The Influence of Topinambur and Inulin Preventive Supplementation on Microbiota, Anxious Behavior, Cognitive Functions and Neurogenesis in Mice Exposed to the Chronic Unpredictable Mild Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Conditions
2.2. Chronic Unpredictable Mild Stress (CUMS)
2.3. Drugs
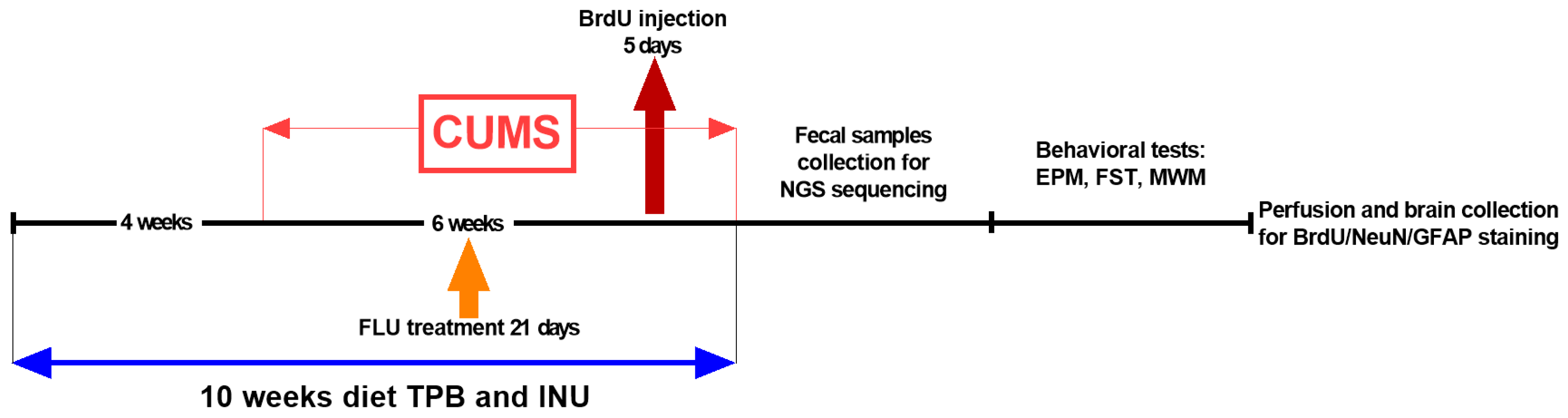
2.4. Experimental Study Design
2.5. Fecal Microbiota Analysis (DNA Extraction, NGS Sequencing, Metagenomic Sequencing)
2.6. Behavioral Tests
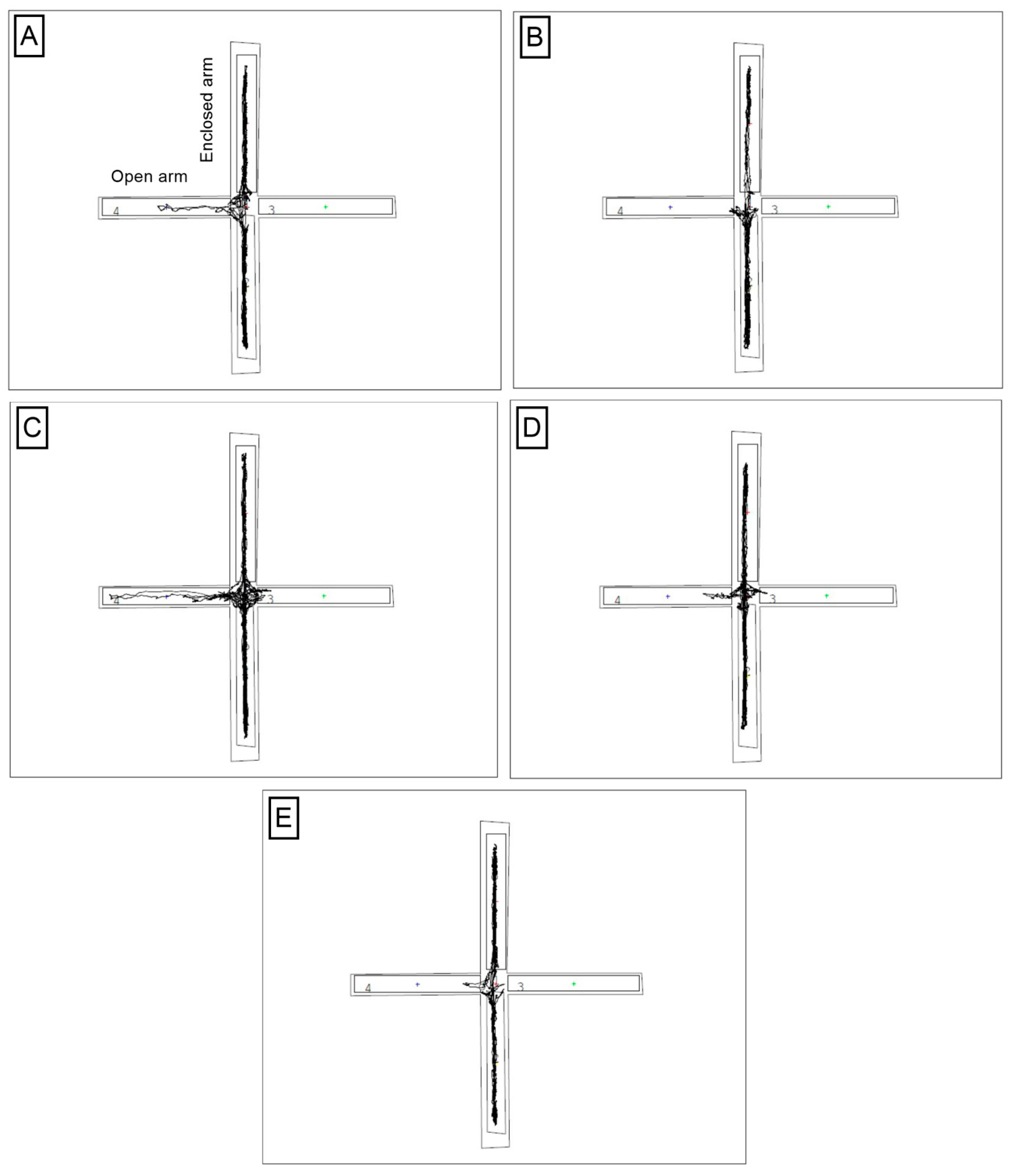
2.6.1. Elevated plus Maze Test, EPM
2.6.2. Forced Swim Test, FST
2.6.3. Morris Water Maze Test, MWM
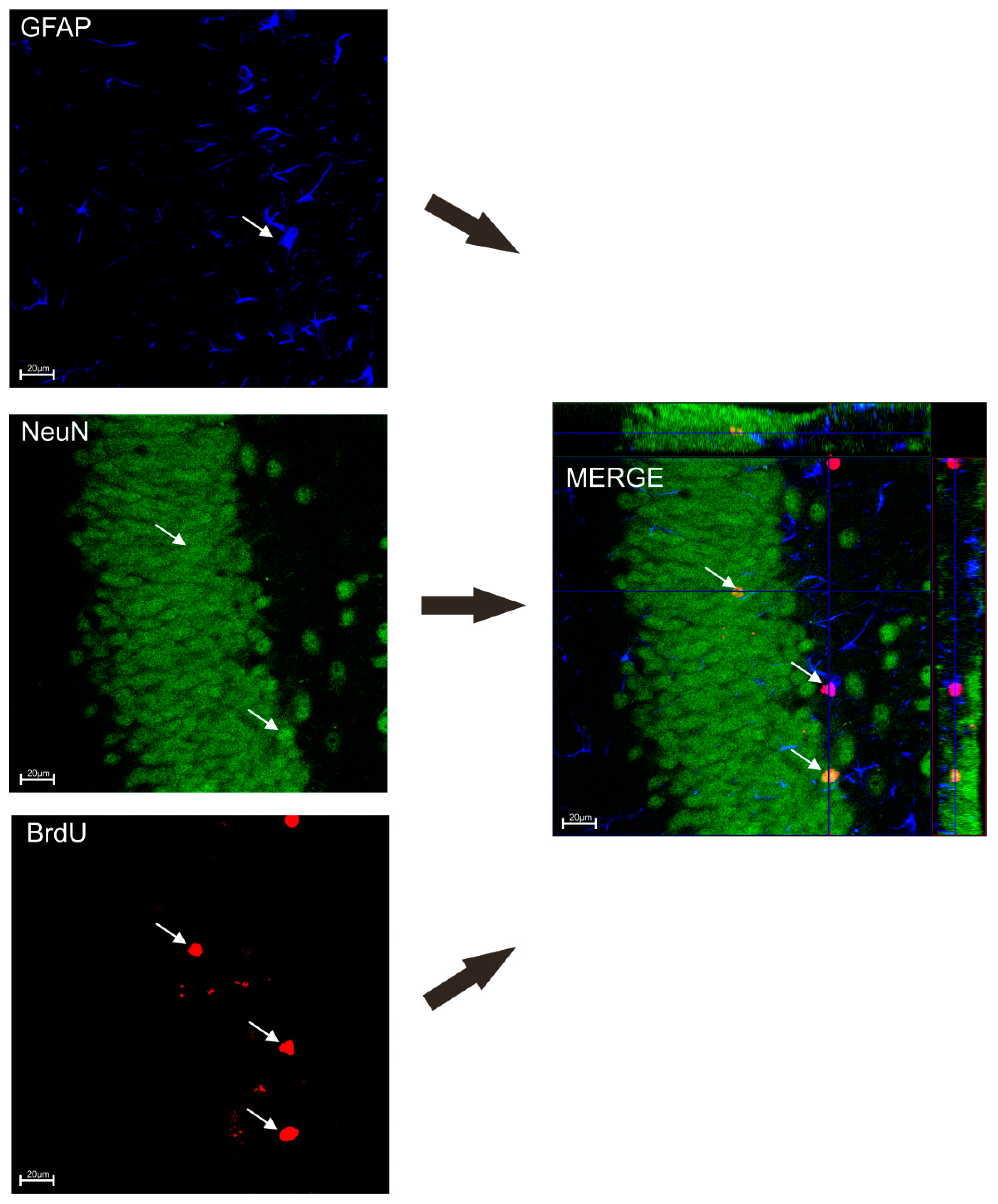
2.7. Immunohistochemistry Staining
BrdU/NeuN/GFAP Staining
2.8. Statistical Analysis of the Results
- -
- Alpha diversity measures using Chao1 and Shannon indices were calculated using the Microbiome Analyst platform. The non-parametric Kruskal-Wallis test was used to compare differences in alpha diversity between different groups.
- -
- Principal Coordinate Analysis (PCoA) was used to show species diversity between samples using Primer 7 software.
- -
- Primer 7 was used for similarity analysis (ANOSIM) and multivariate analysis of variance based on permutations (PERMANOWA).
- -
- The Past 4.0 program was used to perform the SIMPER analysis.
- -
- LEfSe analysis was performed using the Microbiome Analyst platform.
3. Results
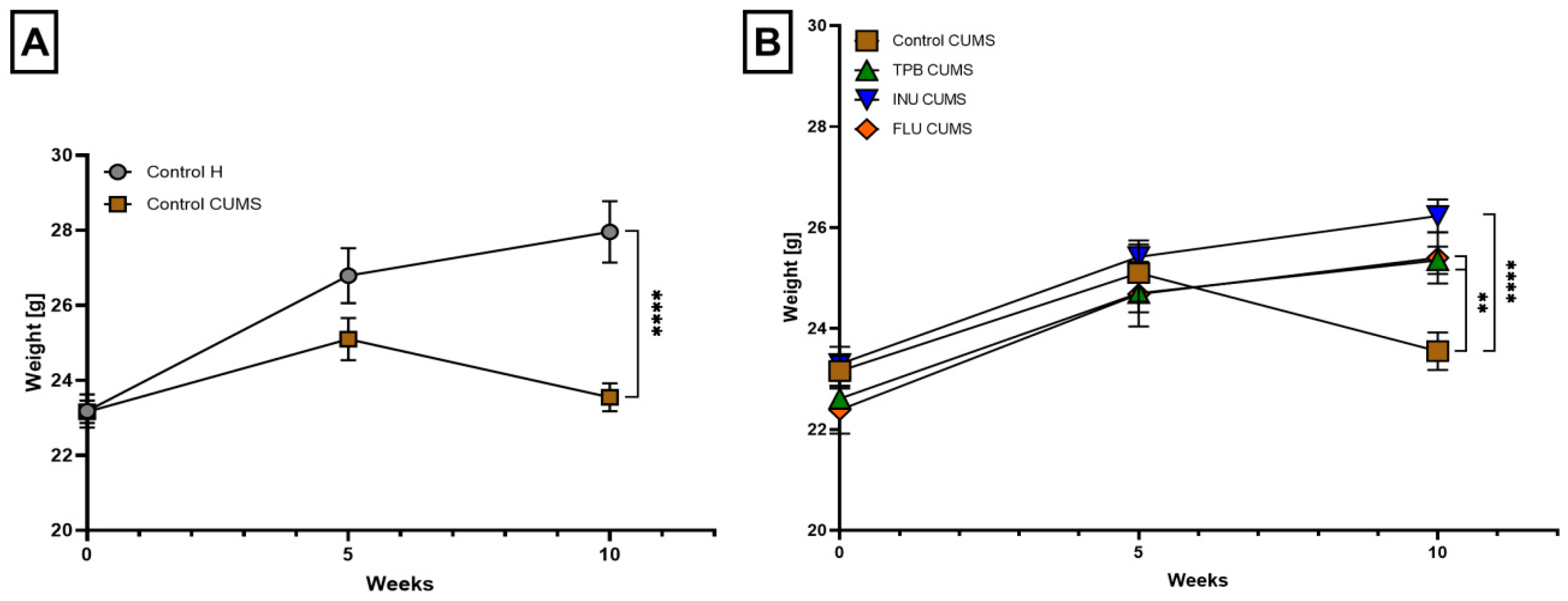
3.1. The Impact of TPB, INU and FLU on the Body Weight of Mice Exposed to CUMS
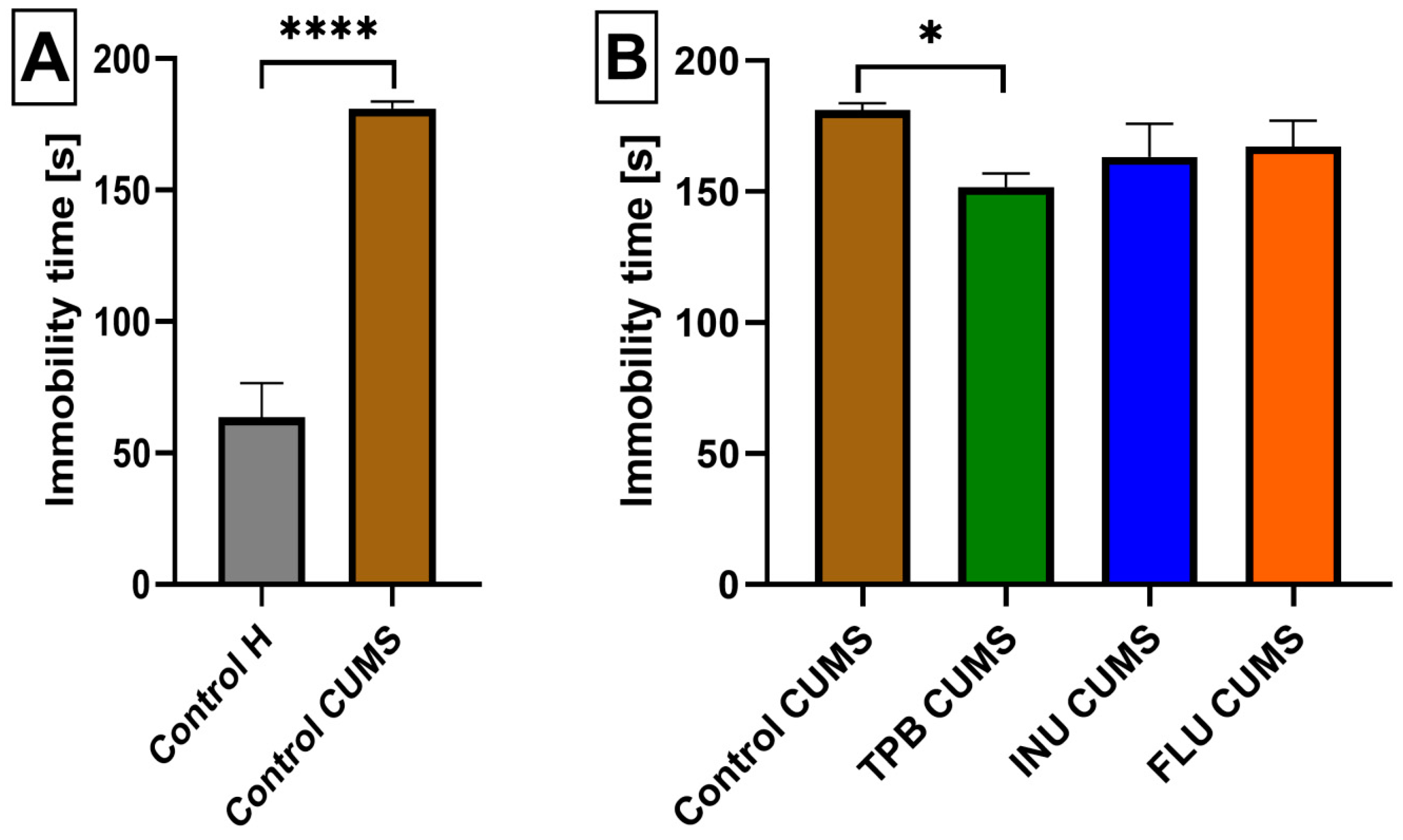
3.2. The Influence of a Prebiotic Diet and FLU on the Depressive Behavior of Mice Exposed to CUMS
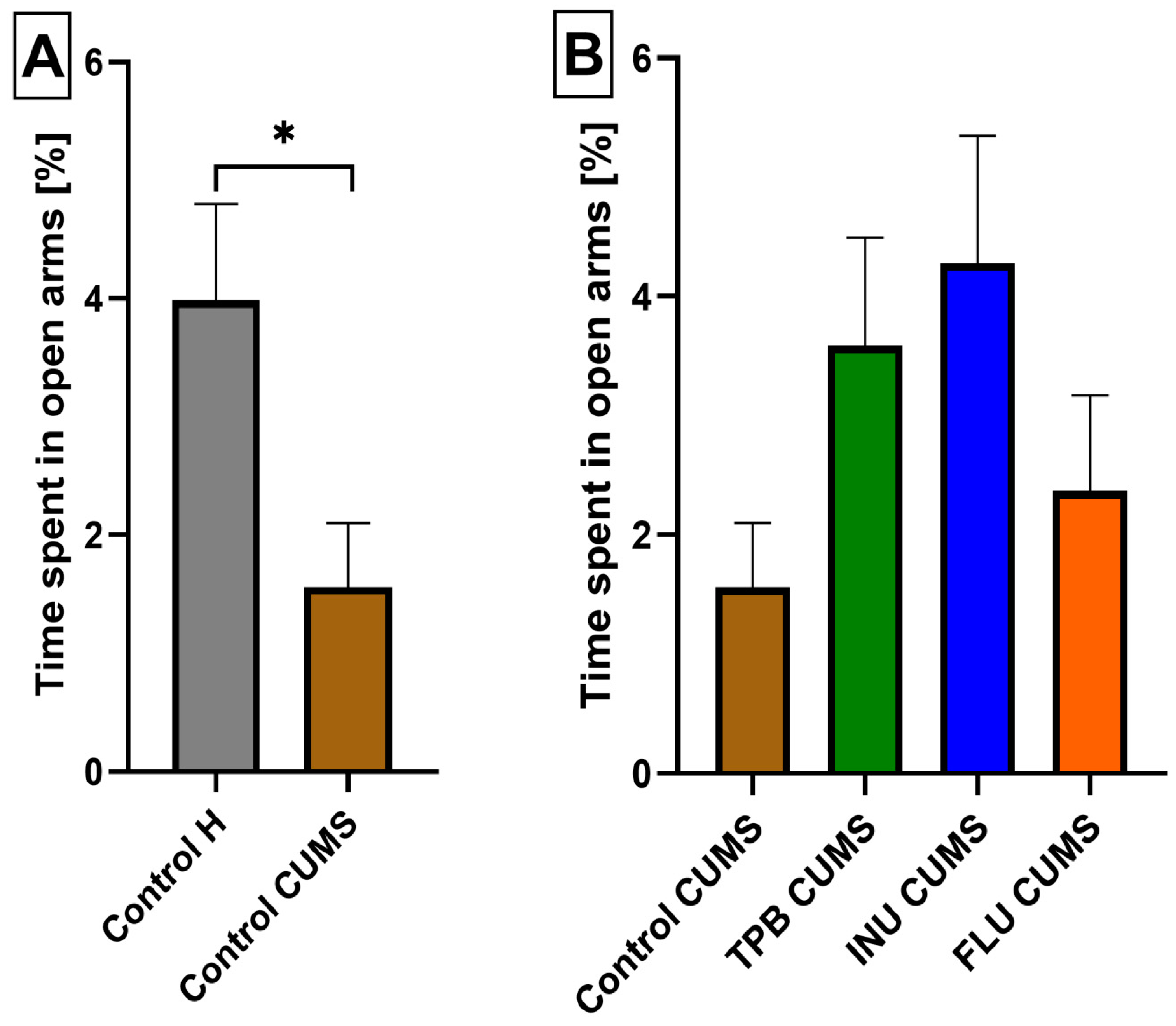
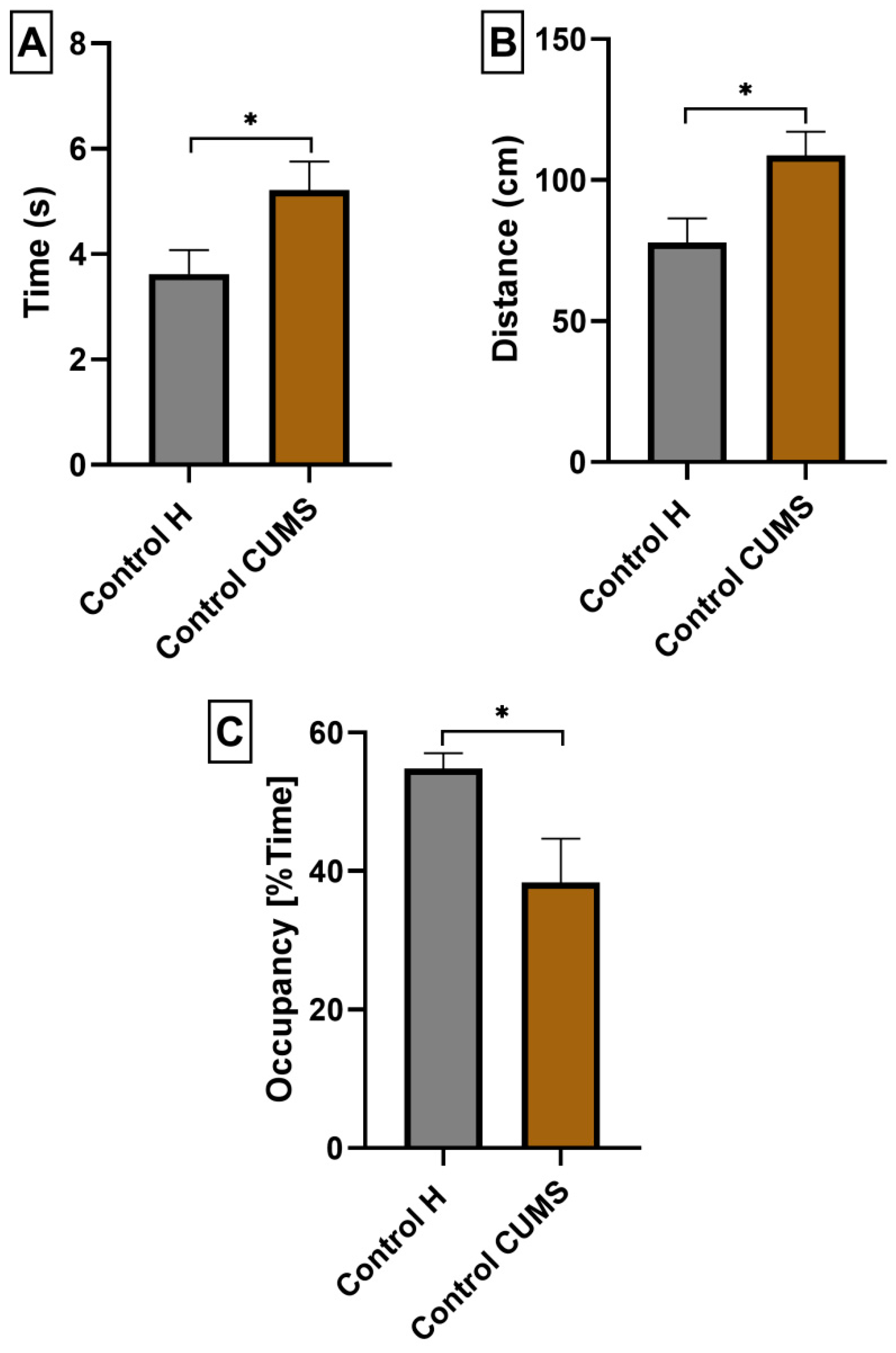
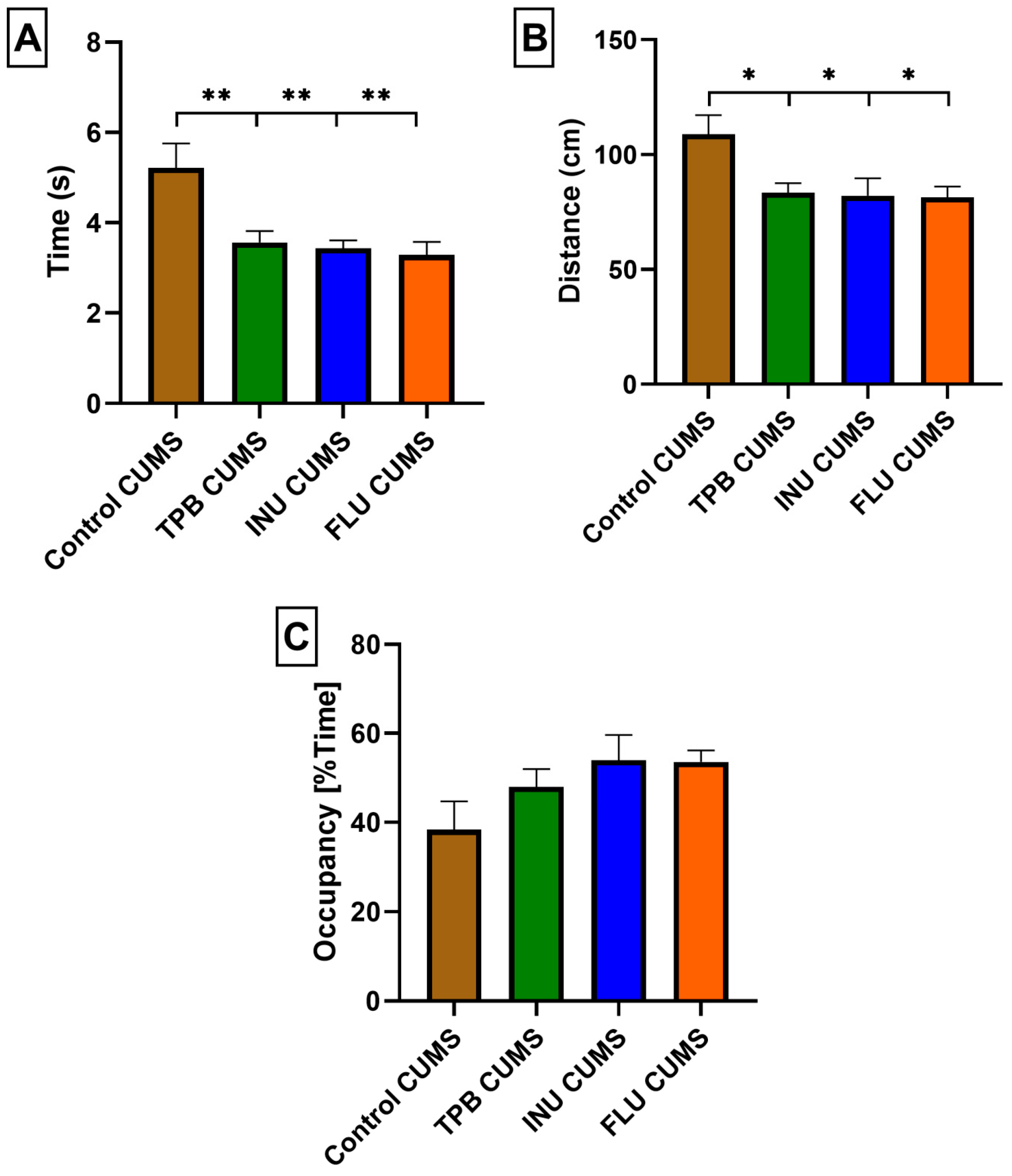
3.3. The Impact of a Prebiotic Diet and FLU on Anxiety Behavior of Mice Exposed to CUMS
3.4. The Effects of a Prebiotic Diet and FLU on Spatial Learning and Memory of Mice Exposed to CUMS
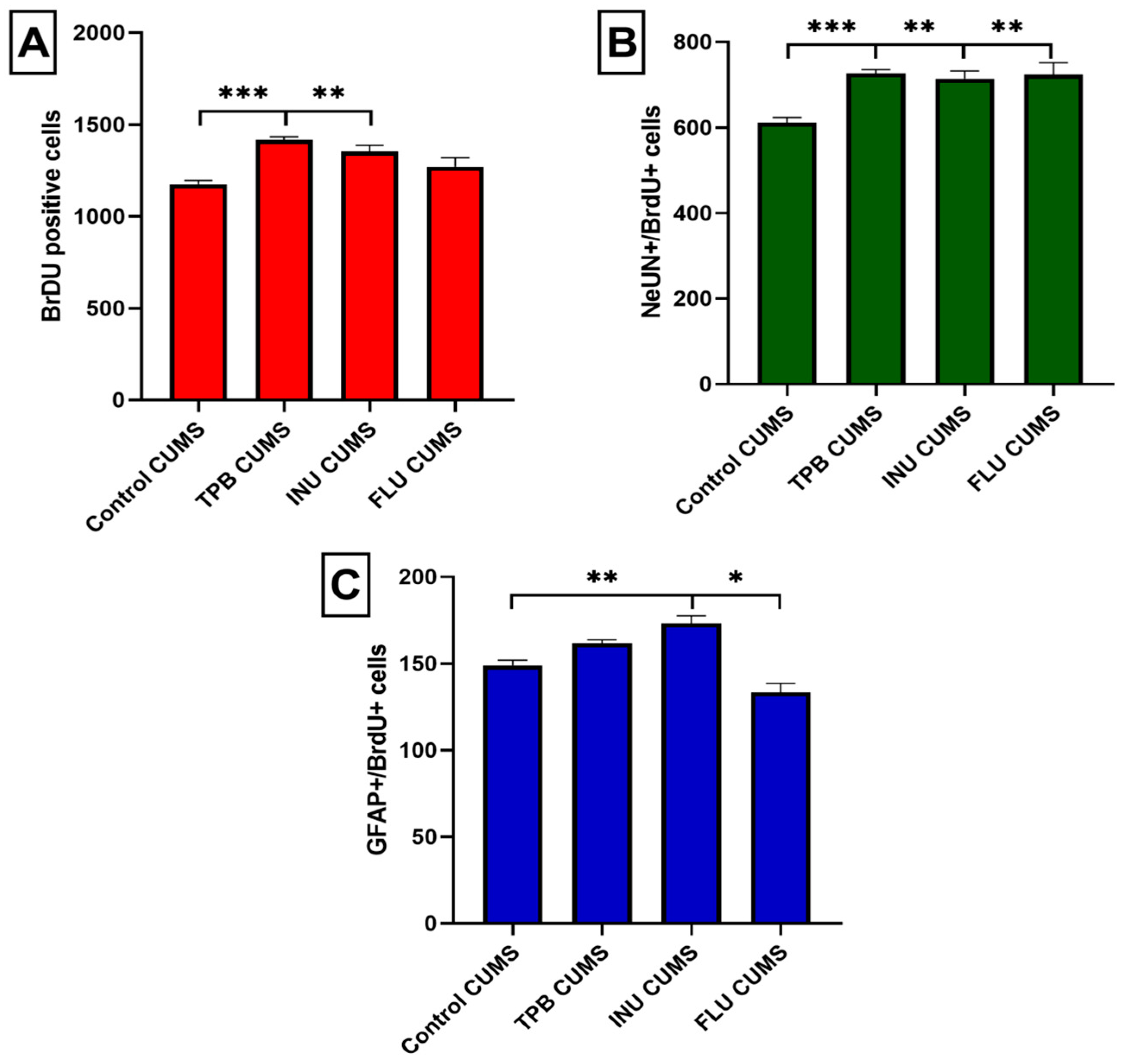
3.5. Effect of TPB, INU and FLU Administration on Neurogenesis in the SGZ and the GCL of theMouse Hippocampus Exposed to CUMS
