | NS | NS |
| Cu/Zn | 13 | 7.5 (6.73–10.5) | 35 | 7.6 (6.6–9.7) | 52 | 8.1. (6.7–9.9) | NS | NS |
| Parameter | T1DM (n = 103) | Control (n = 65) | p-Value (T1DM vs. Control) | |||||
|---|---|---|---|---|---|---|---|---|
| Me (Q1–Q3) | Percentage of Subjects (%) | Me (Q1–Q3) | Percentage of Subjects (%) | |||||
| Type of Norm | Norm | Below (Above *) Norm | Norm | Below (Above *) Norm | ||||
| Cu (mg) | 0.97 (0.77–1.1) | EAR | 87 | 13 | 1.0 (0.88–1.3) | 97 | 3 | NS |
| Mn (mg) | 3.4 (2.3–4.4) | AI | 97 | 3 | 3.1 (2.8–4.4) | 98 | 2 | NS |
| Zn (mg) | 9.0 (7.6–11) | EAR | 77 | 23 | 9.6 (8.2–12) | 74 | 26 | NS |
| UL | 97 | 2 * | 92 | 8 * | ||||
| Vitamin A (µg) | 722 (568–899) | EAR | 77 | 23 | 882 (689–1184) | 86 | 14 | <0.01 |
| UL | 88 | 12 * | 85 | 15 * | ||||
| Retinol (µg) | 260 (197–367) | n/d | – | – | 280 (215–340) | – | – | NS |
| β-carotene (µg) | 2574 (1754–3594) | n/d | – | – | 3522 (2479–5178) | – | – | <0.01 |
| Vitamin C (mg) | 65 (37–108) | EAR | 58 | 42 | 70 (48–110) | 62 | 38 | NS |
| Vitamin E (mg) | 5.5 (4.3–6.8) | AI | 12 | 88 | 6.1 (4.5–7.9) | 15 | 85 | NS |
| Parameter 1 | Parameter 2 | R | p-Value |
|---|---|---|---|
| HbA1c | TAS | −0.3 | <0.01 |
| OSI | 0.3 | <0.001 | |
| Zn | Cu | 0.2 | <0.05 |
| Cu | Hg | 0.3 | <0.01 |
| Cu/Zn ratio | Hg | 0.2 | <0.01 |
| Dietary Zn | −0.3 | <0.001 | |
| Dietary Cu | −0.3 | <0.001 | |
| Dietary Mn | −0.3 | <0.001 | |
| Dietary retinol | −0.3 | <0.01 | |
| Dietary vitamin E | −0.2 | <0.05 | |
| Se | TAS | 0.4 | <0.001 |
| CAT | 0.2 | <0.01 | |
| Dietary vitamin A | 0.2 | <0.01 | |
| Dietary β-carotene | 0.3 | <0.01 | |
| TAS | Cd | −0.3 | <0.01 |
| CAT | 0.2 | <0.01 | |
| SOD | 0.2 | <0.05 | |
| Cd | OSI | 0.2 | <0.05 |
| MDA | 0.3 | <0.01 | |
| GPx | −0.3 | <0.01 | |
| CAT | SOD | 0.4 | <0.001 |
| Parameter | TAS | TOS | OSI |
|---|---|---|---|
| AUC (CI) | 0.77 (0.7–0.84) | 0.83 (0.77–0.90) | 0.87 (0.82–0.93) |
| p-Value AUC | <0.001 | <0.001 | <0.001 |
| Cut-off point | 1.450 | 5.892 | 0.375 |
| Sensitivity | 72% | 79% | 89% |
| Specificity | 77% | 77% | 74% |
| Youden index | 0.488 | 0.556 | 0.632 |
| +LR | 0.231 | 0.231 | 0.262 |
| −LR | 0.282 | 0.214 | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabia, M.; Socha, K.; Soroczyńska, J.; Bossowski, A.; Markiewicz-Żukowska, R. Determinants Related to Oxidative Stress Parameters in Pediatric Patients with Type 1 Diabetes Mellitus. Nutrients 2023, 15, 2084. https://doi.org/10.3390/nu15092084
Grabia M, Socha K, Soroczyńska J, Bossowski A, Markiewicz-Żukowska R. Determinants Related to Oxidative Stress Parameters in Pediatric Patients with Type 1 Diabetes Mellitus. Nutrients. 2023; 15(9):2084. https://doi.org/10.3390/nu15092084
Chicago/Turabian StyleGrabia, Monika, Katarzyna Socha, Jolanta Soroczyńska, Artur Bossowski, and Renata Markiewicz-Żukowska. 2023. "Determinants Related to Oxidative Stress Parameters in Pediatric Patients with Type 1 Diabetes Mellitus" Nutrients 15, no. 9: 2084. https://doi.org/10.3390/nu15092084
APA StyleGrabia, M., Socha, K., Soroczyńska, J., Bossowski, A., & Markiewicz-Żukowska, R. (2023). Determinants Related to Oxidative Stress Parameters in Pediatric Patients with Type 1 Diabetes Mellitus. Nutrients, 15(9), 2084. https://doi.org/10.3390/nu15092084