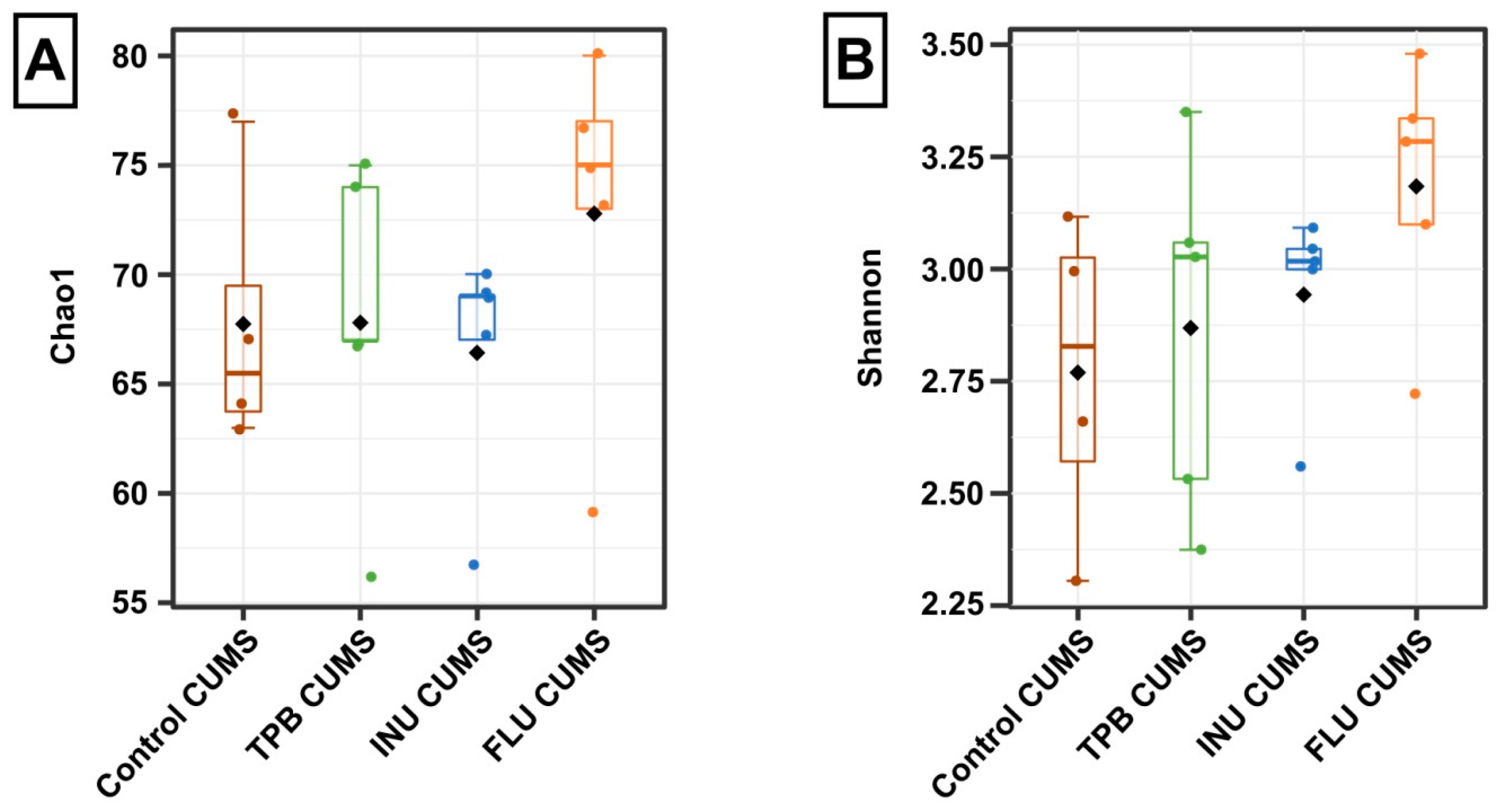
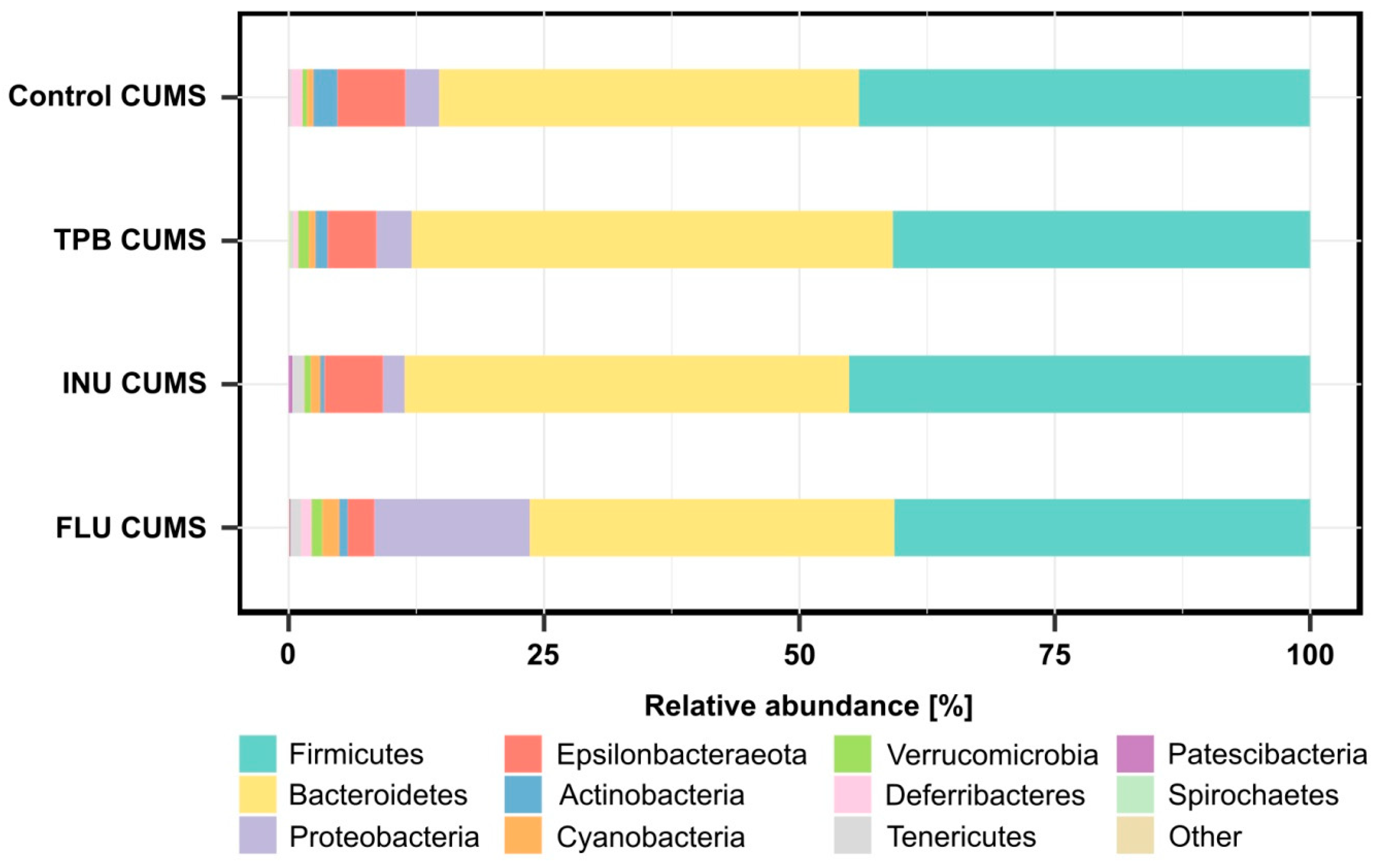
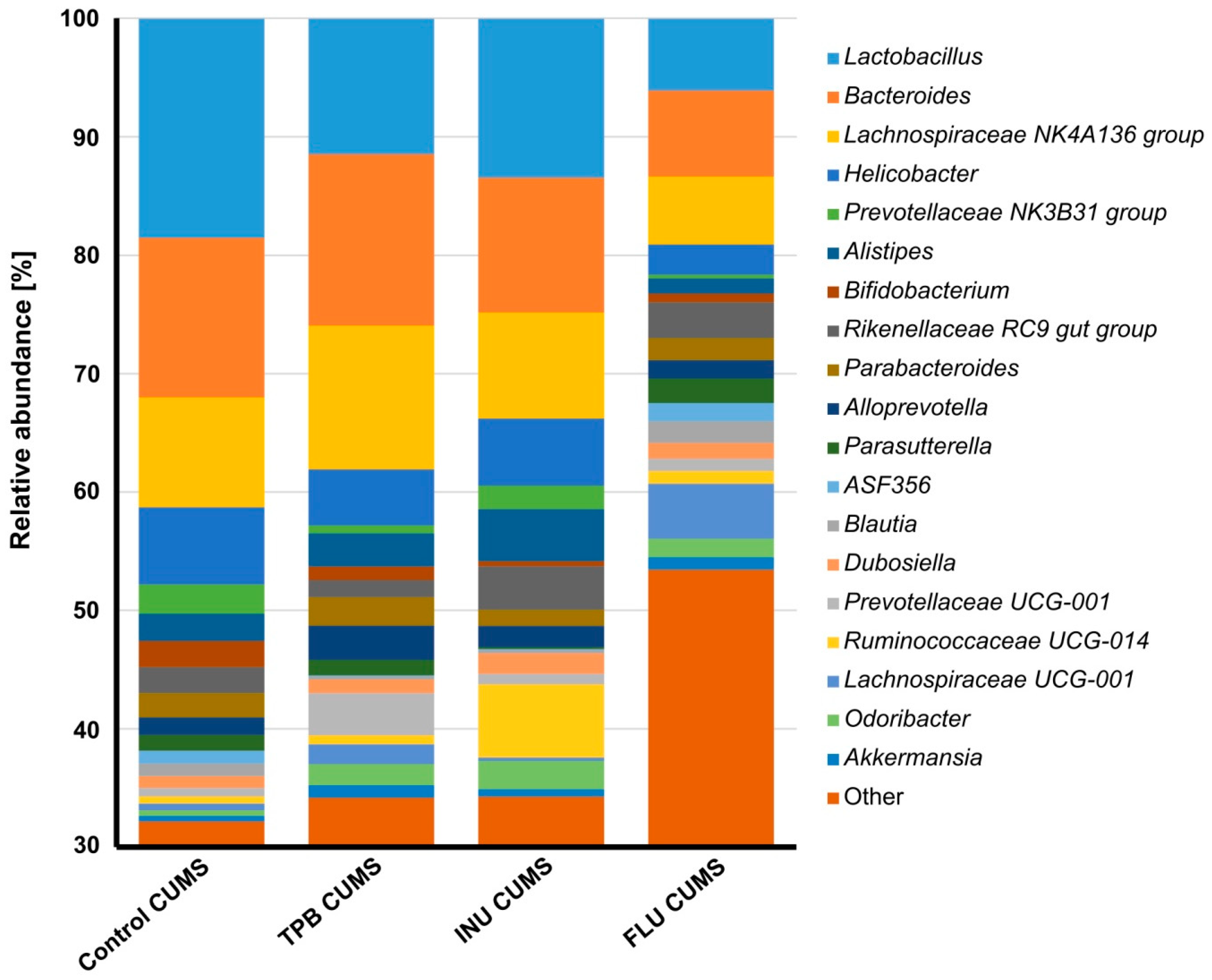
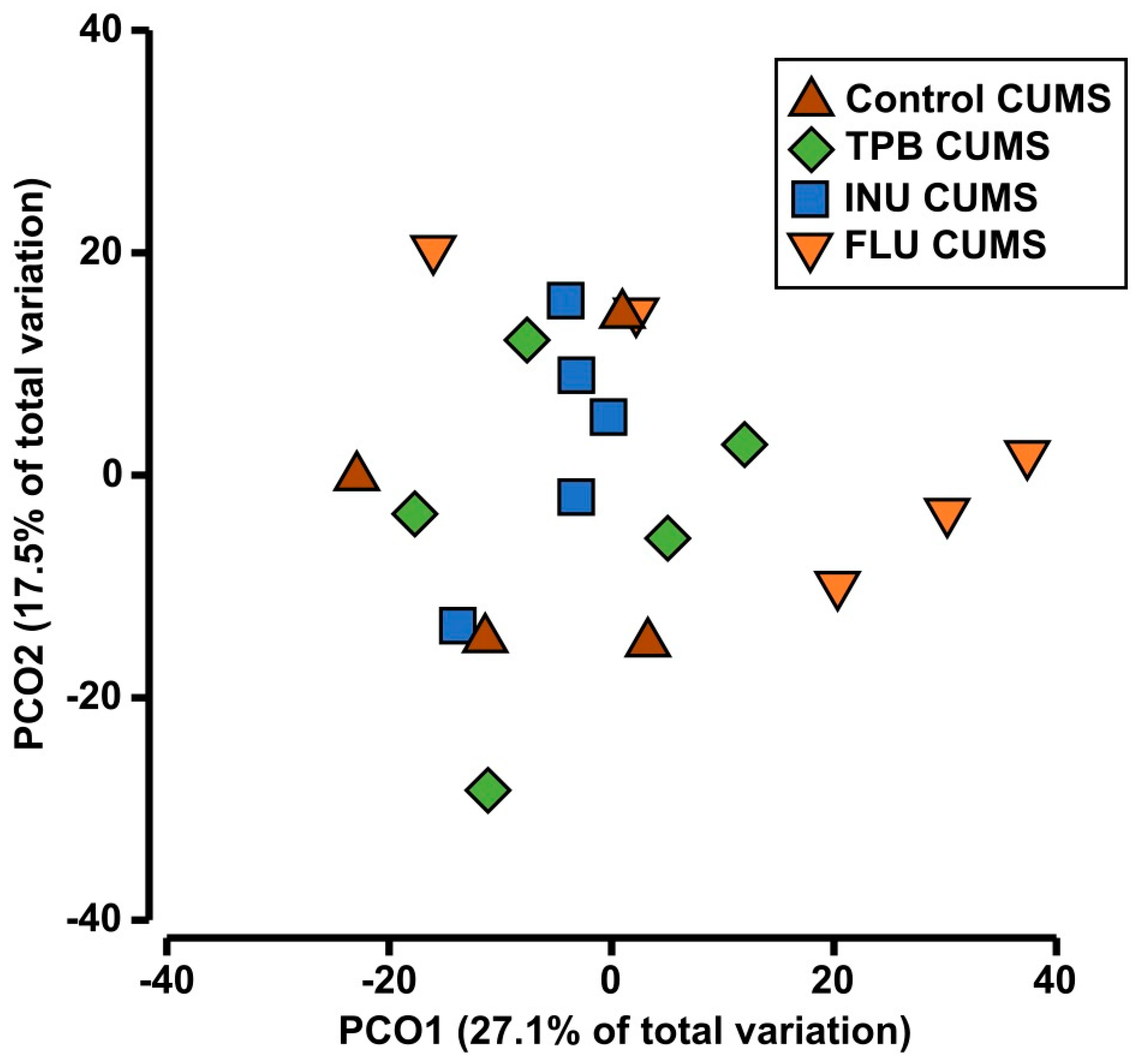
3.6. Effect of TPB, INU and FLU on the Composition of the Intestinal Microbiota in Mice Exposed to CUMS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Yan, J. Editorial: Stress and cognition. Front. Psychol. 2017, 8, 970. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.; Duman, R.S. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology 2003, 28, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Warner-Schmidt, J.L.; Duman, R.S. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus 2006, 16, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tong, J.; Zhang, J.; Farahvar, A.; Wang, E.; Yang, J.; Samadani, U.; Smith, D.H.; Huang, J.H. Imipramine treatment improves cognitive outcome associated with enhanced hippocampal neurogenesis after traumatic brain injury in mice. J. Neurotrauma 2011, 28, 995–1007. [Google Scholar] [CrossRef]
- Anacker, C.; Zunszain, P.A.; Cattaneo, A.; Carvalho, L.A.; Garabedian, M.J.; Thuret, S.; Price, J.; Pariante, C.M. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry 2011, 16, 738–750. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Burokas, A.; Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Microbiota regulation of the Mammalian gut–brain axis. Adv. Appl. Microbiol. 2015, 91, 1–62. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on gut microbiome dysbiosis and depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Gordon, J.I. Metagenomicanalysis in humanizedgnotobiotic mice. Sci.-Translat. Med. 2009, 1, 1–19. [Google Scholar]
- Rogers, G.B.; Keating, D.; Young, R.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.H.; Albenberg, L.G.; Nessel, L.; Golroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology and the microbiota-gut-brain Axis. Adv. Exp. Med. Biol. 2014, 817, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Schmidt, K.; Cowen, P.; Harmer, C.; Tzortzis, G.; Errington, S.; Burnet, P.W.J. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology 2014, 232, 1793–1801. [Google Scholar] [CrossRef]
- Kang, D.; Adams, J.B.; Gregory, A.C.; Borody, T.J.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; Mcdonough-Means, S.I.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.H.; Golubeva, A.V.; et al. The microbiota-gut-brainaxis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Meyer, T.S.M.; Miguel, A.S.M.; Fernández, D.E.R.; Ortiz, G.M.D. Biotechnological Production of Oligosaccharides—Applications in the Food Industry. Food Prod. Ind. 2015, 2, 25–78. [Google Scholar] [CrossRef]
- Johansson, E.; Prade, T.; Angelidaki, I.; Svensson, S.-E.; Newson, W.R.; Gunnarsson, I.B.; Hovmalm, H.P. Economically Viable Components from Jerusalem Artichoke (Helianthus tuberosus L.) in a Biorefinery Concept. Int. J. Mol. Sci. 2015, 16, 8997–9016. [Google Scholar] [CrossRef]
- Lee, Y.R. Analysis of Nutritional Components and Antioxidant Activity of Roasting Wooung (Burdock, Arctiumlappa L.) and Jerusalem Artichoke (Helianthus tuberosus L.). Korean J. Food Nutr. 2016, 29, 870–877. [Google Scholar] [CrossRef]
- Saengthongpinit, W.; Sajjaanantakul, T. Influence of harvest time and storage temperature on characteristics of inulin from Jerusalem artichoke (Helianthus tuberosus L.) tubers. Postharvest Biol. Technol. 2005, 37, 93–100. [Google Scholar] [CrossRef]
- Willner, P.; Muscat, R.; Papp, M. Chronic mild stress-induced anhedonia: A realistic animal model of depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- Ducottet, C.; Belzung, C. Behaviour in the elevated plus-maze predicts coping after subchronic mild stress in mice. Physiol. Behav. 2004, 81, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Belzung, C.; Crusio, W.E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006, 175, 43–50. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Kaczmarczyk-Ziemba, A.; Zagaja, M.; Wagner, G.K.; Pietrykowska-Tudruj, E.; Staniec, B. First Insight into Microbiome Profiles of Myrmecophilous Beetles and Their Host, Red Wood Ant Formica polyctena (Hymenoptera: Formicidae)—A Case Study. Insects 2020, 11, 134. [Google Scholar] [CrossRef]
- Szewczyk, A.; Andres-Mach, M.; Zagaja, M.; Kaczmarczyk-Ziemba, A.; Maj, M.; Szala-Rycaj, J. The Effect of a Diet Enriched with Jerusalem artichoke, Inulin, and Fluoxetine on Cognitive Functions, Neurogenesis, and the Composition of the Intestinal Microbiota in Mice. Curr. Issues Mol. Biol. 2023, 45, 2561–2579. [Google Scholar] [CrossRef]
- El Marzouki, H.; Aboussaleh, Y.; Najimi, M.; Chigr, F.; Ahami, A. Effect of Cold Stress on Neurobehavioral and Physiological Parameters in Rats. Front. Physiol. 2021, 12, 660124. [Google Scholar] [CrossRef]
- Detke, M.J.; Johnson, J.; Lucki, I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp. Clin. Psychopharmacol. 1997, 5, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar] [CrossRef]
- Yan, T.; Wang, N.; Liu, B.; Wu, B.; Xiao, F.; He, B.; Jia, Y. Schisandra chinensis ameliorates depressive-like behaviors by regulating microbiota-gut-brain axis via its anti-inflammation activity. Phytother. Res. 2020, 35, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mach, M.; Szewczyk, A.; Zagaja, M.; Luszczki, J.; Maj, M.; Rola, R.; Abram, M.; Kaminski, K. Evaluation of the impact of compound C11 a new anticonvulsant candidate on cognitive functions and hippocampal neurogenesis in mouse brain. Neuropharmacology 2020, 163, 107849. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mach, M.; Haratym-Maj, A.; Zagaja, M.; Rola, R.; Maj, M.; Chrościńska-Krawczyk, M.; Luszczki, J.J. ACEA (a highly selective cannabinoid CB1 receptor agonist) stimulates hippocampal neurogenesis in mice treated with antiepileptic drugs. Brain Res. 2015, 1624, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mach, M.; Zagaja, M.; Haratym-Maj, A.; Rola, R.; Maj, M.; Haratym, J.; Dudra-Jastrzębska, M.; Łuszczki, J.J. A long-term treatment with arachidonyl-2’-chloroethylamide combined with valproate increases neurogenesis in a mouse pilocarpine model of epilepsy. Int. J. Mol. Sci. 2017, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mach, M.; Szewczyk, A.; Zagaja, M.; Szala-Rycaj, J.; Lemieszek, M.K.; Maj, M.; Abram, M.; Kaminski, K. Preclinical Assessment of a New Hybrid Compound C11 Efficacy on Neurogenesis and Cognitive Functions after Pilocarpine Induced Status Epilepticus in Mice. Int. J. Mol. Sci. 2021, 22, 3240. [Google Scholar] [CrossRef]
- Paiva, I.H.R.; Duarte-Silva, E.; Peixoto, C.A. The role of prebiotics in cognition, anxiety, and depression. Eur. Neuropsychopharmacol. 2020, 34, 1–18. [Google Scholar] [CrossRef]
- Yang, X.-D.; Wang, L.-K.; Wu, H.-Y.; Jiao, L. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiol. 2018, 18, 177. [Google Scholar] [CrossRef]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romani-Pérez, M.; Sanz, Y. Interplay between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Weidinger, E.; Leitner, B.; Schwarz, M.J. The role of inflammation in schizophrenia. Front. Neurosci. 2015, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.P.; De Miranda, A.S.; Teixeira, A.L. Insights into Neuroinflammation in Parkinson’s Disease: From Biomarkers to Anti-Inflammatory Based Therapies. BioMed Res. Int. 2015, 2015, 628192. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.-K.; Liu, C.-H.; Gao, Z.-W.; He, J.-L.; Liu, X.; Wei, Q.-L.; Chen, J.-S. The inulin-type oligosaccharides extract from morinda officinalis, a traditional Chinese herb, ameliorated behavioral deficits in an animal model of post-traumatic stress disorder. Metab. Brain Dis. 2016, 31, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Mika, A.; Day, H.E.W.; Martinez, A.; Rumian, N.L.; Greenwood, B.N.; Chichlowski, M.; Berg, B.M.; Fleshner, M. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur. J. Neurosci. 2017, 45, 342–357. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef]
- Neufeld, K.-A.M.; O’Mahony, S.M.; Hoban, A.E.; Waworuntu, R.V.; Berg, B.M.; Dinan, T.G.; Cryan, J.F. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr. Neurosci. 2019, 22, 425–434. [Google Scholar] [CrossRef]
- Chi, L.; Khan, I.; Lin, Z.; Zhang, J.; Lee, M.Y.S.; Leong, W.; Hsiao, W.L.W.; Zheng, Y. Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine 2020, 67, 153157. [Google Scholar] [CrossRef]
- An, L.; Yang, J.-C.; Yin, H.; Xue, R.; Wang, Q.; Sun, Y.C.; Zhang, Y.-Z.; Yang, M. Inulin-Type Oligosaccharides Extracted from Yacon Produce Antidepressant-like Effects in Behavioral Models of Depression. Phytother. Res. 2016, 30, 1937–1942. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, P.; Zhang, X.; Yang, Y.; Yang, M.; Wang, T.; Lu, H.; Tian, H.; Wang, H.; Liu, J. Inulin ameliorates schizophrenia via modulation of the gut microbiota and anti-inflammation in mice. Food Funct. 2021, 12, 1156–1175. [Google Scholar] [CrossRef]
- Cruz-Pereira, J.S.; Moloney, G.M.; Bastiaanssen, T.F.; Boscaini, S.; Tofani, G.; Borras-Bisa, J.; van de Wouw, M.; Fitzgerald, P.; Dinan, T.G.; Clarke, G.; et al. Prebiotic supplementation modulates selective effects of stress on behavior and brain metabolome in aged mice. Neurobiol. Stress 2022, 21, 100501. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozcan, E.; Peng, C.-Y.; Zhu, Y.; Dunlop, S.R.; Contractor, A.; Kessler, J.A. Activating newborn neurons suppresses depression and anxiety-like behaviors. Nat. Commun. 2019, 10, 3768. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Rozan, P.; Nejdi, A.; Hidalgo, S.; Desor, D. Behavioural and cognitive effects of oligofructose-enriched inulin in rats. Br. J. Nutr. 2005, 93, S27–S30. [Google Scholar] [CrossRef] [PubMed]
- David, D.J.; Samuels, B.A.; Rainer, Q.; Wang, J.-W.; Marsteller, D.; Mendez, I.; Drew, M.; Craig, D.A.; Guiard, B.P.; Guilloux, J.-P.; et al. Neurogenesis-Dependent and -Independent Effects of Fluoxetine in an Animal Model of Anxiety/Depression. Neuron 2009, 62, 479–493. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Rong, H.; Xie, X.-H.; Zhao, J.; Lai, W.-T.; Wang, M.-B.; Xu, D.; Liu, Y.-H.; Guo, Y.-Y.; Xu, S.-X.; Deng, W.-F.; et al. Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef]
- Zhang, M.; Li, A.; Yang, Q.; Li, J.; Wang, L.; Liu, X.; Huang, Y.; Liu, L. Beneficial Effect of Alkaloids from Sophora alopecuroides L. on CUMS-Induced Depression Model Mice via Modulating Gut Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 665159. [Google Scholar] [CrossRef]
- Yamane, T.; Handa, S.; Imai, M.; Harada, N.; Sakamoto, T.; Ishida, T.; Nakagaki, T.; Nakano, Y. Exopolysaccharides from a Scandinavian fermented milk viili increase butyric acid and Muribaculum members in the mouse gut. Food Chem. Mol. Sci. 2021, 3, 100042. [Google Scholar] [CrossRef]
- Nakayama, J.; Yamamoto, A.; Palermo-Conde, L.A.; Higashi, K.; Sonomoto, K.; Tan, J.; Lee, Y.-K. Impact of Westernized Diet on Gut Microbiota in Children on Leyte Island. Front. Microbiol. 2017, 8, 197. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Wang, X.; Xing, T.; Li, J.; Zhu, X.; Zhang, L.; Gao, F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 2020, 100, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-Like Effects of the Histone Deacetylase Inhibitor, Sodium Butyrate, in the Mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef]
- Kim, C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell. Mol. Immunol. 2021, 18, 1161–1171. [Google Scholar] [CrossRef]
- Walsh, J.; Griffin, B.T.; Clarke, G.; Hyland, N.P. Drug-gut microbiota interactions: Implications for neuropharmacology. Br. J. Pharmacol. 2018, 175, 4415–4429. [Google Scholar] [CrossRef]
- Evrensel, A.; Ünsalver, B.; Ceylan, M.E. Immune-Kynurenine Pathways and the Gut Microbiota-Brain Axis in Anxiety Disorders. Anxiety Disord. Rethink. Underst. Recent Discov. 2020, 1191, 155–167. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Cao, Y.; Wang, C.; Zhao, C.; Wang, H.; Cui, G.; Wang, M.; Pan, Y.; Shi, Y.; et al. Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int. J. Med. Sci. 2019, 16, 1260–1270. [Google Scholar] [CrossRef]
- Lawson, P.A.; Song, Y.; Liu, C.; Molitoris, D.R.; Vaisanen, M.L.; Collins, M.D.; Finegold, S.M. Anaerotruncus colihominis gen. nov., sp. nov., from human faeces. Int. J. Syst. Evol. Microbiol. 2004, 54, 413–417. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Futur. Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Bhutia, Y.D.; Yang, S.; Ganapathy, V. Short-chain fatty acid transporters: Role in colonic homeostasis. Compr. Physiol. 2017, 8, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Panthi, S.; Manandhar, S.; Gautam, K. Hydrogen sulfide, nitric oxide, and neurodegenerative disorders. Transl. Neurodegener. 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Lasaro, M.; Liu, Z.; Bishar, R.; Kelly, K.; Chattopadhyay, S.; Paul, S.; Sokurenko, E.; Zhu, J.; Goulian, M. Escherichia coli isolate for studying colonization of the mouse intestine and its application to two-component signaling knockouts. J. Bacteriol. 2014, 196, 1723–1732. [Google Scholar] [CrossRef] [PubMed]

| Days | CUMS | |
|---|---|---|
| Stress Factor | Duration of the Stimulus | |
| 1 | Tilt the cage (45°) | 4 h |
| 2 | Wet litter | 24 h |
| 3 | Light at night | 12 h |
| 4 | Restraint | 2 h |
| 5 | Electric buzzer 90 dB | 5 min |
| 6 | Water deprivation | 24 h |
| 7 | Food deprivation | 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szala-Rycaj, J.; Szewczyk, A.; Zagaja, M.; Kaczmarczyk-Ziemba, A.; Maj, M.; Andres-Mach, M. The Influence of Topinambur and Inulin Preventive Supplementation on Microbiota, Anxious Behavior, Cognitive Functions and Neurogenesis in Mice Exposed to the Chronic Unpredictable Mild Stress. Nutrients 2023, 15, 2041. https://doi.org/10.3390/nu15092041
Szala-Rycaj J, Szewczyk A, Zagaja M, Kaczmarczyk-Ziemba A, Maj M, Andres-Mach M. The Influence of Topinambur and Inulin Preventive Supplementation on Microbiota, Anxious Behavior, Cognitive Functions and Neurogenesis in Mice Exposed to the Chronic Unpredictable Mild Stress. Nutrients. 2023; 15(9):2041. https://doi.org/10.3390/nu15092041
Chicago/Turabian StyleSzala-Rycaj, Joanna, Aleksandra Szewczyk, Mirosław Zagaja, Agnieszka Kaczmarczyk-Ziemba, Maciej Maj, and Marta Andres-Mach. 2023. "The Influence of Topinambur and Inulin Preventive Supplementation on Microbiota, Anxious Behavior, Cognitive Functions and Neurogenesis in Mice Exposed to the Chronic Unpredictable Mild Stress" Nutrients 15, no. 9: 2041. https://doi.org/10.3390/nu15092041
APA StyleSzala-Rycaj, J., Szewczyk, A., Zagaja, M., Kaczmarczyk-Ziemba, A., Maj, M., & Andres-Mach, M. (2023). The Influence of Topinambur and Inulin Preventive Supplementation on Microbiota, Anxious Behavior, Cognitive Functions and Neurogenesis in Mice Exposed to the Chronic Unpredictable Mild Stress. Nutrients, 15(9), 2041. https://doi.org/10.3390/nu15092041






